Abstract
Radioecological modeling requires information about the transfer of different elements and their radionuclides. These models have traditionally used radionuclide concentrations in water to predict concentrations in aquatic organisms. In addition, these models often assume this accumulation to be linear. In this study, we investigated the transfer of Ba, Co, Ni, Sr, U, and Zn from sediment and artificial freshwater to laboratory-reared benthic Oligochaeta (Lumbriculus variegatus). Total elemental concentrations were used as substitute measurements for specific radionuclides. The radioactive isotopes of these metals are an important part of the nuclear fuel cycle. During various parts of this cycle, they can end up in aquatic environments, mostly at low concentrations. Sediment samples were collected from three small lakes connected to a former uranium mine in Eastern Finland, as well as from a reference lake from a different drainage area nearby. A 28-day bioaccumulation experiment was conducted using collected samples and artificial freshwater. Concentrations measured from sediment, porewater, overlying water, and L. variegatus indicated importance of sediment as a source of uptake for all tested metals. Linear accumulation assumption (constant concentration ratio) also did not appear to be correct for most metals. Metal uptake by L. variegatus seemed to reduce at higher sediment concentrations for most metals, except for Ba and Co. Thus, the common assumptions related to radioecological modeling were not supported by our findings for majority of tested metals and accumulation sources. Further basic research is required to develop more accurate and robust radioecological models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Radioecological modeling is needed to predict the transfer of radionuclides in ecosystems and to assess risks they pose to humans and environment (Shaw, 2005; Caffrey et al., 2014; Haanes et al. 2020). These models require great amounts of quantitative data of radionuclide transfer to be able to produce accurate results. Limited amount of data of radionuclide transfer is currently available for boreal freshwater environments. The existing information is also largely focused on radionuclide concentrations in water, rather than sediment, which could serve as an important exposure source to many organisms in aquatic food webs (Besser et al., 2015; Konovalenko et al., 2017).
Many current radioecological models use concentration ratios (CR) for predicting radionuclide concentrations in biota (IAEA, 2010). CR is defined as the ratio of radionuclide concentration in biota to the corresponding concentration in soil, sediment, or water. An assumption related to CR is that it remains constant, regardless of environmental concentration of contaminant. In other words, the concentration in biota is assumed to increase when environmental concentration increases, and vice versa. However, several studies have shown that this may not be a valid assumption in case of many radionuclides and organisms (Pyle and Clulow, 1997; Tuovinen et al., 2011, 2013, 2016a, 2016b; Keum et al., 2013). Similar results were recently observed for fish at the site from where this study obtained its samples (Majlesi et al., 2021).
The number of radionuclides, which are important from the point of view of radiological protection, is vast, and many occur in nature only after accidental releases. Stable isotopes can be used to obtain data on radionuclides of the same element (Konovalenko et al., 2017; Brown et al., 2019; Beresford et al., 2020). This is based on an assumption that both radioactive and stable isotopes of the same element behave similarly in equilibrium state (IAEA, 2010). Former and existing metal mining areas offer a possibility to study the behavior of many relevant elements because the waste rocks and tailings can leach harmful substances to the surrounding environment. A small-scale uranium (U) mine was in operation from 1959 to 1961 at Paukkajanvaara, Eastern Finland, and the area was restored in 1993 (Colpaert, 2006). Recent studies have shown that the U concentrations in small lakes and their sediments below the mine are much higher than background (Tuovinen et al., 2016c, 2019; Majlesi et al., 2020). In addition, Majlesi et al. (2021) have found that the U concentrations in fishes and benthic organisms of these lakes are many times higher than the average Finnish equivalents. This makes the site excellent for studies on metal contamination and accumulation, especially U, in aquatic environments.
In addition to U, the studied metals were barium (Ba), cobalt (Co), nickel (Ni), strontium (Sr), and zinc (Zn). All these metals have radionuclides, which can be released to the environment during nuclear fuel mining operations (Ba, U), normal nuclear power plant operations and decommissioning (Co, Ni, Sr, Zn) or final disposal of spent nuclear fuel (Sr, U) (IAEA, 2005, 2021; OECD, 2006; Posiva, 2012). Under normal nuclear power plant operations, these metals have set exemption levels for release, which are strictly monitored (IAEA, 2012). During various accidents, the amount of these metals released can vary greatly, however. Half of the studied metals (Co, Ni, Zn) serve a biological function in living organisms in trace amounts but can become harmful to them in larger quantities (Gad S, 2014a, 2014b, 2014c). The other half (Ba, Sr, U) serve no known biological function and are also harmful in larger amounts to living organisms (Gad, 2014d, 2014e; Brugge, 2014).
The aim of this work was to study transfer of radionuclides from sediment to benthic organisms using total elemental metal concentrations as substitute measures. A 28-day bioaccumulation test was conducted in a laboratory using sediments collected from lakes near the former U mine. CR values for metal transfer between sediment/water and test organism Lumbriculus variegatus were determined, and the dependency of the CR values on the environmental metal concentrations was studied. L. variegatus was chosen due to its common occurrence in nature and novelty as a test organism in this type of experiment (Govedich et al. 2009; Gustafsson et al. 2009; Wallin et al., 2021). Our hypothesis was that metal transfer from the environment to L. variegatus is nonlinear, and thus, the constant CR assumption is not valid. We also suspected that sediment may have larger role in metal accumulation than previously thought for benthic invertebrates.
2 Materials and Methods
2.1 Test Organism
The test organism used in this experiment, oligochaete Lumbriculus variegatus, is widely spread. It is an important part of many aquatic food webs as a bottom feeder for small particles, and as prey for many organisms higher in the food web (Xie et al., 2016; Wallin et al., 2018). L. variegatus is also resilient, making it well suited for bioaccumulation testing (Sardo et al., 2007). Thus, the results will be widely applicable. As a benthic organism, it also provides us a way of studying the importance of sediment uptake in contaminant transfer compared to the uptake from water. The individuals of L. variegatus used in this experiment were reared at the University of Eastern Finland in the Department of Environmental and Biological Sciences. The worms were grown in tanks filled with 1M (hardness: Ca + Mg = 1 mM, which equals 100 mg/Ɩ of CaCO3) artificial freshwater (MgSO4 (24.65 g/Ɩ) + CaCl2 (58.80 g/Ɩ) + KCl (1.15 g/Ɩ) + NaHCO3 (12.95 g/Ɩ) + deionized water) and soaked and shredded paper sheets (Avalon, Walki Medical). The organisms were transferred to test beakers in 50-mL beakers filled with approximately 20-mƖ artificial freshwater, and each containing approximately 80 individuals. These beakers had been acid-washed in 1 M HCl (Fisher chemical, H/1200/PB17X, UN1789) prior to use.
2.2 Sediments
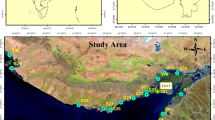
Sediment samples were collected mid-October in 2019 from four small lakes located in Eno, Eastern Finland. These lakes were Iso Hiislampi (62° 55.8272′ N, 30° 01.2871′ E, WGS84), Pieni Hiislampi (62° 55.9973′ N, 30° 01.2685′ E, WGS84), Saarilampi (62° 56.38792′ N, 30° 1.98008′ E, WGS84), and Ruosmanlampi (62° 56.2811′ N, 30° 03.0491′ E, WGS84) (Fig. 1). Three of the lakes are downstream from the former mining site (62° 55.81235′ N, 30° 01.03677′ E, WGS84) and connected to each other in the order of Iso Hiislampi, Pieni Hiislampi, and Saarilampi. Lake Ruosmanlampi was used as a reference, as it is located near the mining area, but was part of a different watershed, and does not receive water from the mining area. Sediment samples were collected from each lake using an Ekman sediment sampler, from following depths: Iso Hiislampi 14 m, Pieni Hiislampi 7 m, Saarilampi 1.5 m, and Ruosmanlampi 6.5 m. Specific sampling locations were chosen primarily based on availability of soft sediment and the data from previous studies from the same area (Tuovinen et al., 2016c; Majlesi et al., 2020, 2021). From each lake, approximately 10 l of sediment were sampled from the top 10 cm. This depth is considered to be active layer for macro-organisms as well. These samples were stored in plastic buckets for transfer and later stored at 4 °C until further analysis, to slow down any possible changes in sediment caused by biogeochemical processes. Before analysis, the sediments were filtered through 2 mm sieve to make them homogenous and to remove possible macro-organisms. From these sediment samples, total organic carbon (TOC) amounts were analyzed with an Analytik Jena TOC analyzer with a solid sample module (Analytik Jena N/C 2100, Jena, Germany). Carbonates were removed from the samples using phosphoric acid (H3PO4) prior to analyses.
A and C Sediment and porewater U concentration in relation to U concentration in L. variegatus in all four lakes. Concentration scale for sediment samples and equivalent L. variegatus samples is mg/kg. For porewater samples, the scale is μg/Ɩ and equivalent L. variegatus samples scale is μg/kg. B and D Environmental U concentration in relation to calculated animal-to-environment CR values for all four lakes. Black solid lines are calculated trend lines, while red dotted trend lines are examples of linear accumulation assumption (A, C) and constant CR assumption (B, D). Blue dotted trend lines are examples of constant animal concentration assumptions. IH = Iso Hiislampi, PH = Pieni Hiislampi, SL = Saarilampi, RL = Ruosmanlampi
2.3 Experimental Setup
This experiment was based on the Standard Guide for Determination of the Bioaccumulation of Sediment-Associated Contaminants by Benthic Invertebrates (ASTM, 2020). The experiment was conducted using a set of 16 acid-washed 3-Ɩ beakers. For each sediment sample, we had three replicate beakers with L. variegatus added and one beaker without test organisms. This allowed us to study possible differences caused by the test organisms in metal concentrations or environmental characteristics. The beakers were filled with 1.5 Ɩ of corresponding lake sediment and 1.5 Ɩ of artificial freshwater added on top without disturbing the sediment. One additional beaker filled with only water was added to the middle of the test setup to monitor average water temperature throughout the experiment. The water temperature in the experimental setup was 19 ± 1°C for the duration of the experiment. Filled beakers were then allowed to settle for 24 h before aeration pipettes were added through the shrink wrap cover of each beaker. Measurements of environmental characteristics from each filled beaker were conducted using WTW Multiline P4 Universal Meter and WTW Polilyte HT 120, WTW SenTix 41 and WTW OxiCal SL electrodes (New York, USA), respectively for sediment pH, water pH and oxygen levels. These measurements were conducted three times; before aeration, one day after the aeration had begun, and immediately after the experiment had been concluded. In addition, conductivity of the water was also measured before starting the aeration using Schott CG 853 conductivity meter (Mainz, Germany). L. variegatus (n = 80/beaker) were added to each designated beaker after 24-h aeration. The beakers were then allowed to be undisturbed for 28 days, barring the addition of deionized water to cover for evaporation.
Once the 28-day bioaccumulation period was over, a sample of 25 mƖ of both overlying water and porewater were taken from each beaker using Rhizon-samplers (Rhizosphere research products, Rhizon CSS, 5-cm porous part, glass fiber wire, 12-cm tubing PVC/PE, male luer lock, flat tip, diameter 2.5 mm) into 9-mƖ vacuum tubes (Vacuum tube, plastic, 9 mƖ, no additives, 4 per overlying water/porewater per beaker), which were stored at 4°C before analysis. After this, the overlying water was removed, and the sediment was sieved using 0.2 mm sieve to extract the L. variegatus from each beaker. They were then transferred to separate, acid-washed 100-mƖ beakers containing artificial freshwater for gut purging over 6 h. Once 6 h had passed, the L. variegatus of each sample were measured for their wet weight and transferred to acid-washed plastic test tubes for storage at −15 °C. One control sample of 80 L. variegatus worms were also taken straight from the growing chambers and frozen in similar manner. After the experiment, only few test beakers contained all 80 (or more, due to reproduction) L. variegatus that were initially placed in them. The lowest number of individuals was 43 in one of the Ruosmanlampi replicates.
2.4 Sample Analysis
All collected samples were sent to commercial services provider (SGS Finland LLE, Kotka, Finland), for elemental analysis. Sediment samples were analyzed using inductively coupled plasma atomic emission spectroscopy (ICP-AES) and aqua regia extraction (standards SFS-EN ISO 11885, SFS-EN 16170, EPA3015A, SFS-EN 16174, ISO 12914), except for U, which was done using inductively coupled plasma mass spectrometry (ICP-MS) (standards SFS-EN 16174, ISO 12914, SFS-EN ISO 17294-2). Overlying water and porewater samples were analyzed using ICP-MS (standard EN ISO 17294-2), except for Sr, which was done using ICP-AES (standard ISO 11885). L. variegatus samples were analyzed using a combination of both ICP-AES (Ba, Co, Ni, Sr, and Zn, modified ISO 11885) and ICP-MS (U, modified EN ISO 17294-2), as well as aqua regia extraction.
2.5 Statistical Analysis
Sediment metal concentrations were statistically analyzed from the original lake samples and artificial overlying water/porewater means per lake were calculated using samples taken from each of the three beakers per lake (n = 3). Means of metal concentrations in L. variegatus were calculated using three replicates per sediment sample (n = 3). Both sediment and L. variegatus results are reported in dry weight. Samples in which metal concentrations were under the detection limit, the value of detection limit was used for calculations and statistical analyses. CR values were calculated as follows: CR = metal concentration in L. variegatus/metal concentration in the environment.
One-way ANOVA (including Welch and Brown-Forsythe ANOVA) with Tukey’s test for pairwise comparison as the post hoc test was used to compare concentrations in artificial overlying water, porewater, and L. variegatus as well as organism-to-environment CR values between lakes. For CR comparisons, log 10 transformations were performed to all values, because of log-normal distributions. The effects of TOC in the lake sediments were analyzed by normalizing the sediment metal concentrations to the TOC amounts and comparing these to the concentrations in L. variegatus using bivariate correlation tests. For all statistical analyses, results were considered to be statistically significant when P value was ≤ 0.05. The dependency of CR values on environmental concentrations was inspected visually. CR and metal concentration visualization graphs were made using GraphPad Prism 8 (Insight Partners), whereas statistical analyses were performed using SPSS 25 for Windows (SPSS Inc., an IBM Company).
3 Results
3.1 Environmental Characteristics
Mean conductivity, pH, and oxygen levels were measured from artificial overlying freshwater samples (n = 3, n = 1 for samples without L. variegatus) collected from experimental beakers before, during and after experiment/aeration (Table 1). TOC and pH were also measured from sediment samples (n = 3). All sediment samples were slightly acidic and Iso Hiislampi TOC amount (7.1 g/kg) was noticeably lower compared to the other three lake samples (> 170 g/kg). The artificial freshwater samples before aeration had somewhat acidic pH and low oxygen level, but pH values quickly rose close to neutral pH only one day after the aeration had begun. After the 28-day bioaccumulation test, the water pH in beakers containing sediment from Ruosmanlampi or Saarilampi was slightly higher than in the beginning of the experiment, but still towards acidic. Both Iso and Pieni Hiislampi pH dropped below 6 by the end of the experiment. Oxygen levels had also risen slightly compared to the pre-aeration values in all test beakers. Water conductivity was similar between all water samples and varied between 84.3 and 103.7 μS/cm.
A and C Sediment and porewater Ba concentration in relation to Ba concentration in L. variegatus in all four lakes. Concentration scale for sediment samples and equivalent L. variegatus samples is mg/kg. For porewater samples, the scale is μg/Ɩ and equivalent L. variegatus samples scale is μg/kg. B and D Environmental Ba concentration in relation to calculated animal-to-environment CR values for all four lakes. Black solid lines are calculated trend lines, while red dotted trend lines are examples of linear accumulation assumption (A, C) and constant CR assumption (B, D). Blue dotted trend lines are examples of constant animal concentration assumptions. IH = Iso Hiislampi, PH = Pieni Hiislampi, SL = Saarilampi, RL = Ruosmanlampi
3.2 Metal Concentrations in Sediment, Water and L. variegatus
The concentrations of the studied metals in sediment samples did not display the expected decreasing trend with increasing distance from the former uranium mine (Table 2). The concentrations in the sample collected from the reference lake Ruosmanlampi were similar to those collected from other lakes, except in the case of U. Uranium concentrations were clearly highest in samples collected from Iso Hiislampi and Saarilampi.
In most cases, beakers containing Iso Hiislampi sediment had the highest metal concentrations in both artificial overlying water and porewater (Tables 3 and 4). The only exceptions were Zn concentration in overlying water and Co concentration in porewater. The differences in artificial overlying water concentrations between beakers containing sediment from different lakes were especially notable for Ba, Sr, and Zn. Ba and Sr concentrations differed significantly among the lakes (P < 0.001), and Zn concentrations were significantly different among the lakes except between Iso and Pieni Hiislampi (Table 3). Variation of Ba concentration between sediment and water was also notable; Iso Hiislampi containing the least Ba in the sediment, while having the highest concentration in water (Tables 2, 3 and 4). Co, Ni, and U concentrations in the overlying water were significantly highest in beakers containing sediment from Iso Hiislampi (Table 3). The differences between the samples from other lakes were not significant, except in the case of Co. The concentrations in porewater were generally lower or similar than in overlying water, except for U (Table 4). The differences between beakers containing sediment from different lakes remained also mostly remained similar as in the case of overlying water. Presence of L. variegatus did not appear to affect the distribution of metals among overlying water, porewater, and sediment (Tables 2, 3 and 4).
Although U concentration in Iso Hiislampi and Saarilampi sediments were noticeably higher compared to the sediments from other two lakes, the concentration of U in L. variegatus did not differ significantly between the lakes after the 28-day exposure (Table 5). The concentrations of other metals in L. variegatus were also quite similar between the beakers containing sediment from different lakes (Table 5). The only statistically significant differences were observed for Ba between Iso Hiislampi and Saarilampi and for Zn between Iso Hiislampi and Pieni Hiislampi. Zn concentrations in L. variegatus were noted to be much higher than environmental concentrations.
When the metal concentrations in the sediment samples were normalized to the amount of organic carbon present, there was no significant variation between metals in terms of accumulation to L. variegatus overall when analyzed using bivariate correlation tests (Supplementary Figure S1). Metals maintained rather similar concentrations in the organisms regardless of the ratio of sediment metal concentration and TOC. However, Ba and Zn concentrations in L. variegatus were lower in Iso Hiislampi treatment, where the TOC amount was clearly lower. Similar effect could also be seen with Ni, but to a lesser extent. Amount of TOC in sediments did not seem to affect the accumulation of Co, Sr, or U to L. variegatus in any noticeable way.
3.3 Concentration Ratios
Calculated animal-to-sediment concentration ratios were noticeably lower compared to either animal-to-water or animal-to-porewater concentration ratios (Table 6). This is due to higher concentration of metals in the sediment than either type of water. Sediment concentrations were in mg/kg scale, whereas concentrations for both types of water were in μg/Ɩ scale. L. variegatus metal concentrations were also much higher than water concentrations, resulting in high animal-to-water CR values.
Considering animal-to-sediment CR values, only Ba and Zn were significantly different between the sediment samples (Table 6). For Ba, Saarilampi had higher value than Pieni Hiislampi and for Zn Iso Hiislampi had higher value than Saarilampi. Compared to the animal-to-sediment CR values, the animal-to-overlying water/porewater CR values varied more between the sediment sampling sites (Table 6). These CR values tended to be lowest for Iso Hiislampi although the differences to other sediment sampling sites were not always statistically significant (Table 6). Many of these CR values for (pore)water were also calculated using detection limit values due to their low metal concentration. This means that the true CR values for those metals could in theory be higher than those listed in Table 6.
3.4 Effects of Environmental Concentration on CR
Most of the studied metals did not transfer to L. variegatus completely following the linear accumulation assumption, with the exception of Ba and Co for some of the environmental phases. If this was the case, CR would have remained constant and the metal concentration in L. variegatus would have increased with the environmental concentration at similar rate. Instead, some of our findings seem to match better with the assumption of stable concentrations in organisms.
Concentration of U in L. variegatus did not increase with increasing environmental concentration in different sediments while under similar pH conditions, which resulted in lower CR values at higher environmental concentrations (Fig. 2, Supplementary Figure S2). Other studied metals that displayed similar behavior in all three environmental phases were Ni and Sr (Supplementary Figures S3 and S4). When standard deviation is considered, Zn concentrations in L. variegatus remain rather constant, even as sediment concentration increases (Supplementary Figure S5). Zn was also the only metal which remained below the detection limit in all porewater samples.
Ba and Co behaved differently from the previously mentioned three metals (Supplementary Figures S6 and S7). For Ba, higher sediment concentration resulted in higher animal-to-sediment CR values, with opposite being true for both overlying water and porewater CR (Fig. 3, Supplementary Figure S7). Co displayed a similar downwards trend to U for animal-to-(pore)water CR values, yet L. variegatus Co concentration also seemed to increase slightly with environmental Co concentrations. However, L. variegatus metal concentrations seemed to follow constant CR and linear accumulation assumptions for Ba and Co when compared to sediment concentrations as the changes in CR were minimal.
4 Discussion
This study consisted of determination of environmental concentration of six metals in sediment, artificial overlying water and porewater, as well as in sediment-exposed benthic organism L. variegatus. All studied metals have radionuclides which are important from the point of view of radiological protection of both humans and biota (OECD, 2006; Posiva, 2012; IAEA, 2021). Transfer from environment to organism was studied by calculating CR values and evaluating their dependency on environmental concentrations. The sediment samples originated from lakes near a former mining site and metal concentrations were expected to decrease with increasing distance from the site, like observed by Majlesi et al. (2021). However, this gradient was not found in this study, which means that the range of environmental metal concentrations was not as wide as expected. For example, U concentration in the sediment from Iso Hiislampi was much lower (496 mg/kg) compared to the concentration (2685 mg/kg) measured by Majlesi et al. (2021). This may be due to patchiness of contamination and changes in sediment quality of the lakes under study.
As the initial artificial freshwater contained no external metal contaminants, all metal contamination in the samples were caused by the initial sediment samples from each lake. After the experiment, metal concentrations in artificial overlying water or porewater were not clearly connected to concentrations in sediments, suggesting that the transfer is affected by other (bio)geochemical factors as well. These factors could be the specific chemical properties and compounds of the tested metals. The measured concentrations also indicated that the transfer from sediment to (pore)water was low in laboratory conditions. The measured metal concentrations in L. variegatus greatly exceeded the concentrations in artificial water, in addition to being in similar range to sediment metal concentrations. This means that the sediment metal concentrations would have had to play miniscule role compared to water if such L. variegatus metal concentrations were to be accumulated via water. Majlesi et al. (2021) also concluded that sediment could play a larger role than currently expected in the uptake of elements compared to water.
As accumulation experiments and the related CR calculations done on benthic invertebrates are currently rare, comparisons were made to previous field studies using chironomids. Both L. variegatus and chironomid larvae share similarities in their benthic lifestyles, feeding largely on sediment particles (Karima, 2021). They also differences, such as L. variegatus burrowing behavior and chironomids mostly living near the surface of the sediment (Karima, 2021). Our animal-to-sediment CR values for Ni, Sr, and Zn were higher than those observed by Majlesi et al. (2021) for Chironomus sp. collected from same lakes, while CRs for U were similar. Another previous study in Finland studied field collected chironomids in sediments also impacted by metal mining (Väänänen et al., 2019). The order of magnitude of CR values for Ni and Zn were found to be lower than those reported in the current study. Only few exceptions were noted, such as animal-to-water CR of Iso Hiislampi for both metals. The actual metal concentrations in overlying and porewater were similar in magnitude between current laboratory study and Väänänen et al. (2016, 2019), aside from our U concentrations. Larger differences could be seen in sediment metal concentrations, especially for Ni, Zn and U between our study and Väänänen et al. (2016, 2019). Ni and Zn concentrations were much higher in their study, while U concentrations were higher in the current study. This could be seen as more possible proof for importance of sediment as an exposure route.
Comparisons were also made to similar laboratory and in situ studies reporting CR, bioconcentration factors (BCF) or bioaccumulation factors (BAF) for various aquatic invertebrates (Supplementary Table S1). As BCFs and BAFs are defined as chemical concentration in organism divided by chemical concentration in surrounding medium with the only difference between the two being that for BCF, uptake is considered only from water, whereas BAF considers uptake from food, soil or sediment as well. Therefore, they are both comparable to similarly calculated CR values (Karlsson et al., 2002). Overall, our animal-to-sediment CR values for most metals were similar or higher than in the previous studies with other aquatic organisms (IAEA, 2010), while animal-to-water CR values were either similar or slightly lower (Hosseini et al., 2008; IAEA, 2010; Takata et al., 2010; Doering et al., 2019; Husson et al., 2019). Exceptions were animal-to-water CR for U and Zn, which were clearly higher in our study compared to other similar studies. It should be noted that great degree of variation for CR/BAF/BCF was visible between various studies, most likely due to variations in environmental conditions and organisms used in testing.
Ni, Sr, and U behaved similarly regarding accumulation to L. variegatus. In all three cases, the observed behavior did not follow the assumption of linear accumulation, but instead the transfer from sediment or water seemed to be lower at higher environmental concentrations. It is possible that L. variegatus accumulates these metals until a certain inner threshold, after which it will actively start to purge them from itself. This kind of behavior has been noted with Chironomus tentans larvae in laboratory conditions (Muscatello and Liber, 2010). Rest of the tested metals (Ba, Co, and Zn) behaved differently. For all three the accumulation from sediment was somewhat linear, and for Co also from water. The transfer of Ni, Sr, and U to L. variegatus differed from linear transfer observed for Chironomus sp. collected from the study lakes (Majlesi et al. 2021). The transfer of Zn was similar in both studies. Our observations on Zn need to be interpreted with caution as we discovered during analysis that there was a large quantity of Zn in the feed used during the rearing of the larvae. Chironomid larvae spend large part of their life living in and on top of the sediment with possibly selective feeding habits (Davies, 1975), whereas L. variegatus burrow deep in sediment, with rather nonselective feeding (Camusso et al., 2012). These behavioral differences in addition to differences in either accumulation routes or elimination capabilities, perhaps both, could explain the differences between our results for these organisms. Differences could also arise from our laboratory conditions in comparison to in situ conditions of Majlesi et al. (2021).
It should be noted that the total element concentration is not the sole explaining factor as the bioavailability of the elements is governed by different factors. Previous studies have shown that a major factor for Ni accumulation in aquatic environments is the ratio of acid volatile sulfides to simultaneously extracted metals (Ankley et al., 1991; Vandegehuchte et al., 2007) while porewater Ni2+ ion concentrations have been found to act as a major accumulation source (Camusso et al., 2012) and correlate with toxicity (Vandegehuchte et al., 2007). Sr accumulation is inversely affected by calcium (Vanderploeg et al., 1975), which in our experiment varied between 14.4 and 31.6 mg/l in (pore)water samples and between 4482 and 13280 mg/kg in sediment samples. This could be one explaining factor to Sr accumulation behavior. U is not very soluble, unless it is in more reactive physicochemical forms, such as U4+ or UO2(OH)+ (Puigdomenech and Bruno, 1988; Markich, 2002). U4+ is a common occurrence in nature alongside uranyl, which in turn can form UO2(OH)+ from oxidized U liquors near neutral pH (Taylor, 1971; Hutchinson and Blackwell, 1984). Organic matter has also been shown to be efficient in binding U (Dunn et al., 1985; Cumberland et al., 2016). However, Iso Hiislampi had the lowest TOC amount, which should theoretically allow greater U mobility. It was also noted that U was the only metal which consistently had higher concentrations in porewater compared to overlying water. Given its low TOC in comparison to other lakes, U speciation in Iso Hiislampi cannot be explained by the amount of organic material in sediment. A recent review article by Smedley and Kinniburgh (2023) has compiled information about U speciation in natural waters. As our water samples were slightly oxidizing and near neutral pH, this would suggest that the dominant U species in our samples could be UO2(OH)+ or some form of tertiary complex, such as (UO2)2CO3(OH)3−. This assumption would also be supported by calculations done by Krestou and Panias (2004). Bioavailability of Ba could be partially explained by its alkaline metal (Kirkpatrick, 1978) and clay affinity for forming salts in soil and sediment, with its solubility increasing at low pH environments (Kabata-Pendias, 2010), such as when ingested by L. variegatus. For Co, low bioavailability could be explained by hypoxic conditions, which have been shown to affect Co uptake on Tubifex worms (Evans et al., 1988), similar to conditions at Paukkajanvaara and possibly with our laboratory setup as well regarding porewater oxygen levels. Aforementioned factors could have partially explained the observed trends in transfer of the studied metals and should be investigated in more detail in the following studies.
Animal-to-water CR values together with soil adsorption coefficients (Kd), which measure sorption affinity of radionuclides to sediment, are currently the most common method to predict concentrations in freshwater organisms (IAEA, 2004; Hosseini et al., 2008; Howard et al., 2013). Relatively few studies involving these values have been conducted to calculate animal-to-sediment CR values, which could be more relevant in many cases. Increasing amounts of evidence has been presented about the existence of “brown food chain” as well, which relies on methane oxidizing bacteria and serves as food for zooplankton and macro-invertebrates (Jones and Grey, 2004; Sanseverino et al., 2012; Grey, 2016). The “green food chain” represents the traditional view where phytoplankton is the basis for aquatic food webs. These two types of food chains have been shown to be closely intertwined, and these interactions are important to the functioning of the whole ecosystem (Zou et al., 2015; Mougi, 2020). As L. variegatus is a detritivore, this would mean that any metal accumulation from sediment to it would also affect higher trophic levels, as per the brown-green food web interaction. This idea is also supported by studies, which compared uptake from water and food for deposit-feeding invertebrates. They concluded that sediment was the main source of metal uptake for those types of organisms (Wang and Fisher, 1999; Rowan et al., 2014). There is also mounting evidence in marine environments for the importance of direct transfer of radioisotopes from the bottom sediment to benthic organisms, and from them to higher trophic levels (Shigenobu et al., 2015; Bezhenar et al., 2016; Wang et al., 2016; Perianez et al., 2019). This gives precedent to the thought that same could be possible in freshwater environments as well. The relevancy of these factors to the sampled lakes was originally brought up by Majlesi et al. (2021), as the food chain of these lakes is comprised mostly of benthic organisms and fish feeding on them. The findings of Majlesi et al. (2021) also support our own findings here regarding L. variegatus.
Overall, our results obtained here do not seem to support the assumption of linearity related to CR values. Consistently with our findings, a recent study indicated that nonlinear transfer models could be more suitable for soil-to-ant transfer (Roivainen et al., 2022). Similar results regarding nonlinear transfer were also obtained by Tuovinen et al. (2011, 2013, 2016a, 2016b) for various plants and animals in terrestrial and aquatic environments. These results together with ours and Majlesi et al. (2021) have provided more credence to the possible re-evaluation of aquatic radioecological models and their current usage and implementation of only (pore)water-based CR values. At least for Ni, Sr, and U the use of constant CR values in models for L. variegatus seems to underestimate the metal concentration in the organism, based on our results. However, it should be remembered that the results discussed here were obtained from laboratory experiments, rather than in situ. Various factors in the field could affect these results, such as pH, oxygen levels, sediment composition, or specific characteristics of different isotopes/metal species.
Possible limitations and weaknesses which we recognized in this study include the lack of expected concentration gradient which affects the study of linearity of transfer, as well as the large amount of Zn found in the feed given for L. variegatus during rearing. This additional Zn can be seen in especially well when comparisons we made between L. variegatus and (pore)water concentrations for Zn and in the resulting CR values as well. Due to this, our results related to Zn may not be entirely accurate. Another possible limitation is the possible effects of different (bio)geochemical or metal-specific factors on accumulation. As these factors were not measured in this experiment, we cannot completely rule out their effects on metal accumulation from sediment and (pore)water to L. variegatus. Although information on the transfer of stable elements is useful for radioecological modelling, further studies could include measurements of specific radionuclides. Finally, our results obtained here are not directly comparable to in situ results, due to laboratory conditions. As such, future repeat experiment in situ at Paukkajanvaara could provide interesting comparison data to this experiment.
5 Conclusions
The findings presented in this study provide more support for the theory on the importance of sediment as a direct uptake source of metals and their radionuclides for benthic invertebrates. The transfer of the most studied elements into L. variegatus seemed to be nonlinear, which in consistent with many other recent findings. Further research is needed to ascertain the observed nonconstant behavior of CR values for these metals, preferably as in situ experiments while accounting for differences from specific metal species. This could involve research on environmental processes that drive both metal and radionuclide transfer in aquatic environments and their uptake to and elimination from aquatic organisms. The results of this and future studies on this subject can be utilized in the development of more robust and accurate radioecological models after validation with, e.g., in situ studies.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Ankley, T., Philips, G., Leonard, E., Benoit, D., Mattson, V., Kosian, P., Cotter, A., Dierkes, J., Hansen, D., & Mahony, J. (1991). Acid-volatile sulfide as a factor mediating cadmium and nickel bioavailability in contaminated sediments. Environmental Science & Technology, 10, 1299–1307. https://doi.org/10.1002/etc.5620101009
ASTM International. (2020). Standard guide for determination of the bioaccumulation of sediment-associated contaminants by benthic invertebrates, E1688-19. https://doi.org/10.1520/E1688-19
Beresford, N., Barnett, C., Gashchak, S., Maksimenko, A., Guliaichenko, E., Wood, M., & Izquierdo, M. (2020). Radionuclide transfer to wildlife at a reference site in the Chernobyl exclusion zone and resultant radiation exposures. Journal of Environmental Radioactivity, 211, 105661. https://doi.org/10.1016/j.jenvrad.2018.02.007
Besser, J., Brumbaugh, W., & Ingersoll, C. (2015). Characterizing toxicity of metal-contaminated sediments from mining areas. Applied Geochemistry, 57, 73–84. https://doi.org/10.1016/j.apgeochem.2014.05.021
Bezhenar, R., Jung, K., Maderich, V., Willemsen, S., de With, G., & Qiao, F. (2016). Transfer of radiocaesium from contaminated bottom sediments to marine organisms through benthic food chain in post-Fukushima and post-Chernobyl periods. Biogeosciences, 13, 3021–3034. https://doi.org/10.5194/bg-13-3021-2016
Brown, J., Beresford, N., & Hevrøy, T. (2019). Exploring taxonomic and phylogenetic relationships to predict radiocaesium transfer to marine biota. Science of the Total Environment, 649, 916–928. https://doi.org/10.1016/j.scitotenv.2018.08.343
Brugge, D. (2014). Uranium. In Wexler et al. (Eds.), Encyclopedia of Toxicology (Vol. 4, 3rd ed., pp. 883–884). Academic Press/Elsevier.
Caffrey, E., Leonard, M., Napier, J., Neville, D., & Higley, K. (2014). Radioecology: why bother? Journal of Environmental Protection, 5, 181–192. https://doi.org/10.4236/jep.2014.53022
Camusso, M., Polesello, S., Valsecchi, S., & Vignati, D. (2012). Importance of dietary uptake of trace elements in benthic deposit-feeding Lumbriculus variegatus. Trends in Analytical Chemistry, 36, 103–112. https://doi.org/10.1016/j.trac.2012.02.010
Colpaert, A. (2006). The Forgotten Uranium Mine of Paukkajanvaara, North Karelia, Finland. Nordia Geographical Publications, 35(2), 31–38. https://nordia.journal.fi/article/view/76199
Cumberland, S., Douglas, G., Grice, K., & Moreau, J. (2016). Uranium mobility in organic matter-rick sediments: a review of geological and geochemical processes. Earth-Science Reviews, 159, 160–185. https://doi.org/10.1016/j.earscirev.2016.05.010
Davies, I. (1975). Selective feeding in some arctic Chironomidae. Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie, 19(4), 3149–3154. https://doi.org/10.1080/03680770.1974.11896425
Doering, C., Carpenter, J., Orr, B., & Urban, D. (2019). Whole organism concentration ratios in freshwater wildlife from an Australian tropical U mining environment and the derivation of a water radiological quality guideline value. Journal of Environmental Radioactivity, 198, 27–35. https://doi.org/10.1016/j.jenvrad.2018.12.011
Dunn C, Ek J, Byman J. 1985. Uranium biogeochemistry: a bibliography and report on the state of the art. IAEA-TECDOC-327
Evans, R., Andrews, D., & Cornett, R. (1988). Chemical fractionation and bioavailability of cobalt-60 to benthic deposit-feeders. Canadian Journal of Fisheries and Aquatic Sciences, 45, 228–236. https://doi.org/10.1139/f88-027
Gad, S. (2014a). Cobalt. In Wexler et al. (Eds.), Encyclopedia of Toxicology (Vol. 1, 3rd ed., pp. 996–998). Academic Press/Elsevier.
Gad, S. (2014b). Nickel and nickel compounds. In Wexler et al. (Eds.), Encyclopedia of Toxicology (Vol. 3, 3rd ed., pp. 506–510). Academic Press/Elsevier.
Gad, S. (2014c). Zinc. In Wexler et al. (Eds.), Encyclopedia of toxicology (Vol. 4, 3rd ed., pp. 997–1000). Academic Press/Elsevier.
Gad, S. (2014d). Barium. In Wexler et al. (Eds.), Encyclopedia of toxicology (Vol. 1, 3rd ed., pp. 368–370). Academic Press/Elsevier.
Gad, S. (2014e). Strontium. In Wexler et al. (Eds.), Encyclopedia of toxicology (Vol. 4, 3rd ed., pp. 405–406). Academic Press/Elsevier.
Govedich, F., Bain, B., Moser, W., Gelder, S., Davies, R., & Brinkhurst, R. (2009). Chapter 12 - Annelida (Clitellata): Oligochaeta, Branchiobdellida, Hirudinida, and Acanthobdellida. In J. Thorp & A. Covich (Eds.), Ecology and Classification of North American Freshwater Invertebrates (3rd ed., pp. 385–436). Academic Press.
Grey, J. (2016). The incredible lightness of being methane-fuelled: stable isotopes reveals alternative energy pathways in aquatic ecosystems and beyond. Frontiers in Ecology and Evolution, 4, 8. https://doi.org/10.3389/fevo.2016.00008
Gustafsson, D., Price, D., & Erseus, C. (2009). Genetic variation in the popular lab worm Lumbriculus variegatus (Annelida: Clitellata: Lumbriculidae) reveals cryptic speciation. Molecular Phylogenetics and Evolution, 51, 182–189. https://doi.org/10.1016/j.ympev.2008.12.016
Haanes, H., Hansen, E., Hevroy, T., Jensen, L., Gjelsvik, R., Jaworska, A., & Bradshaw, C. (2020). Realism and usefulness of multispecies experiment designs with regard to application in radioecology: a review. Science of the Total Environment, 718, 134485. https://doi.org/10.1016/j.scitotenv.2019.134485
Hosseini, A., Thorring, H., Brown, J., Saxen, R., & Ilus, E. (2008). Transfer of radionuclides in aquatic ecosystems - default concentration ratios for aquatic biota in the ERICA tool. Journal of Environmental Radioactivity, 99, 1408–1429. https://doi.org/10.1016/j.jenvrad.2008.01.012
Howard, B., Beresford, N., Copplestone, D., Telleria, D., Proehl, G., Fesenko, S., Jeffree, R., Yankovich, T., Brown, J., Higley, K., Johansen, M., Mulye, H., Vandenhove, H., Gaschak, S., Wood, M., Takata, H., Andersson, P., Dale, P., Ryan, J., et al. (2013). The IAEA handbook on radionuclide transfer to wildlife. Journal of Environmental Radioactivity, 121, 55–74. https://doi.org/10.1016/j.jenvrad.2012.01.027
Husson, A., Leermakers, M., Descostes, M., & Lagneau, V. (2019). Environmental geochemistry and bioaccumulation/bioavailability of uranium in a post-mining context - the Bois-Noirs Limouzat mine (France). Chemosphere, 236, 124341. https://doi.org/10.1016/j.Chemosphere.2019.124341
Hutchinson, R., & Blackwell, J. (1984). Time, crustal evolution and generation of uranium deposits. In Uranium geochemistry, mineralogy, geology, exploration and resources, 89-100. Springer. https://doi.org/10.1007/978-94-009-6060-2_7
IAEA (International Atomic Energy Agency). (2004). Sediment distribution coefficients and concentration factors for biota in the marine environment. Technical report series, no. 422. IAEA.
IAEA (International Atomic Energy Agency). (2005). Environmental contamination from uranium production facilities and their remediation. In Proceedings of and International Workshop, Lisbon 11-13. STI/PUB/1228. International Atomic Energy Agency.
IAEA (International Atomic Energy Agency). (2010). Handbook of parameter values for the prediction of radionuclide transfer in terrestrial and freshwater environments. Technical reports series, no. 472. IAEA STI/DOC/010/472.
IAEA (International Atomic Energy Agency). (2012). Monitoring for compliance with exemption and clearance levels. Safety Reports Series, no. 67. IAEA STI/PUB/1511.
IAEA (International Atomic Energy Agency). 2021. Approaches for modelling of radioecological data to identify key radionuclides and associated parameter values for human and wildlife exposure assessment. IAEA-TECDOC-1950
Jones, R., & Grey, J. (2004). Stable isotope analysis of chironomid larvae from some Finnish forest lakes indicates dietary contribution from biogenic methane. Boreal Environment Research, 9, 17–24.
Kabata-Pendias, A. (2010). Trace elements in Soils and Plants (4th ed.). CRC Press. https://doi.org/10.1201/b10158
Karima, Z. (2021). Chironomidae: biology ecology and systematics. In F. Perveen (Ed.), The Wonders of Diptera – Characteristics, Diversity, and Significance for the World’s Ecosystems. IntechOpen.
Karlsson S, Meili M, Bergström U, Studsvik Eco & Safety AB. 2002. Bioaccumulation factors in aquatic ecosystems: a critical review. Svensk Kärnbränslehantering AB, R-02-36.
Keum, D., Jun, I., Lim, K., Choi, Y., & Howard, B. (2013). Time-dependent transfer of 137Cs, 85Sr and 65Zn to earthworms in highly contaminated soils. Journal of Environmental Radioactivity, 126, 427–433. https://doi.org/10.1016/j.jenvrad.2012.07.017
Kirkpatrick, T. (1978). Barium compounds. Kirk-Othmer encyclopedia of chemical technology (Vol. 3, 3rd ed., pp. 463–479). John Wiley and Sons.
Konovalenko, L., Bradshaw, C., Andersson, E., & Kautsky, U. (2017). Application of an ecosystem model to evaluate the importance of different processes and food web structure for transfer of 13 elements in a shallow lake. Journal of Environmental Radioactivity, 169, 85–97. https://doi.org/10.1016/j.jenvrad.2016.12.016
Krestou, A., & Panias, D. (2004). Uranium (VI) speciation diagrams in the UO22+ / CO32- / H2O system at 25 °C. The European Journal of Mineral Processing and Environmental Protection, 4(2), 113–129.
Majlesi, S., Akkanen, J., Roivainen, P., Tuovinen, T., Sorvari, J., Naarala, J., & Juutilainen, J. (2021). Transfer of elements relevant to radioactive waste into chironomids and fish in boreal freshwater bodies. Science of the Total Environment, 791, 148218. https://doi.org/10.1016/j.scitotenv.2021.148218
Majlesi, S., Carrasco-Navarro, V., Sorvari, J., Panzuto, S., Naarala, J., Akkanen, J., & Juutilainen, J. (2020). Is developmental instability in chironomids a sensitive endpoint for testing uranium mine-affected sediments? Science of the Total Environment, 720, 137496. https://doi.org/10.1016/j.scitotenv.2020.137496
Markich, S. (2002). Uranium speciation and bioavailability in aquatic systems: an overview. The Scientific World Journal, 2, 707–729. https://doi.org/10.1100/tsw.2002.130
Mougi, A. (2020). Coupling of green and brown food webs and ecosystem stability. Ecology and Evolution, 10, 9192–9199. https://doi.org/10.1002/ece3.6586
Muscatello, J., & Liber, K. (2010). Uranium uptake and depuration in the aquatic invertebrate Chironomus tentans. Environmental Pollution, 158, 1696–1701. https://doi.org/10.1016/j.envpol.2009.11.032
OECD (Organization for Economic Co-operation and Development). (2006). Nuclear energy agency (OECD/NEA). Advanced nuclear fuel cycles and radioactive waste management. NEA#5990. Organization for Economic Co-operation and Development.
Perianez, R., Bezhenar, R., Brovchenko, I., Duffa, C., Iosjpe, M., Jung, K., Kim, K., Kobayashi, T., Liptak, L., Little, A., Maderich, V., McGinnity, P., Min, B., Nies, H., Osvath, I., Suh, K., & de With, G. (2019). Marine radionuclide transport modelling: recent developments, problems and challenges. Environmental Modelling and Software, 122, 104523. https://doi.org/10.1016/j.envsoft.2019.104523
Posiva, (2012). Olkiluoto Biosphere Description 2012. Posiva Report 2012-06. Posiva Oy, Eurajoki.
Puigdomenech, I., & Bruno, J. (1988). Modelling uranium solubilities in aqueous solutions: validation of a thermodynamic database for the EQ3/6 geochemical codes. Swedish Nuclear Fuel and Waste Management Co. SKB-TR-88-21.
Pyle, G., & Clulow, F. (1997). Non-linear radionuclide transfer from the aquatic environment to fish. Health Physics, 73, 488–493. https://doi.org/10.1097/00004032-199709000-00007
Roivainen, P., Muurinen, S.-M., Sorvari, J., Juutilainen, J., Naarala, J., & Salomaa, S. (2022). Transfer of elements into boreal forest ants at former uranium mining site. Environmental Pollution, 304, 119231. https://doi.org/10.1016/j.envpol.2022.119231
Rowan, D., Silke, R., & Carr, J. (2014). Biota-sediment accumulation factors (BSAF) for radionuclides and sediment associated biota of the Ottawa River. Nuclear Review, 2, 3–15. https://doi.org/10.12943/ANR.2013.00013
Sanseverino, A., Bastviken, D., Sundh, I., Pickova, J., & Enrich-Prast, A. (2012). Methane carbon supports aquatic food webs on the fish level. PLoS One, 7, e42723. https://doi.org/10.1371/journal.pone.0042723
Sardo, A., Soares, A., & Gerhardt, A. (2007). Behavior, growth and reproduction of Lumbriculus variegatus (Oligochaetae) in different sediment types. Human and Ecological Risk Assessment, 13, 519–526. https://doi.org/10.1080/10807030701341043
Shaw, G. (2005). Applying radioecology in a world of multiple contaminants. Journal of Environmental Radioactivity, 81, 117–130. https://doi.org/10.1016/j.jenvrad.2005.01.001
Shigenobu, Y., Ambe, D., Kaeriyama, H., Sohtome, T., Mizuno, T., Koshiishi, Y., Yamasaki, S., & Ono, T. (2015). Investigation of radiocaesium translation from contaminated sediment to benthic organisms. In Impacts of the Fukushima Nuclear Accident on Fish and Fishing Grounds (pp. 91–98). Springer. https://doi.org/10.1007/978-4-431-55537-7_7
Smedley, P., & Kinniburgh, D. (2023). Uranium in natural waters and the environment: distribution, speciation and impact. Applied Geochemistry, 148, 105534. https://doi.org/10.1016/j.apgeochem.2022.105534
Takata, H., Aono, T., Tagami, K., & Uchida, S. (2010). Concentration ratios of stable elements for selected biota in Japanese estuaries area. Radiation and Environmental Biophysics, 49, 591–601. https://doi.org/10.1007/s00411-010-0317-x
Taylor, J. (1971). The structure of the α form of Uranyl Hydroxide. Acta Crystallographica Section B, B27, 1088.
Tuovinen, H., Pohjolainen, E., Vesterbacka, D., Kaksonen, K., Virkanen, J., Solatie, D., Lehto, J., & Read, D. (2016c). Release of radionuclides from waste rock and tailings at a former pilot uranium mine in eastern Finland. Boreal Environment Research, 21, 471–480. http://hdl.handle.net/10138/172377
Tuovinen, H., Vesterbacka, D., Kaksonen, K., Virkanen, J., Read, D., Solatie, D., & Lehto, J. (2019). Release of base and potentially harmful metals from the waste deposits at a former uranium mine in eastern Finland. International Journal of Environmental Monitoring and Analysis, 7(4), 85–92. https://doi.org/10.11648/j.ijema.20190704.12
Tuovinen, T., Kasurinen, A., Häikiö, E., Tervahauta, A., Makkonen, S., Holopainen, T., & Juutilainen, J. (2016a). Transfer of elements relevant to nuclear fuel cycle from soil to boreal plants and animals in experimental meso- and microcosms. Science of the Total Environment, 539, 252–261. https://doi.org/10.1016/j.scitotenv.2015.08.157
Tuovinen, T., Kolehmainen, M., Roivainen, P., Kumlin, T., Makkonen, S., Holopainen, T., & Juutilainen, J. (2016b). Nonlinear transfer of elements from soil to plants: impact on radioecological modeling. Radiation and Environmental Biophysics, 55(3), 393–400. https://doi.org/10.1007/s00411-016-0655-4
Tuovinen, T., Roivainen, P., Makkonen, S., Kolehmainen, M., Holopainen, T., & Juutilainen, J. (2011). Soil-to-plant transfer of elements is not linear: results for five elements relevant to radioactive waste in five boreal species. Science of the Total Environment, 410, 191–197. https://doi.org/10.1016/j.scitotenv.2011.09.043
Tuovinen, T., Saengkul, C., Ylipieti, J., Solatie, D., & Juutilainen, J. (2013). Transfer of 137Cs from water to fish is not linear in two northern lakes. Hydrobiologia, 700, 131–139. https://doi.org/10.1007/s10750-012-1224-8
Väänänen, K., Abel, S., Oksanen, T., Nybom, I., Leppänen, M., Asikainen, H., Rasilainen, M., Karjalainen, A., & Akkanen, J. (2019). Ecotoxicity assessment of boreal lake sediments affected by metal mining: sediment quality triad approach complemented with metal bioavailability and body residue studies. Science of the Total Environment, 662, 88–98. https://doi.org/10.1016/j.scitotenv.2019.01.209
Väänänen, K., Kauppila, T., Mäkinen, J., Leppänen, M., Lyytikäinen, M., & Akkanen, J. (2016). Ecological risk assessment of boreal sediments affected by metal mining: metal geochemistry, seasonality and comparisons of several risk assessment methods. Integrated Environmental Assessment and Management, 12, 759–771. https://doi.org/10.1002/ieam.1751
Vandegehuchte, M., Roman, Y., Nguyen, L., Janssen, C., & De Schamphelaere, K. (2007). Toxicological availability of nickel to the benthic oligochaete Lumbriculus variegatus. Environment International, 33, 736–742. https://doi.org/10.1016/j.envint.2007.02.006
Vanderploeg, H., Parzyck, D., Wilcox, W., Kercher, J., & Kaye, S. (1975). Bioaccumulation factors for radionuclides in freshwater biota. United States Department of Energy Technical Report ORNL-5002.
Wallin, J., Karjalainen, J., Väisänen, A., & Karjalainen, A. (2021). Toxicity of mining-contaminated lake sediments to Lumbriculus variegatus. Water, Air, & Soil Pollution, 232, 202. https://doi.org/10.1007/s11270-021-05157-5
Wallin, J., Vuori, K. M., Väisänen, A., Salmelin, J., & Karjalainen, A. (2018). Lumbriculus variegatus (Annelida) biological responses and sediment sequential extractions indicate ecotoxicity of lake sediments contaminated by biomining. Science of the Total Environment, 645, 1253–1263. https://doi.org/10.1016/j.scitotenv.2018.07.117
Wang, C., Baumann, Z., Madigan, D., & Fisher, N. (2016). Contaminated marine sediments as a source of cesium radioisotopes for benthic fauna near Fukushima. Environmental Science & Technology, 50, 10448–10455. https://doi.org/10.1021/acs.est.6b02984
Wang, W., & Fisher, N. (1999). Delineating metal accumulation pathways for marine invertebrates. Science of the Total Environment, 237, 459–472. https://doi.org/10.1016/S0048-9697(99)00158-8
Xie, L., Wu, X., Chen, H., Luo, Y., Guo, Z., Mu, J., Blankson, E., Dong, W., & Klerks, P. (2016). The bioaccumulation and effects of selenium in the oligochaete Lumbriculus variegatus vie dissolved and dietary exposure routes. Aquatic Toxicology, 178, 1–7. https://doi.org/10.1016/j.aquatox.2016.07.008
Zou, K., Thebault, E., Lacroix, G., & Barot, S. (2015). Interactions between the green and the brown food web determine ecosystem functioning. Functional Ecology, 30, 1454–1465. https://doi.org/10.1111/1365-2435.12626
Acknowledgements
Pyry Pihlasvaara is acknowledged for the sediment sampling.
Funding
Open access funding provided by University of Eastern Finland (including Kuopio University Hospital). This study was funded by the Finnish Research Programme on Nuclear Waste Management KYT2022 (Decision numbers TEM/1507/08.09.02/2018 and VN/14858/2021), Project no. 18 – Improved radioecology for biosphere modeling.
Author information
Authors and Affiliations
Contributions
Marko Ylönen is acknowledged for conceptualization, methodology, formal analysis, investigation, data curation, and writing of original draft. Päivi Roivainen is acknowledged for methodology, writing review and editing, and supervision. Jonne Naarala is acknowledged for writing review and editing and supervision. Jarkko Akkanen is acknowledged for conceptualization, methodology, resources, writing review and editing, supervision, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 914 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ylönen, M., Roivainen, P., Naarala, J. et al. Transfer of Metals Relevant to the Nuclear Fuel Cycle into Lumbriculus variegatus from Boreal Freshwater Sediments. Water Air Soil Pollut 234, 749 (2023). https://doi.org/10.1007/s11270-023-06750-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06750-6