Abstract
We measured the total mercury (THg) concentration in the muscle of northern pike (Esox lucius L.) from 12 boreal headwater lakes once per decade during the 1980s to 2010s. Relations of pike THg to regional environmental changes, such as reduction of acid deposition and increase in water colour (i.e. browning), were examined as well as the effects of local lake-specific changes caused by introduced beavers (Castor canadensis) and intensive fishing of pike. No consistent decrease in pike THg took place during 1983–2013 despite the reductions in mercury emissions from European sources since the 1990s and the associated decreased mercury deposition in Finland. In most of the lakes, individual pike exceeded the recommended limit for human consumption (1 mg kg−1, wet weight). The highest THg levels were recorded in 2002 following intensive browning of the lakes in the 1990s in connection with decreased sulphate deposition. The effects of acid deposition were only seen as the highest pike THg in the two most acidic lakes in the early 1980s. After taking length effects into account, pike THg had a negative relationship with growth (i.e. growth dilution), but a positive relationship with water colour, iron, nutrients and maximum lake depth. Females grew faster and had lower THg than males. Pike THg was elevated in beaver-impacted lakes. Experimental removal of pike from three study lakes during 2005–2013 resulted in a decrease of pike THg. These findings emphasize the significance of local abiotic and biotic drivers when interpreting the regional long-term trends in pike THg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mercury (Hg) is a toxic metal occurring naturally in the environment. Since the beginning of the industrial revolution, mercury has been widely released into the environment due to human activities like mining, industrial point sources, agriculture and atmospheric deposition from burning of fossil fuels, especially coal (e.g. Streets et al., 2017). Mercury occurs in various forms in nature: elemental mercury (Hg0), inorganic mercury salts (HgI and HgII) and organic mercury compounds, especially toxic methylmercury (MeHg). The symptoms of acute mercury poisoning are connected to disturbances in the central nervous system. Because of the risk to human health, upper limits for the mercury content of edible fish have been set widely (EU, 2006). More recently, the Minamata Convention on Mercury entered into force in August 2017 (Wang et al., 2019). This treaty obligates the signed countries to control Hg emissions and the release of Hg to ambient air, water bodies and soil. It addresses several aspects necessary to reduce Hg use globally and to protect human health and the environment from the adverse effects of mercury.
Fish accounts for most of the mercury intake by humans from daily food, and over 95 % of the Hg contained in adult predatory fish muscle is MeHg (Bloom, 1992; but see Lescord et al., 2018) that bioaccumulates in organisms and biomagnifies in aquatic food web (e.g. Lavoie et al., 2013). High fish mercury concentrations, exceeding the recommended limits for human consumption (1 mg kg−1), were measured in Nordic lakes during the 1960s and 1970s (Häsänen and Sjöblom, 1968; Lindeström, 2001). Since then, a general decreasing trend, although fluctuating, has been reported (Åkerblom et al., 2014; Braaten et al., 2019). This is due to the restrictions of use of Hg in industrial processes (Lodenius, 1991; Lindeström, 2001) and due to the decrease of mercury emissions from European sources since the early 1990s (Travnikov et al., 2012) followed by decreased mercury deposition (Wängberg et al., 2010; Ruoho-Airola et al., 2014).
In headwater lakes, where mercury mainly originates from atmospheric deposition to the catchment and directly to the lake surface, high and even increasing fish Hg levels were reported in recent studies (Wyn et al., 2010; Braaten et al., 2014; Rask et al., 2021). This is connected to long-term environmental changes, such as recovery of lakes from acidification after reduced sulphate deposition (Keller et al., 2007; Rask et al., 2021), subsequent browning of lake waters due to increased load of dissolved organic carbon (DOC) compounds (Vuorenmaa et al., 2006; Monteith et al., 2007; Arvola et al., 2010; Blanchet et al., 2022) and different effects of climate change (Gandhi et al., 2014; Ahonen et al., 2018; Eagles-Smith et al., 2018; de Wit et al., 2021). As a consequence of these changes, legacy mercury load from catchments to lakes has increased due to complexation reactions between DOC and Hg compounds. This has often resulted in increased mercury concentration of fish (Hongve et al., 2012; Braaten et al., 2018; Moslemi-Aqdam et al., 2023).
Besides the large-scale atmospheric deposition and climatic effects, DOC and Hg dynamics can be affected locally via beaver activities and by forestry (e.g. Blanchet et al., 2022). The effects of beavers are typically strongest during the first 3 years of flooding (Vehkaoja et al., 2015; Nummi et al., 2018). Increasing DOC flux from riparian areas brings bound mercury and subsequently lowers growth rate and elevate mercury levels in food webs especially in top predatory fish, such as northern pike Esox lucius L. (Moslemi-Aqdam et al., 2021, Moslemi-Aqdam et al., 2022). Forest harvesting as an important form of land use in countries of boreal region can increase the DOC and mercury load to lakes and bioaccumulation of mercury to fish (Rask et al., 1994; Garcia & Carignan, 2000; Porvari et al., 2003; Wu et al., 2018; Ahonen et al., 2018; Eklöf et al., 2018).
Pike is one of the most common fish species in the boreal lakes of the Nordic countries (Tammi et al., 2003). As a top predator of food chains in boreal lakes, pike often show the highest fish mercury concentrations (Häsänen and Sjöblom, 1968; Göthberg, 1983; Heiskary and Helwig, 1986; Verta, 1990; Porvari, 1998; Lindeström, 2001; Kozak et al., 2021). Available prey resources will directly affect the growth rate of predatory fish, which is generally inversely correlated with the total mercury (THg) content, and is often referred to as mercury growth dilution, as described for many different species (Keva et al., 2017; Rask et al., 2021; Moslemi-Aqdam et al., 2022). Fish growth is dependent on both environmental and biotic factors, where intensive fishing of the fish community including predatory fish has often led to reduction of the mercury content of top predator fish such as pike (Göthberg, 1983; Verta, 1990; Sharma et al., 2008).
Considering the Hg dynamics of the entire food web of a lake ecosystem, the bioaccumulation of MeHg in organisms at the base of the food chain is an important controller of Hg concentrations in taxa at higher trophic levels (Yu et al., 2011; Painter et al., 2015; Kahilainen et al., 2017; Kozak et al., 2021). Habitat- and species-specific differences in Hg bioavailability are affecting the fish Hg levels, and the dominance of pelagic energy sources is often related to a higher mercury content in fish (Karimi et al., 2016; Kahilainen et al., 2016; Arcagni et al., 2018; Moslemi-Aqdam et al., 2022). Microbial communities in stratified lakes with anoxic hypolimnion are also important (Verta et al., 2010; Rask et al., 2010; Karhunen et al., 2013) because sulphate-reducing bacteria under anoxic conditions are mainly responsible for the methylation of inorganic mercury to MeHg in freshwater ecosystems (Ullrich et al., 2001; Eckley et al., 2005; Bravo et al., 2017).
We measured pike THg content in 12 small headwater lakes in the Evo forest area, southern Finland, during 1983–2013. During these four decades, sulphate and Hg deposition decreased (Vuorenmaa, 2004; Wängberg et al., 2010; Ruoho-Airola et al., 2014), and browning of lakes took place, especially in the 1990s (Vuorenmaa et al., 2006, Arvola et al., 2010; Arzel et al., 2020). Questions related to the potential effects of these long-term environmental changes include the following: (1) did the pike mercury decrease due to lower Hg deposition (Ruoho-Airola et al., 2014) or due to reduced methylation activity of sulphate-reducing bacteria after decreased sulphate deposition (Hrabik & Watras, 2002; Braaten et al., 2020)? Or (2) did pike mercury concentrations increase in the course of lake browning due to increased dissolved organic matter load from catchments to the lakes accompanied by increased load of historical mercury (Hongve et al., 2012; Braaten et al., 2018; Moslemi-Aqdam et al., 2022)? In addition, some of our study lakes have been subject to two important local changes that could affect the overall Hg dynamics of lakes. First, beavers have occupied several lakes over the years (Vehkaoja et al., 2015) with a subsequent increase in DOC fluxes and availability of mercury in the ecosystem (Painter et al., 2015; Ecke et al., 2017). We here address the question, (3) did beaver floods increase pike mercury in these lakes? Second, in a lake-scale experimental research on sustainable fishery management (Lehtonen et al., 2017), some lakes were subject to effective fishing and here the question is, (4) did intensive pike removal decrease pike mercury due to growth dilution (cf. Lavigne et al., 2010; Moslemi-Aqdam et al., 2022)?
2 Material and Methods
2.1 Study Lakes
We sampled pike from 12 natural headwater lakes for THg analyses at circa 10-year intervals between 1983 and 2013. The lakes are located in the Evo forest area, southern Finland (Fig. 1), which is part of the Lammi Long-Term Ecological Research area (LTER, Rask et al., 2014a). The catchment areas of the lakes are 0.04–62 km2 and mainly covered by coniferous boreal forest on peatland, sandy or rocky landscape. The lakes are small with surface area between 1 and 45 ha, maximum depth 6–14 m, and altitude 126–156 m above sea level (Table 1). All 12 lakes are inhabited by pike and perch (Perca fluviatilis L.) and 10 of them also by roach (Rutilus rutilus L.). Other species like ruffe (Gymnocephalus cernuus L.), bream (Abramis brama L.) and bleak (Alburnus alburnus L.) were present to a lesser extent (Olin et al., 2017a). A diet study on three of the lakes indicated that perch and roach clearly dominated the diet of pike (Tiainen, 2017). Water quality data for 1983 were from Arvola et al. (1990) and Rask and Metsälä (1991), and for the years 1993, 2002 and 2013 from the databases of the Lammi Biological Station, University of Helsinki (for details, see Arvola et al., 2010). Briefly, the lakes were acidic with a pH range 4.9–6.7 and alkalinity from 0 to 0.25 mmol L−1. Water colour ranged from very clear to highly humic (7−495 mg Pt L−1), and the nutrient levels of lakes varied from oligotrophic to eutrophic (tot P 2–73 µg L−1 and tot N 218–1107 µg L−1). Ca ranged between 0.7 and 6.6 mg L−1 and iron from 0.1 to 1.6 mg L−1 (Table 1).
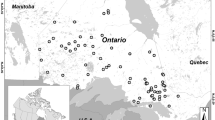
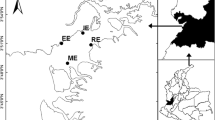
The location of the 12 study lakes in southern Finland and in the Evo area. The lake numbers are as given in Table 1; chain lakes 1–6 and headwater lakes 7–12
Half of the lakes (numbers 1–6 in Table 1) belong to the main lake chain of the Evo area and are subsequently referred to as “chain lakes”, whereas the rest (lakes 7–12) are seepage lakes or uppermost drainage lakes with an outlet, but no inlet, and designated as “headwater lakes”. The chain lakes are larger (12–45 ha) than the headwater lakes (0.9–4.2 ha) and are therefore less vulnerable to lake-specific changes due to direct human (forestry, fishing) or beaver (flooding) activities in their catchments. Most of the lakes are mainly fed by surface runoff, but two of them (L. Syrjänalunen and L. Iso Valkjärvi) are groundwater lakes with clear water. The headwater lakes L. Iso Valkjärvi and L. Valkea-Kotinen acidified to pH levels of <5 during the 1980s (Rask & Metsälä, 1991) but have recovered from the acidification since the early 1990s.
Introduced beavers (Castor canadensis) are common in the lakes of the study area (Vehkaoja et al., 2015; Kivinen et al., 2020) and have temporarily caused floods on the shores of certain lakes (L. Haarajärvi, L. Haukijärvi, L. Majajärvi, L. Huhmari), potentially affecting the Hg dynamics in the lake-catchment systems. During 2005–2013, three lakes in the present study (L. Haarajärvi, L. Haukijärvi, L. Majajärvi) were included in the project “Ecologically sustainable fishing” (Lehtonen et al., 2017). This project offered an opportunity to assess the effect of intensive pike fishing (Tiainen et al., 2017) on mercury concentration.
2.2 Sampling of Fish
Fish for mercury analyses were caught in May–September 1983/1984, 1993, 2002 and 2013 with wire traps, fyke nets and hook and line (5–21 pike per lake and per year, 26 to 60 per lake in total and 490 in the whole study, Table 2). Samples from the years 1983 (n = 108) and 1984 (n = 45) were pooled together to represent the pike THg level of the early 1980s. For 2002, missing samples from L. Valkea-Kotinen were replaced with those taken in 1999. No samples from L. Huhmari were available in 2013.
Total length and weight were measured with an accuracy of 1 cm and 1 g for each fish, and sex was determined when possible (117 females and 95 males). Samples for mercury analyses were taken from dorsal white muscle and frozen (−20 °C) until analysed. Cleithrum bones of fish were taken for age determination (age data available for 93 females and 77 males) and back-calculation of growth (Casselman, 1996). As fish growth rate is known to affect mercury bioaccumulation (Göthberg, 1983; Verta, 1990), a simple proxy of pike growth for each lake was calculated by dividing the length of sampled fish by their age (mean annual length increment, Table 2).
2.3 Mercury Analyses
The mercury content of pike samples was determined in the Department of Biological and Environmental Sciences, University of Helsinki. In 1983–2002, a cold vapour atomic absorption spectrophotometric technique and a PerkinElmer/Bacharac Coleman 50b MAS analyser (detection limit 0.01 µg Hg g−1) was used. Prior to and after the analyses, the calibration of the instrument was checked with a mercury standard solution, and acid-permanganate solution was used as blank samples during the analyses (Rask & Metsälä, 1991; Rask et al., 2007). Duplicate samples (0.1–0.25 g, ww) from each fish were analysed, and the mean content of duplicates was used in subsequent analyses. The values of fish mercury concentrations are given as THg, mg kg−1 of fresh muscle tissue (wet weight, ww). For ascertaining the quality of analyses, two common reference materials were used: dogfish muscle (National Research Council Canada, Certified Reference Material DORM-1, mercury content 0.794 ± 0.074 mg kg−1) and cod muscle (European Commission Community Bureau of Reference, BCR-422, mercury content 0.559 ± 0.016 mg kg−1).
In 2013, an atom absorption spectrometer direct mercury analyser (Milestone DMA-80, Sorisole, Italy) was used. Certified reference materials DORM-4 fish protein (National Research Council, Canada, mercury concentration 0.410 ± 0.055 mg kg−1) and cod muscle (European Commission Community Bureau of Reference, BCR-422, mercury content 0.559 ± 0.016 mg kg−1) were used for quality assurance of the analyses at the beginning and end of each run.
2.4 Statistical Analyses
The effects of year (1983, 1993, 2002 and 2013), lake (12 lakes), fish sex, fish length (covariate) and their interactions on THg concentration was analysed with a general linear mixed model (GLMM).
The study design is a four-timepoint (year) repeated measurement structure. Within each timepoint, 4–21 THg measurements from random samples of pike of different sizes (12–100 cm, n = 490) were obtained from 12 lakes. The fish length was regarded as a continuous variable, and therefore, the data were analysed with a random slope model in which a separate slope was fitted for each year and lake. A general linear mixed model was fitted using a normal distribution (identity link) (Stroup, 2013), and the Kenward-Roger approximation was used for analysis of degrees of freedom. The distribution of pike THg values was right skewed and therefore ln-transformation was applied. All analyses were conducted in log scale, and the results were back transformed with an exponential function and presented in the original scale.
The symmetricity of residual distribution and model fit was checked from the shape of Pearson residuals and from the observed vs predicted plots. The effect of outliers was checked by reanalysing datasets in which observations with Pearson residual > |2.5| were excluded. Results were not changed, and therefore, the original dataset was used for analysis. The modelling was performed by the GLIMMIX procedure of the SAS/STAT software (version 9.4, SAS Institute, 2018).
The THg model structure for lake and year effects is:
where F stands for fixed effect, C for covariate and R for random effect. Because the fish for each lake and year were random samples from the fish population, the data were analysed with a random slope (fish length) model in which a separate slope was fitted for each year and lake.
To estimate the effects of sex, growth (mean annual length increment) and environmental variables (lake depth, water colour, alkalinity, total phosphorus, total nitrogen, Fe and Ca) on pike THg, the parameters were separately added to the linear model as an additional covariate. Inter-correlation among the environmental variables (Table 3) was not taken into account.
The model-fitted year THg estimates are covariate-adjusted, and they answer to the question about association between pollution, climate and natural factors and THg concentration. The slope estimates of covariates show the direction (decrease, increase) and strength of the effect. The association cannot be directly seen because of different age and size distributions of fish in different lakes but needs to be modelled.
In total, 10 different THg models were fitted and are presented in Table 4 (models 1–10). For year and lake comparisons (Table 4, model 1), the model-estimated averages were obtained from contrast specification which consists of model effects (year, lake, length) and G-side random effects (length) and their coefficients (ESTIMATE statement in SAS PROC GLIMMIX). The pairwise comparisons of years and years within lakes were calculated with linear contrasts including fixed effects, covariates and random slope effects (ESTIMATE statement). Due to multiple statistical tests, p-values and confidence intervals in each test set were adjusted by using a simulation-based correction in a step-down fashion (Westfall, 1997, SAS 9.4 documentation: PROC GLIMMIX, ESTIMATE statement). This is a less conservative approach compared to the Bonferroni method. Conclusions from statistical comparisons are based on adjusted p-values and confidence intervals when applicable.
For year, lake and sex comparisons (Table 4, models 1 and 2), the fish length was adjusted to 54.0 cm, which corresponds to a 1-kg fish (Rask & Metsälä, 1991). With the rest of the THg models (Table 4), the adjustment was done for a 43.9-cm length (data average). For details of the THg model calculations, see the supplementary information (Tables SI 1 and SI 2).
The effects of year (1983 and 2013), lake (12 lakes), fish sex, fish length or age (covariate) and their interactions on growth were analysed with a GLMM in the same manner as above. Two different growth models were fitted (Table SI 3), and statistical models are presented in Table 4 (models 11 and 12).
3 Results
3.1 No Consistent Change in the Mean Pike THg During 1983–2013
The model-estimated annual average pike THg of all 12 lakes, length-corrected to 54 cm, was 0.72–0.88 mg kg−1 (ww) during 1983–2013. The highest concentrations occurred in 2002 and were significantly higher than those in 1983 (GLMM, t = 4.41, p < 0.0001) and 2013 (GLMM, t = 2.86, p < 0.0231, Fig. 2a, Table SI 1). Average estimated THg concentrations of different lakes over the four-decade study period varied between 0.26 and 1.33 mg kg−1 (ww) (Fig. 2b, Table SI 1), and concentrations measured from individual pike ranged from 0.05 to 2.87 mg kg−1 (Table 2). The lake, year and pike length (Fig. 2c) statistically significantly affected the pike THg concentration (GLMM, Table SI 2). Length was always the most important variable in all GLMM.
Average (±95 % CI) model-estimated and length-corrected (54 cm) pike THg a of all study lakes in the four sampling campaigns and b in single lakes over the study period of four decades, with lake numbers as in Table 1 and Fig. 1, and the relation of pike THg to c fish length and d growth (=average annual length increment) of pike
There was a statistically significant difference in the model-estimated average pike THg between the sexes (GLMM, t = −2.94, p < 0.0038), the concentrations (mean ± 95% CI) being higher in males, 0.66 (n = 95, 0.48–0.90) mg kg−1 (ww) than in females 0.59 (n = 117, 0.43–0.81) mg kg−1 (Table SI 1).
The growth of pike was slow, with the mean total length of 5-year-old fish ranging between 34.5 and 43.5 cm among the different lakes (Table 2). Nevertheless, the growth, given as the average annual length increment (Fig. 2d), still had a negative effect on the THg concentration of pike (GLMM, F = 32.35, p < 0.0001, Table SI 2), with a 4.7 % (95 % cl 3.1–6.3) decrease per 1-cm increase in the annual growth of pike. The average growth was significantly higher for females than males, 8.1 and 7.5 cm per year, respectively (GLMM, t = 3.07, p = 0.0025, fish age as a covariate (Table SI 3)).
3.2 Pike THg and Lake Characteristics
Lake depth statistically significantly (GLMM, F = 7.01, p < 0.0116) affected the pike THg, with an average increase of 0.05 mg kg−1 (ww) THg per 1-m increase in the maximum depth of lakes (Fig. 3a, Tables 5 and SI 2). Among water quality parameters, pike THg concentrations increased significantly with increases in DOC-related water colour (GLMM, F = 26.91, p < 0.0001, Fig. 3b), total phosphorus, total nitrogen and iron (Tables 5 and SI 2). Pike THg was not significantly related to the acidification-related parameters alkalinity (GLMM, F = 2.50, p = 0.123, Fig. 3c) and calcium (Tables 5 and S2).
3.3 Lake-Specific Responses in Pike THg
The model-estimated average pike THg in the chain lakes (lakes 1–6 in Table 1 and Fig. 1) was 0.56–0.84 mg kg−1 in the first sampling campaign of 1983–1984 (Fig. 4a). In all chain lakes, the highest average pike THg occurred in 2002, with the highest levels of 1.41 mg kg−1 (ww) in L. Savijärvi and the lowest in L. Alinen Rautjärvi (0.82 mg kg−1 ww). In 2013 the average pike THg of chain lakes was 0.80–1.01 mg kg−1 (ww). The pattern of pike THg was fairly similar throughout the lake chain (Figure 4a), and statistically significant differences in pairwise comparisons of decades were found in each lake (Table 6).
In the headwater lakes, the variation in pike THg was wider than that in the chain lakes (Fig. 4b), from measured concentrations of 0.05–0.46 mg kg−1 (ww) in the clear groundwater lake L. Syrjänalunen to 0.15–2.67 mg kg−1 (ww) and 0.67–1.95 mg kg−1 (ww) in the brown water lakes L. Haukijärvi and L. Majajärvi, respectively (Table 2).
In the acidified lakes L. Iso Valkjärvi and L. Valkea-Kotinen, significantly higher pike THg concentrations were measured in 1983 (1.19 and 0.81 mg kg−1, respectively) than in the following sampling campaigns (GLMM, p < 0.001, Table 6), coinciding with the years of lowest pH and alkalinity of the lakes (Table 1). Since then, the pike THg in these lakes varied between 0.51 and 0.77 mg kg−1 (ww), with a slight decrease in the clear water lake L. Iso Valkjärvi and with an increasing trend in the brown water lake L. Valkea-Kotinen (Fig. 4b).
The outlets of the chain lake Haarajärvi and the headwater lakes Huhmari, Haukijärvi and Majajärvi were dammed by beavers temporarily in the 1980s, resulting in rises of floodwater into the surrounding riparian forest and wetland. The beaver floods were followed by high variation in the pike THg of these lakes (Fig. 4b). The clearest example of beaver effect was L. Huhmari, where the first beaver flood took place in 1990 and doubled the pike THg content from 0.50 mg kg−1 (ww) in 1983 to 1.05 mg kg−1 (ww) in 1993 (GLMM, p = 0.0001). After disappearance of beavers, the pike THg dropped again, to 0.56 mg kg−1 (ww) in 2002 (GLMM, p = 0.0027 (Tables 6 and SI 1).
In the three lakes (L. Haarajärvi, L. Haukijärvi, L. Majajärvi) targeted for intensive pike fishing during 2008–2012, the pike THg decreased from 2002 to 2013 more than in any other study lake, excluding L. Savijärvi (Fig. 4). In L. Haarajärvi, the uppermost lake of the lake chain, the decrease in pike THg from 2002 to 2013 was 0.44 mg kg−1 (ww) whereas the decrease was 0.02–0.26 mg kg−1 in other chain lakes except L. Savijärvi with a 0.52 mg kg−1 (ww) drop. In the headwater lakes L. Haukijärvi and L. Majajärvi, the decrease in pike THg from 2002 to 2013 was 0.68 and 0.70 mg kg−1 (ww), respectively. The three fish-manipulated lakes, L. Haarajärvi, L. Haukijärvi and L. Majajärvi, together with L. Savijärvi, were the only ones with a statistically significant decrease of pike THg from 2002 to 2013 (GLMM, p = 0.0019, 0.0007, 0.0007 and 0.0095, respectively, Tables 6 and SI 1).
4 Discussion
Our study clearly demonstrates that mercury concentrations of pike in small boreal headwater lakes of southern Finland have remained high despite the ca. 50 % decrease of mercury concentration in deposition from 11 µg L−1 in the late 1980s (Iverfeldt, 1991) to levels 4–6 µg L−1 in the 2000s (Wängberg et al., 2010; Ruoho-Airola et al., 2014). Pike THg was strongly linked to biological characteristics like length, growth and sex of fish as observed in many earlier studies (e.g. Madenjian et al., 2014; Moslemi-Aqdam et al., 2022). Among environmental factors, water colour was important, as in most lakes the highest pike THg concentrations were recorded in 2002 after consistent browning of lakes during the 1990s due to decreased sulphate deposition (Arvola et al., 2010; Arzel et al., 2020), suggesting a dominant role of DOC in the lake water Hg dynamics.
Furthermore, the model-estimated average mercury concentrations exceeded the EU recommended health limit (1.0 mg kg−1, ww) for human consumption of pike (EU, 2006) at least in one sampling campaign in 9 out of the 12 lakes. These concentrations are higher than those measured from large lakes and coastal brackish waters of Baltic Sea, from where the majority of pike for human consumption are caught (Ahonen et al., 2018; Airaksinen et al., 2018; Braaten et al., 2019).
4.1 Browning of Lakes
Pike THg content was significantly affected by water colour, emphasizing the importance of increased organic carbon load to mercury dynamics in catchment-lake systems (Porvari, 1998; Braaten et al., 2020; Moslemi-Aqdam et al., 2022). The occurrence of the highest pike THg levels during the 2002 sampling campaigns followed the most intensive period of browning of the lakes in the study area which took place during the 1990s (Arvola et al., 2010; Arzel et al., 2020; Moslemi-Aqdam et al., 2022). Significant positive relations of total P, total N and iron to the pike THg in the lakes of the present study were probably caused by their coupling with the humic substances as well (Kortelainen et al., 1986; Kozak et al., 2023). An increasing DOC concentration shifts lakes from phytoplankton primary production towards heterotrophic bacterial production (Forsström et al., 2013) that may also promote both within-lake methylation processes and more efficient Hg transfer in the food chain (Moslemi-Aqdam et al., 2023). The rise of DOC concentrations in lakes has also been considered to be the major reason for recent increases in mercury concentrations in the fish of lakes recovered from acidification (Wyn et al., 2010; Hongve et al., 2012;), but in some cases, the growth dilution phenomenon has been shown to be more important (Keva et al., 2017; Moslemi-Aqdam et al., 2021; Rask et al., 2021).
4.2 Acidified Lakes
In our study, the two lakes with pH < 5.0 during the 1980s, L. Iso Valkjärvi and L. Valkea-Kotinen (Rask & Metsälä, 1991), were the only ones with the highest pike THg measured during the first sampling campaign. This supports the role of acidification as a reason for high pike THg concentrations in the 1980s during the years of the highest sulphate deposition (Verta et al., 1990; Rask et al., 2021). In L. Iso Valkjärvi, liming in the early 1990s (Rask et al., 1996) enhanced the decrease of pike THg in the lake but a decrease also took place in the control basin of the lake (Rask et al., 2007). Later, during 2007–2012, intensive fishing of perch (Olin et al., 2017b) may have contributed to the mercury dynamics and continuous decrease of pike THg in the lake, as happened also with the perch (Rask et al., 2007). In L. Valkea-Kotinen, the significant increase of pike THg up to the last sampling campaign in 2013 is apparently related to the browning of the lake (Vuorenmaa et al., 2014; Rask et al., 2021).
4.3 Beavers
Beaver floods affect the water chemistry and biota of small headwater lakes (Vehkaoja et al., 2015; Nummi et al., 2018; Nummi et al., 2021). The results of Vehkaoja et al. (2015) showed that water colour increased especially during the first three beaver-impoundment years, while concentrations of dissolved oxygen simultaneously decreased. In the beaver lakes, increased leaching of mercury from riparian areas and low oxygen concentration in the hypolimnion of the lakes associated with the flooding may increase within-lake methylation processes (Eckley et al., 2005), jointly promoting mercury biomagnification through the food web and culminates as increased pike THg (Moslemi-Aqdam et al., 2023). Our observations are in line with those by Painter et al. (2015), who showed that beaver impoundments can increase the availability and subsequent uptake of MeHg by basal food web organisms in beaver ponds. It has also been found that, during the comeback of beavers after extirpation, the pioneer inundation caused elevated MeHg concentrations in the system, but later re-floodings produced only negligible increases (Levanoni et al., 2015). Moreover, young beaver ponds, but not the old ones, tend to be a source of methylmercury in water (Ecke et al., 2017). In our study, the first beaver flood in L. Huhmari took place in 1990 and resulted in a sharp increase of pike THg.
4.4 Intensive Fishing
Fish removal has been suggested as a method to promote fish growth and hence decrease the Hg levels in remaining fish, and the risk to humans of fish consumption (Göthberg, 1983; Verta, 1990, Sharma et al., 2008). Three of our study lakes, L. Haarajärvi, L. Haukijärvi and L. Majajärvi, were targeted for effective pike removal during 2008–2012, with 30–50 % of pike biomass removed annually (Tiainen et al., 2017). An increase in the growth of pike was recorded in all lakes, especially in L. Haarajärvi, where the increase was statistically significant in all age groups (Tiainen, 2017). Thus, the growth dilution apparently had a role in explaining the decrease of pike THg in these lakes from 2002 to 2013. Apparently, this was also the reason for the difference in THg concentration between the sexes in our study, as female pike grew significantly faster than males (see also Casselman, 1996). The three pike manipulation lakes were also inhabited by beavers in certain years (Vehkaoja et al., 2015), but during the “Ecologically sustainable fishing” project 2005–2013, beaver floods took place only in L. Haukijärvi. The decrease of pike THg also in this lake from 2002 to 2013 emphasizes the importance of pike population for the mercury dynamics of the lakes (see Sharma et al., 2008).
4.5 Forest Management
Although the sampling programme for our study did not offer sufficient context for detailed assessment of the impacts of forest harvesting on pike THg, the forestry in the lake catchments likely has increased DOC and mercury load to the lakes and overall variation in the pike THg concentrations (Moslemi-Aqdam et al., 2022; Moslemi-Aqdam et al., 2023). An earlier study showed that catchment deforestation (46 % of the catchment area) and associated burning of logging remains resulted in changes in water quality, increases in algal primary production and decomposition of organic matter in one small uppermost lake in our study area (Rask et al., 1993). This was accompanied by an increase in THg in cladoceran zooplankton and fish (Rask et al., 1994). A Canadian study (Garcia & Carignan, 2000) reported an effect of forest clearcutting on fish THg, with Hg levels of northern pike in logged lakes twice as high as those in reference lakes. A Swedish study (Wu et al., 2018) reported an average increase of 26 % in Hg concentrations of large perch from lakes after clearcutting of their catchments.
4.6 Climate Change
Effects of climate change in our study area have been recorded as a statistically significant increase in annual and spring mean air temperature, thinning of annual mean snow depth and shortening of the lake-ice season (Jylhä et al., 2014). Dissolved organic carbon concentrations in lakes have increased, partly due to decreasing sulphate deposition and partly by climate change–induced hydrological changes with increasing runoff and leaching of substances from catchments to lakes (Arvola et al., 2010; Vuorenmaa et al., 2014). The consequent browning of lake waters has led to stronger stratification and lowered oxygen concentration (Vuorenmaa et al., 2014; Couture et al., 2015), which may elevate the mercury concentration of perch and pike (Rask et al., 2010; Ahonen et al., 2018; Kozak et al., 2021). The higher pike THg concentrations in our deeper study lakes may have been due to the relatively larger volume of anoxic hypolimnetic water in the deeper lakes. In L. Valkea-Kotinen, the primary production decreased during the 20-year period 1990–2009 mainly due to climatic forcing and consequent hydrological changes and increased DOC loading (Arvola et al., 2014). The decrease of general productivity of the lake resulted in slower growth of perch (Rask et al. 2014b), which may have contributed to the increase in perch THg (Rask et al., 2010; 2021) and also explains the increase of pike THg (Moslemi-Aqdam et al., 2021) as recorded in the present study.
4.7 Concluding Remarks
The results of the present study indicated high pike mercury levels in pristine boreal headwater lakes from the 1980s to 2010s. The ca. 50 % decrease of mercury deposition during this period did not result in generally lower pike THg levels. The reasons for this, and even increased pike THg until the shift of the millennium, were most probably connected to the regional effects of decreased atmospheric SO4 deposition on increased DOC mobility and leaching and on mercury dynamics in lakes and their catchments, together with the complex effects of climate change. Thus, the answer to our original study question 1 is no, as no regular decrease in pike THg took place over decades. The answer to question 2 is yes, as pike THg concentrations increased during the course of lake browning. The answer to questions 3 and 4 on local effects is yes, as beaver floods resulted in increases of pike THg, while intensive pike removal decreased pike THg. Taking into account deposition (mercury and other elements), environmental (lake and catchment) and biotic (size, sex, age and growth of fish) variables is thus crucial for disentangling the most important factors behind decadal trends in fish mercury.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Ahonen, S. A., Hayden, B., Leppänen, J. J., & Kahilainen, K. K. (2018). Climate and productivity affect total mercury concentration and bioaccumulation rate of fish along a spatial gradient of subarctic lakes. Science of the Total Environment, 637–638, 1586–1596.
Airaksinen, R., Jestoi, M., Keinänen, M., Kiviranta, H., Koponen, J., Mannio, J., Myllylä, T., Nieminen, J., Raitaniemi, J., Rantakokko, P., Ruokojärvi, P., Venäläinen, E.-R. & Vuorinen, P.J. (2018). Changes in the levels of environmental contaminants of Finnish wild caught fish. Publications of the Government´s analysis, assessment and research activities 51/2018, 71 p. (In Finnish).
Arcagni, M., Juncos, R., Rizzo, A., Pavlin, M., Fajon, V., Arribere, M. A., Horvat, M., & Guevara, S. R. (2018). Species- and habitat-specific bioaccumulation of total mercury and methylmercury in the food web of a deep oligotrophic lake. Science of the Total Environment, 612, 1311–1319.
Arvola, L., Metsälä, T.-R., Similä, A., & Rask, M. (1990). Seasonal fluctuations in the chemistry of small forest lakes in southern Finland with special reference to acidity. Aqua Fennica, 20, 71–79.
Arvola, L., Rask, M., Ruuhijärvi, J., Tulonen, T., Vuorenmaa, J., Ruoho-Airola, T., & Tulonen, J. (2010). Long-term patterns in pH and colour in small acidic boreal lakes of varying hydrological and landscape settings. Biogeochemistry, 101, 269–279. https://doi.org/10.1007/s10533-010-9473-y
Arvola, L., Salonen, K., Keskitalo, J., Tulonen, T., Järvinen, M. & Huotari, J. (2014). Plankton metabolism and sedimentation in a small boreal lake – A long-term perspective. Boreal Environment Research 19 (Supplement A), 83-96.
Arzel, C., Nummi, P., Arvola, L., Pöysä, H., Davranche, A., Rask, M., Olin, M., Holopainen, S., Viitala, R., Einola, E., & Manninen-Johansen, S. (2020). Invertebrates are declining in boreal aquatic habitat: The effect of brownification? Science of the Total Environment, 724, 138199. https://doi.org/10.1016/j.scitotenv.2020.1381990048-9697
Blanchet, C. C., Arzel, C., Davranche, A., Kahilainen, K. K., Secondi, J., Taipale, S., Lindberg, H., Loehr, J., Manninen-Johansen, S., Sundell, J., Maanan, M., & Nummi, P. (2022). Ecology and extent of freshwater browning - What we know and what should be studied next in the context of global change. Science of The Total Environment, 812, 15240. https://doi.org/10.1016/j.scitotenv.2021.152420
Bloom, N. S. (1992). On the chemical form of mercury in edible fish and marine invertebrate tissue. Canadian Journal of Fisheries and Aquatic Sciences, 49, 1010–1017.
Braaten, H. F. V., deWit, H. A., Fjeld, E., Rognerud, S., Lydersen, E., & Larssen, T. (2014). Environmental factors influencing mercury speciation in subarctic and boreal lakes. Science of the Total Environment, 467–477, 336–345.
Braaten, H. F. V., de Wit, H. A., Larssen, T., & Poste, A. E. (2018). Mercury in fish from Norwegian lakes: The complex influence of aqueous organic carbon. Science of the Total Environment, 627, 341–348.
Braaten, H. F. V., Åkerblom, S., Kahilainen, K. K., Rask, M., Vuorenmaa, J., Mannio, J., Malinen, T., Lydersen, E., Poste, A. E., Amundsen, P.-A., Kashulin, N., Kashulina, T., Terentyev, P., Christensen, G., & de Wit, H. A. (2019). Improved environmental status: 50 years of declining fish mercury levels in boreal and subarctic Fennoscandia. Environmental Science and Technology, 53, 1834–1843. https://doi.org/10.1021/acs.est.8b06399
Braaten, H. F. V., Lindholm, M., & de Wit, H. A. (2020). Five decades if declining methylmercury concentrations in boreal food webs suggest pivotal role for sulphate deposition. Science of the Total Environment, 714, 136774.
Bravo, A. G., Bouchet, S., Tolu, J., Björn, E., Mateos-Rivera, A., & Bertilsson, S. (2017). Molecular composition of organic matter controls methylmercury formation in boreal lakes. Nature Communications, 8, 14255. https://doi.org/10.1038/ncomms14255
Casselman, J. M. (1996). Age, growth and environmental requirements of pike. In J. F. Craig (Ed.), Pike: Biology and exploitation (pp. 69–101). Chapman & Hall.
Couture, R.-M., de Wit, H., Tominaga, K., Kiuru, P., & Markelov, I. (2015). Oxygen dynamics in a boreal lake responds to long-term changes in climate, ice phenology, and DOC inputs. Journal of Geophysical Research: Biogeosciences, 120, 2441–2456.
de Wit, H. A., Stoddard, J. L., Monteith, D. T., Sample, J. E., Austnes, K., Couture, S., Fölster, J., Higgins, S. N., Houle, D., Hruška, J., Krám, P., Kopáček, J., Paterson, A. M., Salar, V., Van Dam, H., Vuorenmaa, J., & Evans, C. D. (2021). Cleaner air reveals growing influence of climate on dissolved organic carbon trends in northern headwaters. Environmental Research Letters, 16, 104009.
Eagles-Smith, C. A., Silbergeld, E. K., Basu, N., Bustamante, P., Diaz-Barriga, F., Hopkins, W. A., Kidd, K. A., & Nyland, J. F. (2018). Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio, 47, 170–197.
Ecke, F., Levanoni, O., Audet, J., Carlson, P., Eklöf, K., Hartman, G., McKie, B., Ledesma, J., Segersten, J., Truchy, A., & Futter, M. (2017). Meta-analysis of environmental effects of beaver in relation to artificial dams. Environmental Research Letters, 12, 113002.
Eckley, C. S., Watras, C. J., Hintelmann, H., Morrison, K., Kent, A. D., & Regnell, O. (2005). Mercury methylation in the hypolimnetic waters of lakes with and without connection to wetlands in northern Wisconsin. Canadian Journal of Fisheries and Aquatic Sciences, 62, 400–411.
Eklöf, K., Bishop, K., Bertilsson, S., Björn, E., Buck, M., Skyllberg, U., et al. (2018). Formation of mercury methylation hotspots as a consequence of forestry operations. Science of the Total Environment, 613–614, 1069–1078.
EU (2006). Setting maximum levels for certain contaminants in foodstuffs. Commission Regulation No 1881/2006.
Forsström, L., Roiha, T., & Rautio, M. (2013). Responses of microbial food web to increased allochthonous DOM in an oligotrophic subarctic lake. Aquatic Microbial Ecology, 68, 171–184. https://doi.org/10.3354/ame01614
Gandhi, N., Tang, R. W. K., Bhavsar, S. P., & Arhonditsis, G. B. (2014). Fish mercury levels appear to be increasing lately: A report from 40 years of monitoring in the province of Ontario, Canada. Environmental Science and Technology, 48, 5404–5414.
Garcia, E., & Carignan, R. (2000). Mercury concentrations in northern pike (Esox lucius) from boreal lakes with logged, burned, or undisturbed catchments. Canadian Journal of Fisheries and Aquatic Sciences, 57(Suppl. 2), 129–135.
Göthberg, A. (1983). Intensive fishing - A way to reduce the mercury level in fish. Ambio, 12, 259–261.
Hongve, D., Haaland, S., Riise, G., Blakar, I., & Norton, S. (2012). Decline of acid rain enhances mercury concentrations in fish. Environmental Science and Technology, 46, 2490–2491.
Heiskary, S. A., & Helwig, D. D. (1986). Mercury levels in northern pike, Esox lucius, relative to water chemistry in northern Minnesota lakes. Lake and Reservoir Management, 2, 33–37.
Hrabik, T. R., & Watras, C. J. (2002). Recent declines in mercury concentration in a freshwater fishery isolating the effects of de-acidification and decreased atmospheric mercury deposition in Little Rock Lake. Science of the Total Environment, 297, 229–237.
Häsänen, E. & Sjöblom, V. (1968). Kalojen elohopeapitoisuus Suomessa vuonna 1967 (Mercury concentrations of fish in Finland in 1967). Suomen Kalatalous 36, 1-24. (in Finnish, with an English summary).
Iverfeldt, Å. (1991). Occurrence and turnover of atmospheric mercury over the Nordic countries. Water, Air, and Soil Pollution, 56, 251–265.
Jylhä, K., Laapas, M., Ruosteenoja, K., Arvola, L., Drebs, A., Kersalo, J., et al. (2014). Climate variability and trends in the Valkea-Kotinen region, southern Finland: Comparisons between the past, current and projected climates. Boreal Environment Research, 19(Supplement A), 4–30.
Kahilainen, K. K., Thomas, S. M., Keva, O., Hayden, B., Knudsen, R., Eloranta, A. P., et al. (2016). Seasonal dietary shift to zooplankton influences stable isotope ratios and total mercury concentrations in Arctic charr (Salvelinus alpinus (L.)). Hydrobiologia, 783, 47–63.
Kahilainen, K. K., Thomas, S. M., Nystedt, E. K. M., Keva, O., Malinen, T., & Hayden, B. (2017). Ecomorphological divergence drives differential mercury bioaccumulation in polymorphic European whitefish (Coregonus lavaretus) populations of subarctic lakes. Science of the Total Environment, 599–600, 1768–1778.
Karhunen, J., Arvola, L., Peura, S., & Tiirola, M. (2013). Green sulphur bacteria as a component of the photosynthetic plankton community in small dimictic humic lakes with an anoxic hypolimnion. Aquatic Microbial Ecology, 68, 267–272.
Karimi, R., Chen, C. Y., & Folt, C. L. (2016). Comparing nearshore benthic and pelagic prey as mercury sources to lake fish: The importance of prey quality and mercury content. Science of the Total Environment, 565, 211–221.
Keller, W., Yan, N. D., Gunn, J. M., & Heneberry, J. (2007). Recovery of acidified lakes: Lessons from Sudbury, Ontario, Canada. Water, Air, and Soil Pollution: Focus, 7, 317–322.
Keva, O., Hayden, B., Harrod, C., & Kahilainen, K. K. (2017). Total mercury concentrations in liver and muscle of European whitefish (Coregonus lavaretus (L.)) in a subarctic lake - Assessing the factors driving year-round variation. Environmental Pollution, 231, 1518–1528.
Kivinen, S., Nummi, P., & Kumpula, T. (2020). Beaver-induced spatiotemporal patch dynamics affect landscape-level environmental heterogeneity. Environmental Research Letters, 15(9), 094065.
Kortelainen, P., Mannio, J., & Pennanen, V. (1986). Characteristics of the organic allochthonous matter of Finnish forest lakes and reservoirs. Publications of the Water Research Institute, National Board of Waters, Finland, 65, 88–97.
Kozak, N., Ahonen, S. A., Keva, O., Østbye, K., Taipale, S. J., Hayden, B., & Kahilainen, K. K. (2021). Environmental and biological factors are joint drivers of mercury biomagnification in subarctic lake food webs along a climate and productivity gradient. Science of the Total Environment, 779, 146261. https://doi.org/10.1016/j.scitotenv.2021.146261
Kozak, N., Kahilainen, K. K., Pakkanen, H. K., Hayden, B., Østbye, K., & Taipale, S. J. (2023). Mercury and amino acid content relations in northern pike (Esox lucius) in subarctic lakes along a climate-productivity gradient. Environmental Research, 233, 116511. https://doi.org/10.1016/j.envres.2023.116511
Lavoie, R. A., Jardine, T. D., Chumchal, M. M., Kidd, K. A., & Campbell, L. M. (2013). Biomagnification of mercury in aquatic food webs: A worldwide meta-analysis. Environmental Science & Technology, 47, 13385–13394.
Lavigne, M., Lucotte, M., & Paquet, S. (2010). Relationship between mercury concentration and growth rates for walleyes, northern pike, and lake trout from Quebec lakes. North American Journal of Fisheries Management, 5, 1221–1237.
Lehtonen H., Estlander S., Horppila J., Nurminen L., Olin M., Rask M., Ruuhijärvi J. & Tiainen J. (2017). Preface to the Special Issue “Ecologically sustainable fishing”: Project KESKALA background, objectives and conclusions. Boreal Environment Research 22 (Special issue 1), 115-118.
Lescord, G. L., Johnston, T. A., Branfireun, B. A., & Gunn, J. M. (2018). Percentage of methylmercury in the muscle tissue of freshwater fish varies with body size and age and among species. Environmental Toxicology and Chemistry, 37, 2682–2691.
Levanoni, O., Bishop, K., Mckie, B. G., Hartman, G., Eklöf, K., & Ecke, F. (2015). Impact of beaver pond colonization history on methylmercury concentrations in surface water. Environmental Science and Technology, 49, 12679–12687.
Lindeström, L. (2001). Mercury in sediment and fish communities of Lake Vänern, Sweden: Recovery from contamination. Ambio, 30, 538–544.
Lodenius, M. (1991). Mercury concentrations in an aquatic ecosystem during twenty years following abatement the pollution source. Water, Air, and Soil Pollution, 56, 323–332.
Madenjian, C. P., Blanchfield, P. J., Hrenchuk, L. E., & van Walleghem, J. L. A. (2014). Mercury elimination rates for adult northern pike Esox lucius: Evidence for a sex effect. Bulletin of Environmental Contamination and Toxicology, 93, 144–148. https://doi.org/10.1007/S00128-014-1256
Monteith, D. T., Stoddard, J. L., Evans, C. D., de Wit, H. A., Forsius, M., Høgåsen, T., et al. (2007). Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature, 450, 537–540.
Moslemi-Aqdam, M., Low, G., Low, M., Branfireun, B. A., & Swanson, H. K. (2021). Catchments affect growth rate of northern pike, Esox lucius, in subarctic lakes. Aquatic Sciences, 83, 1–14. https://doi.org/10.1007/S00027-021-00817-4/FIGURES/5
Moslemi-Aqdam, M., Baker, L., Baltzer, J., Branfireun, B., Evans, M., Laird, B., Low, G., Low, M., & Swanson, H. K. (2022). Understanding among-lake variability of mercury concentrations in northern pike (Esox lucius): A whole-ecosystem study in subarctic lakes. Science of the Total Environment, 822, 153430. https://doi.org/10.1016/J.SCITOTENV.2022.153430
Moslemi-Aqdam, M., Low, G., Low, M., Laird, B. D., Branfireun, B. A., & Swanson, H. K. (2023). Estimates, spatial variability, and environmental drivers of mercury biomagnification rates through lake food webs in the Canadian subarctic. Environmental Research, 217, 114835. https://doi.org/10.1016/j.envres.2022.114835
Nummi, P., Vehkaoja, M., Pumpanen, J., & Ojala, A. (2018). Beavers affect carbon biogeochemistry: Both short-term and long-term processes are involved. Mammal Review, 48, 298–311.
Nummi, P., Liao, W., van der Schoor, J., & Loehr, J. (2021). Beaver creates early successional hotspots for water beetles. Biodiversity and Conservation, 30, 2655–2670.
Olin, M., Rask, M., Estlander, S., Horppila, J., Nurminen, L., Tiainen, J., Vinni, M., & Lehtonen, H. (2017). Roach (Rutilus rutilus) populations respond to varying environment by altering size structure and growth rate. Boreal Environment Research, 22(Special Issue 1), 119–136.
Olin, M., Tiainen, J., Rask, M., Vinni, M., Nyberg, K., & Lehtonen, H. (2017). Effects of non-selective and size-selective fishing on perch populations in a small lake. Boreal Environment Research, 22(Special Issue 1), 137–155.
Painter, K. J., Westbrook, C. J., Hall, B. D., O’Driscoll, N. J., & Jardine, T. D. (2015). Effects of in-channel beaver impoundments on mercury bioaccumulation in Rocky Mountain stream food webs. Ecosphere, 6, 194. https://doi.org/10.1890/ES15-00167.1
Porvari, P. (1998). Development of fish mercury concentrations in Finnish reservoirs from 1979 to 1994. Science of the Total Environment, 213, 279–290.
Porvari, P., Verta, M., Munthe, J., & Haapanen, M. (2003). Forestry practices increase mercury and methylmercury output from boreal forest catchments. Environmental Science and Technology, 37, 2389–2393.
Rask, M., & Metsälä, T.-R. (1991). Mercury concentrations in northern pike, Esox lucius L. in small lakes of Evo Area, southern Finland. Water, Air, and Soil Pollution, 56, 369–378.
Rask, M., Arvola, L., & Salonen, K. (1993). Effects of catchment deforestation and burning on the limnology of a small forest lake in southern Finland. Verhandlungen des Internationale Vereinigung fur Limnologie, 25, 525–528.
Rask, M., Metsälä, T.-R., & Salonen, K. (1994). Mercury in the food chains of a small polyhumic forest lake in southern Finland. In C. J. Watras & J. W. Huckabee (Eds.), Mercury pollution: Integration and synthesis (pp. 409–416). Lewis Publishers.
Rask, M., Järvinen, M., Kuoppamäki, K., & Pöysä, H. (1996). Limnological responses to the collapse of the perch population in a small lake. Annales Zoologici Fennici, 33, 517–524.
Rask, M., Jones, R. I., Järvinen, M., Paloheimo, A., Salonen, M., Syväranta, J., et al. (2007). Changes in fish mercury concentrations over 20 years in an acidified lake subject to experimental liming. Applied Geochemistry, 22, 1229–1240.
Rask, M., Verta, M., Korhonen, M., Salo, S., Forsius, M., Arvola, L., Jones, R. I., & Kiljunen, M. (2010). Does lake thermocline depth affect methyl mercury concentrations in fish? Biogeochemistry, 101, 311–322. https://doi.org/10.1007/s10533-010-9487-5
Rask, M., Arvola, L., Forsius, M., & Vuorenmaa, J. (2014). Preface to the special issue: Integrated monitoring in Valkea-Kotinen Catchment during 1990–2009: Abiotic and biotic responses to changes in air pollution and climate. Boreal Environment Research, 19(Supplement A), 1–3.
Rask, M., Sairanen, S., Vesala, S., Arvola, L., Estlander, S., & Olin, M. (2014). Population dynamics and growth of perch in a small, humic lake over a 20-year period – Importance of abiotic and biotic factors. Boreal Environment Research, 19(Supplement A), 112–123.
Rask, M., Malinen, T., Olin, M., Nyberg, K., Ruuhijärvi, J., Kahilainen, K. K., Verta, M., Vuorenmaa, J., Blauberg, T.-R., & Arvola, L. (2021). High mercury concentrations of European perch (Perca fluviatilis) in boreal headwater lakes with variable history of acidification and recovery. Water, Air, and Soil Pollution, 232, 382. https://doi.org/10.1007/s11270-021-05303-z
Ruoho-Airola, T., Hatakka, T., Kyllönen, K., Makkonen, U., & Porvari, P. (2014). Temporal trends in the bulk deposition and atmospheric concentration of acidifying compounds and trace metals in the Finnish integrated monitoring catchment Valkea-Kotinen during 1988–2011. Boreal Environment Research, 19(Supplement A), 31–46.
Sharma, C. M., Borgstrom, R., Huitfeldt, J., & Rosseland, B. O. (2008). Selective exploitation of large pike Esox lucius-effects on mercury concentrations in fish populations. Science of the Total Environment, 399, 33–40.
Streets, D. G., Horowitz, H. M., Jacob, D. J., Lu, Z., Levin, L., ter Schure, A. F. H., & Sunderland, E. M. (2017). Total mercury released to the environment by human activities. Environmental Science & Technology, 51, 5969–5977.
Stroup, W. (2013). Generalized linear mixed models, modern concepts, method and applications. Chapman & Hall/CRC.
Tammi, J., Appelberg, M., Hesthagen, T., Beier, U., Lappalainen, A., & Rask, M. (2003). Fish status survey of Nordic lakes: Effects of acidification, eutrophication and stocking activity on present fish species composition. Ambio, 32, 98–105.
Tiainen, J. (2017). Let there be pike! Effects of fishing on the dynamics of pike (Esox lucius) populations. PhD thesis, University of Helsinki.
Tiainen, J., Olin, M., Lehtonen, H., Nyberg, K., & Ruuhijärvi, J. (2017). The capability of harvestable slot-length limit regulation in conserving large and old pike (Esox lucius). Boreal Environment Research, 22, 169–186.
Travnikov, O., Ilyin, I., Rozovskaya, O., Varygina, M., Aas, W., Uggerud, H.T. et al. (2012). Long-term changes of heavy metal transboundary pollution of the environment (1990–2010). EMEP contribution to the revision of the Heavy Metal Protocol. EMEP MSC-E & CCC & CEIP Status Report 2/2012.
Ullrich, S. M., Tanton, T. W., & Abdrashitova, S. A. (2001). Mercury in the aquatic environment: A review of factors affecting methylation. Critical Reviews in Environmental Science and Technology, 31, 241–293.
Vehkaoja, M., Nummi, P., Rask, M., & Arvola, L. (2015). Spatiotemporal dynamics of boreal landscapes with ecosystem engineers: Beavers influence the biogeochemistry of small lakes. Biogeochemistry, 124, 405–415. https://doi.org/10.1007/s10533-015-0105-4
Verta, M. (1990). Changes in fish mercury concentrations in an intensively fished lake. Canadian Journal of Fisheries and Aquatic Sciences, 47, 1888–1897.
Verta, M., Mannio, J., Iivonen, P., Hirvi, J.-P., Järvinen, O., & Piepponen, S. (1990). Trace metals in Finnish headwater lakes – Effects of acidification and airborne load. In P. Kauppi, P. Anttila, & K. Kenttämies (Eds.), Acidification in Finland (pp. 883–908). Springer-Verlag.
Verta, M., Salo, S., Korhonen, M., Porvari, P., Paloheimo, A., & Munthe, J. (2010). Climate induced thermocline change has an effect on methyl mercury cycle in small boreal lakes. Science of the Total Environment, 408, 3639–3647.
Vuorenmaa, J. (2004). Long-term changes of acidifying deposition in Finland (1973–2000). Environmental Pollution, 128, 351–362.
Vuorenmaa, J., Forsius, M., & Mannio, J. (2006). Increasing trends in total organic carbon concentrations in small forest lakes in Finland from 1987 to 2003. Science of the Total Environment, 365, 47–65.
Vuorenmaa, J., Salonen, K., Arvola, L., Mannio, J., Rask, M., & Horppila, P. (2014). Water quality of a small headwater lake reflects long-term variations in deposition, climate and in-lake processes. Boreal Environment Research, 19(Supplement A), 47–65.
Wang, F., Outridge, P. M., Feng, X., Meng, B., Heimbürger-Boavida, L.-E., & Mason, R. P. (2019). How closely do mercury trends in fish and other aquatic wildlife track those in the atmosphere? – Implications for evaluating the effectiveness of the Minamata Convention. Science of the Total Environment, 674, 58–70.
Wängberg, I. Aspmo., Pfaffhuber, K., Berg, T., Hakola, H., Kyllönen, K., Munthe, J., et al. (2010). Atmospheric and catchment mercury concentrations and fluxes in Fennoscandia. TemaNord, 2010(594), 1–56.
Westfall, P. H. (1997). Multiple testing of general contrasts using logical constraints and correlations. Journal of the American Statistical Association, 92(437), 299–306. https://doi.org/10.1080/01621459.1997.1047362
Wu, P., Bishop, K., von Brömssen, C., Eklöf, K., Futter, M., Hultberg, H., et al. (2018). Does forest harvest increase the mercury concentrations in fish? Science of the Total Environment, 622–623, 1353–1362.
Wyn, B., Kidd, K. A., Burgess, N. M., Curry, R. A., & Munkittrick, K. R. (2010). Increasing mercury in yellow perch at a hotspot in Atlantic Canada, Kejimkujik National Park. Environmental Science and Technology, 44, 9176–9181.
Yu, X., Driscoll, C. T., Montesdeoca, M., Evers, D., Duron, M., Williams, K., et al. (2011). Spatial patterns of mercury in biota of Adirondack, New York lakes. Ecotoxicology, 20, 1543–1554.
Åkerblom, S., Bignert, A., Meili, M., Sonesten, L., & Sundbom, M. (2014). Half a century of changing mercury levels in Swedish freshwater fish. Ambio, 43, 91–103.
Acknowledgements
The authors thank all persons involved in collecting fish samples and analysing mercury over the years. The facilities for this long-term study were provided by the Lammi Biological Station and the Department of Biological and Environmental Sciences of the University of Helsinki, the Finnish Environment Institute and the Finnish Game and Fisheries Research Institute (now part of the Natural Resources Institute Finland). Jussi Huotari worked on the map for Fig. 1, and Roger Jones kindly checked the English language of the manuscript.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). No specific funding was obtained for this study, but the work was done as a part of various research projects in the Lammi Biological Station and the Department of Biological and Environmental Sciences of the University of Helsinki, the Finnish Environment Institute and the Natural Resources Institute Finland.
Author information
Authors and Affiliations
Contributions
Martti Rask — conceptualization, study design, data acquisition, data analysis, lead writing, editing, supervision
Tommi Malinen — conceptualization, data acquisition, writing, review
Kari Nyberg — conceptualization, data acquisition, writing, review
Mikko Olin — conceptualization, data acquisition, data analysis, writing, review
Mika Kurkilahti — conceptualization, data analysis, writing, review
Tarja-Riitta Blauberg — conceptualization, data acquisition, writing, review
Maiju Salonen — conceptualization, data acquisition, writing, review
Sami Vesala — conceptualization, data acquisition, writing, review
Jukka Ruuhijärvi — conceptualization, data acquisition, writing, review
Joni Tiainen — conceptualization, data acquisition, writing, review
Jussi Vuorenmaa — conceptualization, writing, review
Martin Lodenius — conceptualization, data analysis, writing, review
Celine Arzel — conceptualization, writing, review
Petri Nummi — conceptualization, writing, review
Kimmo K. Kahilainen — conceptualization, writing, review
Matti Verta — conceptualization, data acquisition, writing, review
Lauri Arvola — conceptualization, data acquisition, writing, editing, review
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rask, M., Malinen, T., Nyberg, K. et al. Pike Mercury Concentration in Small Boreal Headwater Lakes During Four Decades of Regional and Local Changes. Water Air Soil Pollut 235, 85 (2024). https://doi.org/10.1007/s11270-024-06894-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-06894-z