Abstract
The presence of urea in wastewater can give rise to many issues, including the proliferation of algae as a consequence of eutrophication as well as the discharge of ammonia, which exerts a detrimental impact on aquatic organisms. To assess the efficacy of several treatment strategies for lowering urea concentrations, this study compared the removing performances of electrocoagulation (EC) with those of conducting electrocoagulation and chemical coagulation in sequence (EC-CC) or vice versa (CC-EC). Many effective parameters of electrocoagulation have been studied, such as current density, spacing between electrodes, electrolyte type, and electrolysis time. A scanning electron microscope was used to investigate the electrode morphology, and a Fourier transform infrared was conducted to analyze the formed sludge. The electrocoagulation was carried out at its optimum conditions at 30 A/m2, and the chemical coagulation was conducted using three types of iron coagulants: FeSO4, Fe2(SO4)3, and FeCl3. The results showed insufficient improvement in urea removal for synthetic and domestic wastewater via EC-CC, regardless of the coagulant type. The urea removal efficiency via EC-CC improved by less than 0.5% and 5.5% for synthetic and domestic wastewater, respectively. In contrast, CC-EC proved a better improvement for urea removal for both synthetic and domestic wastewater, but only for FeCl3. Treatment by CC-EC at 30 A/m2 for 60 min using iron electrodes and 0.5 g/L of FeCl3 resulted in an improvement in the removal efficiency of urea by about 3.4% and 10.40% for synthetic and domestic wastewater, respectively. CC-EC achieved better removal of COD from domestic wastewater than that achieved by EC-CC by 6%. The results obtained from the study indicate that the CC-EC process is a cost-effective method for removing urea from both synthetic and domestic wastewater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The conservation of water resources is a significant challenge in the twenty-first century. The subject at hand is confronted with a multitude of challenges, including but not limited to population expansion, deforestation, accelerated urbanization, industrial development, and the phenomenon of global climate change. Freshwater supply and aquatic ecosystems are both severely impacted by water pollution, and access to clean drinking water is restricted and under stress these days (Hakizimana et al., 2017). Microplastics, pharmaceuticals, pesticides, inorganic anions, heavy metals, and organic compounds are all significant contaminants that pose a threat to the environment and must be eliminated (Dolatabadi & Ahmadzadeh, 2020; Dolatabadi et al., 2022, 2023; Jing et al., 2021; Urbańczyk et al., 2016). Hence, it is imperative to devise effective technologies and strategies for the treatment and control of wastewater to uphold its quality and enhance its volume on a significant scale, all the while ensuring the preservation of the environment and the promotion of sustainability. This includes handling the treatment and management of various types of wastewater, such as those originating from urban, industrial, and agricultural sources (Dolatabadi et al., 2023; Hakizimana et al., 2017).
Among the organic compounds present in many types of wastewater is urea. The extensive use of urea results in the production of substantial quantities of urea. Urea exists in the environment by being used as a raw material in plants and industries, human wastewater, and leachate from agriculture fields and farms (Urbańczyk et al., 2016). Urea itself is not toxic, but its hydrolysis to ammonia causes eutrophication, which causes damage to marine life (Urbańczyk et al., 2016). As part of stricter environmental regulations, it is imperative to reduce urea concentrations in effluent from wastewater treatment facilities. The maximum acceptable urea concentration in effluent is 10 mg/L (Hernlem, 2005). Many techniques have been applied for urea removal, such as chemical and electrochemical oxidation, adsorption, hydrolysis of urea, decomposition by a strong oxidant, biological treatment, and catalytic decomposition (El Gheriany et al., 2022; Urbańczyk et al., 2016; Zaher & Shehata, 2021). According to previous studies, the electrochemical approach holds promise as it can oxidize urea even in mild working circumstances (Cataldo Hernández et al., 2014; Singla et al., 2017, 2018, 2019; Urbańczyk et al., 2016; Zhang et al., 2018). Furthermore, harmless gases (N2 and CO2) are the primary products of decomposition. Renewable energy sources, such as photovoltaic panels, can be used to power the process, making it environmentally friendly because electrons are used to oxidize the urea (Cho et al., 2014; Millán et al., 2021; Perez-Rodriguez et al., 2018). Nicolau et al. in 2014, recovered urea and converted it to power using a urea bioreactor (GAC-urease) (Nicolau et al., 2014).
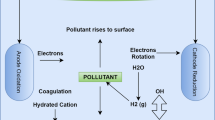
Electrocoagulation (EC) is an efficient method for treating different types of pollutants by forming active coagulants that destabilize the suspended and dissolved pollutants (Gafoor et al., 2020; Moussa et al., 2017). EC merges the advantages of coagulation, floatation, and electrochemistry, so it is a promising technology in wastewater treatment (Bajpai et al., 2022). EC was used for the removal of different types of pollutants, such as organic and inorganic pollutants, heavy metals, suspended solids, and emerging contaminants (Dolatabadi et al., 2021; Jing et al., 2021). Compared to chemical coagulation, electrocoagulation relies on the in situ generation of coagulant species by passing an electric current through a sacrificial anode (Hakizimana et al., 2017; Moussa et al., 2017). In addition, the sludge produced from the electrocoagulation process is much less than that produced from chemical coagulation (Moussa et al., 2017). However, there are some disadvantages to using electrocoagulation, such as electrode consumption and energy utilization (Shamaei et al., 2018). The main processes that occurred during the electrocoagulation process are as follows: (i) oxidation at the anode; (ii) formation of gas bubbles at the cathode; (iii) precipitation and floatation of the formed metal hydroxides (Hakizimana et al., 2017; Mollah et al., 2004). During applying electric current through the electrodes, oxidation and reduction reactions occur at the anode and the cathode, respectively (Hakizimana et al., 2017; Mollah et al., 2004). The destabilization of suspended solids depends on the existence of metal hydroxides formed by the oxidation reactions, which produce the cations. During electrocoagulation, the following reactions take place at the electrodes:
-
1.
Fe → Fe+2 + 2e− for coagulation (at the anode)
-
2.
2H2O + 2e−→ H2 + 2OH− for flotation (at the cathode)
Likewise, the electro-oxidation reactions of urea occur as follows:
-
3.
CO(NH2)2 (aq) + 6OH−(aq) → N2 (g) + 5H2O (1) + CO2 (g) + 6e− (at the anode)
-
4.
6H2O (l) + 6e−→ 3H2 (g) + 6OH−(aq) (at the cathode)
-
5.
CO(NH2)2 (aq) + H2O (l) → N2 (g) + 3H2 (g) + CO2 (g) (overall)
The elimination of urea has been investigated by the implementation of an electrocoagulation technique employing various electrode materials (Mamdouh et al., 2021; Safwat et al., 2020; Safwat & Matta, 2020). In the year 2020, Safwat and Matta successfully accomplished a 66% elimination of urea from synthetic wastewater using zinc as an anode (Safwat & Matta, 2020). In a study conducted by Safwat et al. (2020), it was shown that the utilization of titanium as an anode resulted in a urea removal efficiency of 59%. Additionally, when aluminum was employed as the anode, the urea removal efficiency was observed to be 40% (Safwat et al., 2020). Copper and iron as anode have been shown to remove 40% and 51% of urea, respectively, from synthetic wastewater (Mamdouh et al., 2021). Based on the results obtained from electrocoagulation investigations conducted on synthetic and domestic wastewater, it is advisable to enhance the process or integrate it with complementary treatment techniques to enhance the efficiency of urea removal.
Many researchers integrated EC with other technologies in wastewater treatment, and some of them demonstrated potential cost, energy savings, and enhanced the removal efficiency such as biological treatment, chemical coagulation, adsorption, reverse osmosis, and membrane filtration (Arambarri et al., 2019; Azerrad et al., 2019; Deveci et al., 2019; Dolatabadi et al., 2022; Hussin et al., 2019; Swain et al., 2020). The combination of electrocoagulation with chemical coagulation (EC-CC) is an emerging water treatment process that merges the merits of both electrocoagulation (EC) and chemical coagulation (CC) techniques (Shamaei et al., 2018). Reducing the separation time, better characteristics of sludge, such as its low water content, and simplifying the process of dewatering are the gained merits of combining EC with CC. The economic viability of implementing a combined electrocoagulation and chemical coagulation (EC-CC) treatment approach for brewery wastewater has been established based on an analysis of energy consumption. This is primarily attributed to the high efficiency of nutrient removal and the resulting reduction in expenses associated with sewer discharge (Swain et al., 2020). Therefore, the primary objective of this research is to examine the efficacy of employing sequential electrocoagulation (EC) and chemical coagulation (CC) techniques for the removal of urea. It is worth noting that this study represents the first attempt to explore the potential of this combination for urea removal.
2 Materials and Methods
2.1 Wastewater Samples
A synthetic wastewater sample was made by adding 1 g/L of urea (99% purity) and 0.5 g/L of sodium chloride (99.50% purity) to 1 L of distilled water. The second type of wastewater was domestic wastewater taken from the Benha wastewater treatment plant, and Table 1 expresses their characteristics.
2.2 Electrocoagulation Reactor Design
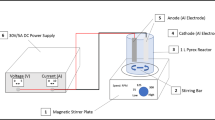
Fig. 1 represents the electrocoagulation reactor setup. The experiment consists of two electrodes submerged in a 1-L glass beaker containing a magnetic stirrer, and the electrodes are connected to a power supply of 31 V/5 A. The two electrodes are made of iron with dimensions of (4 cm × 12.5 cm). The dimensions of the immersed part of the electrodes are (4 cm × 9 cm). Iron material was used in our study as a result of its availability, reliability, and nontoxicity (Elazzouzi et al., 2019). The experiment was done in a batch reactor for 60 min. The first trial of the experiment was done at a gap distance of 3 cm, and then two further alternative spacings (2 and 4 cm) were investigated. The stirring speed was set at a modest level of 100 rpm to avoid shearing the flocs. (Attour et al., 2014). The electrodes were washed with distilled water and cleaned before every trial to remove any impurities from the surface of the electrodes. The samples were taken at 2, 5, 10, 20, 40, and 60 min and these samples were filtered to remove any sludge formed during the process. Current density variations, spacing between electrodes, electrolyte type, and electrolysis time have been examined. Using energy-dispersive X-ray spectroscopy (EDX) to analyze the anode electrode as shown in Fig. 2.
2.3 Chemical Coagulation with Different Types of Coagulants
To determine the most effective coagulant for urea elimination, a comparative analysis was conducted using three different types of iron coagulants. This analysis involved doing a jar test, which allowed for the evaluation of each coagulant’s performance and determining the optimal dosage. An investigation was conducted to examine the impact of ferrous sulfate, ferric sulfate, and ferric chloride at concentrations ranging from 0.1 to 0.5 g/L on the elimination of urea. The mixing procedure consisted of three stages: an initial period of quick mixing at 125 rpm for 30 s, followed by a subsequent stage of mixing at 75 rpm for a duration of 2 min, and finally, a final stage of mixing at 25 rpm for a duration of 5 min. After the mixing process, the reactors were left undisturbed for a duration of 30 min to facilitate the settling of the flocs (Swain et al., 2020).
2.4 Electrocoagulation and Post-Chemical Coagulation (EC-CC)
The experimental setup involved the initial implementation of the EC process, followed by the application of chemical coagulation. The EC involves the examination of four significant factors, including current density, electrode spacing, electrolyte type, and electrolysis time, with the objective of identifying the optimal conditions for each parameter variation. The EC was repeated until the following durations were reached: 2 min, 5 min, 10 min, 20 min, 40 min, and 60 min, to determine the optimal time for urea removal. Following each electrocoagulation process, a subsequent step involves the application of conventional chemical coagulation. This is achieved through the addition of an optimal dosage of coagulant, as determined through the jar test. The optimum dose of coagulant was determined to be 0.5 g/L of every type of coagulant, as shown in Fig. 3.
2.5 Chemical Coagulation Followed by Electrocoagulation (CC-EC)
The current configuration involved the first implementation of the CC procedure, followed by the subsequent execution of the EC procedure. Both procedures adhered to the identical sequence of stages as delineated in the preceding section.
2.6 Analysis
Each experiment was performed twice, and the analyses were conducted with three replicates. The results are expressed as mean values. The determination of urea was done using HPLC. COD was determined using the closed reflux titrimetric method. Using a multi-meter to determine pH, TDS, conductivity, and temperature. Based on the formula: \(\textrm{removal}\ \textrm{efficiency}\ \left(\%\right)=\frac{C_0-{C}_e}{C_0}\) ×100, the percentage of urea and COD removal was calculated, where C0 is the influent concentration of urea or COD and Ce is the effluent concentration of urea or COD. A Fourier-transform infrared (FTIR) spectrometer (Bruker VERTEX 80 (Germany) combined Platinum Diamond ATR, comprising a diamond disk as that of an internal reflector in the range 4000–400 cm−1 with resolution 4 cm−1, refractive index 2.4) was used to analyze the sludge formed during the process and scanning electron microscopy (SEM) (JEOL JSM 6510 lv) to inspect the morphology of the electrode.
3 Results and Discussion
3.1 Effect of Current Density on Urea Removal
The impact of altering current density on the elimination of urea from synthetic wastewater is presented in Fig. 4. For all different current densities, there was a significant increase in urea removal during the initial phase of the treatment period, followed by a modest rise during the latter phase of the process. During the initial phase of the process, the observed augmentation in the percentage of urea removal can be attributed to the dissolution of the anode and the subsequent formation of gas bubbles because of the applied current on the anode (Hakizimana et al., 2017; Mollah et al., 2004). The desorption phenomena in the final stage of the process significantly influence the level at which urea is eliminated. Additionally, it is possible that the presence of a passivation layer on the anode surface also impacts the rate of urea removal (Hakizimana et al., 2017; Mollah et al., 2004). In our investigation, it was observed that a rise in current density resulted in an increase in the presence of metal hydroxides, which can absorb urea, leading to a corresponding increase in urea elimination (Hakizimana et al., 2017; Shahedi et al., 2020). The maximum urea elimination rate was observed to be 49.90% at a current density of 30 A/m2 over a duration of 60 min.
3.2 Effect of Inter-Electrodes Spacing
The electrocoagulation process is significantly impacted by the gap distance between the electrodes, which also has an impact on the electrocoagulation cell’s ohmic potential and energy depletion (Gafoor et al., 2020; Safwat et al., 2019). The impact of altering the spacing between the two electrodes on urea elimination at a current density of 30 A/m2 is depicted in Fig. 5. The maximum elimination of urea was achieved at 3 cm. The configuration of the system may exert the most significant influence on initiating this phenomenon. The utilization of a circular reactor results in the 3 cm distance between the electrodes being equivalent to the separation between the electrodes and the beaker walls. This relationship is directly linked to the cross-section of the reactor. The uniformity of spacing at the reactor implies that (i) the flocs distribution is homogeneous, (ii) minimizing the flocs disruption that may occur in the case of 2 or 4 cm as a gap distance (Mamdouh et al., 2021; Safwat et al., 2020). The urea removal efficiency experiences a decline at the lowest gap distance, primarily due to the degradation of flocs generated for urea removal by settling. This degradation is attributed to the collision resulting from the heightened electrostatic attraction (Naje et al., 2017; Nandi & Patel, 2017; Safwat & Matta, 2020). Furthermore, the electrical resistance between the electrodes exhibits a positive correlation with the distance between the electrodes (Gafoor et al., 2020). The optimal urea removal efficiency is 56% when the distance between the electrodes is 3 cm.
3.3 Effect of Electrolyte Type
The assessment of EC efficiency and overall energy consumption is significantly influenced by the conductivity of the solution. The augmentation in conductivity yields a corresponding enhancement in pollution removal efficacy while concurrently leading to a reduction in electricity usage (Nandi & Patel, 2017; Tahreen et al., 2020). The type and concentration of the electrolyte have a major impact on the conductivity of an aqueous solution (Mahmoud et al., 2013; Moussa et al., 2017). The elimination efficiency of urea is significantly influenced by the electrolyte (Mamdouh et al., 2021; Safwat et al., 2020). The investigation focused on two electrolyte types, namely NaCl and Na2SO4, primarily chosen for their widespread availability and minimal toxicity (Acharya et al., 2022). Fig. 6 shows the effect of the electrolyte type on the removal of urea using electrocoagulation. Both forms of electrolytes are present at a concentration of 0.5 g/L in the electrocoagulation process’s aqueous solution. The elimination of urea exhibits a positive correlation with time for both NaCl and Na2SO4; however, NaCl demonstrates the highest level of accomplished urea removal. The observed discrepancy in results can be attributed to the greater conductivity of NaCl compared to Na2SO4, as well as the presence of chloride ions. These factors contribute to a reduction in the required voltage for achieving a certain current density due to the mitigation of the IR drop (El Gheriany et al., 2022). The experimental results indicate that the elimination efficiencies of urea were determined to be 56.05% and 40.20% for NaCl and Na2SO4, respectively.
3.4 Electrolysis Time Effect
The electrocoagulation treatment time plays a major role in affecting the removal efficiency of pollutants (Bajpai et al., 2022). The removal efficiency has a positive correlation with time until it reaches the optimal electrolysis duration. However, subsequent to this point, the removal efficiency remains constant due to the abundance of flocs (Bajpai et al., 2022; Jing et al., 2021). The experiment involved studying the electrocoagulation process for a duration of 120 min, utilizing a current density of 30 A/m2, and maintaining a 3-cm internal distance between the electrodes. Fig. 7 illustrates the influence of electrolysis time on the elimination of urea from synthetic wastewater. The maximum elimination efficiency of urea observed was 60.50% over a duration of 120 min. After a duration of 60 min, the electrocoagulation process exhibited a marginal increase in the removal efficiency of urea, rising from 56 to 60.50%. This agrees with prior research, indicating that the rate of urea oxidation decreases as the electrolysis time increases. This phenomenon can be related to the sluggish adsorption of urea on the surface of the electrodes, which subsequently reduces the rate of the anodic reaction and leads to increased polarization (El Gheriany et al., 2022). The duration of one hour for treatment may be deemed significant due to the marginal improvement in urea elimination observed with an additional hour.
3.5 Characterization of the Formed By-products Obtained from EC using FTIR
To specify the characteristics of EC sludge, Fourier transform infrared spectral analysis was performed. Fig. 8 shows the comparison of the FTIR of the sludge formed in the absence and presence of urea through an EC experiment conducted at 30 A/m2 for 60 min. The sludge was subjected to filtration using filter paper and subsequently air-dried for a duration of 24 h at ambient room temperature, then investigated by FTIR. It is shown that there are significant differences between the FTIR of the two samples because of the presence of urea in the second sample. For the second sample sludge, some bands emphasize the presence of urea compound groups. The presence of the OH group is indicated by the significant range of frequencies seen between 2800 and 3900 cm−1, as well as between 1400 and 1800 cm−1. The presence of the OH group confirms the incidence of adsorption, which is one of the removal mechanisms of urea (Mamdouh et al., 2021; Safwat et al., 2020). The frequencies of the area between 3300 and 3400 cm−1 belong to the N-H stretching. The C=O in urea is shown at a band of 1626 cm−1 and the C−N stretching is shown at 1465 cm−1.
3.6 Investigation of the Morphology of Electrode
Fig. 9 shows the SEM images for the iron electrode before and after the electrocoagulation process which was conducted at 30 A/m2. The electrocoagulation process causes corrosion, which ensures the occurrence of the treatment. The active side of the anode electrode creates oxygen at the surface, which consumes the electrode materials and leads to the formation of large voids (Safwat et al., 2019; Yılmaz Nayır & Kara, 2018). The electrode surface pitting causes non-uniform corrosion, which is considered worse than uniform corrosion because it cannot be predicted (Mamdouh et al., 2021).
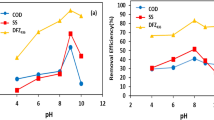
3.7 Performance of (EC-CC) and (CC-EC) with Synthetic Wastewater for Removal of Urea
The implementation of CC-EC yielded the highest overall efficiency in removing urea from synthetic wastewater, as depicted in Fig. 10. The highest recorded efficiency for urea removal was 59.40% when employing chemical coagulation with 0.5 g/L of FeCl3, followed by a 60-min electrocoagulation process at 30 A/m2. This slight enhancement can be attributed to the rise in chloride ion concentration, leading to an augmentation in chlorine current efficiency. In their study conducted in 2022, El Gheriany et al. observed a positive correlation between chlorine current efficiency and the rate of urea decomposition, which in turn influenced the rate of urea oxidation (El Gheriany et al., 2022). The application of ferrous sulfate in CC prior to conducting EC yields reduced results due to diminished conductivity levels. Moreover, the application of ferric sulfate as a coagulant prior to performing EC results in a reduced rate of urea removal compared to EC alone, potentially attributed to the lack of chloride ions. The results of the implementation of EC-CC indicated that there was a lack of significant enhancement in urea elimination for synthetic wastewater, irrespective of the specific coagulant utilized, as illustrated in Fig. 10. The insufficient utilization of coagulant dosage may be the underlying cause for the limited enhancement in the rate of urea removal.
3.8 Performance of EC-CC/CC-EC with Domestic Wastewater for Removal Urea and COD
The application of CC-EC demonstrated the most optimal efficacy in the elimination of urea and COD from domestic wastewater, as illustrated in Figs. 11 and 12, respectively. The maximum efficiency observed for the elimination of urea was 39.10%, achieved with the application of CC (0.5 g/L of FeCl3)-EC under the previously determined optimal conditions. As previously stated, the application of FeCl3 enhances the decomposition of urea because of the increased efficiency of chlorine current (El Gheriany et al., 2022). The addition of FeSO4 or Fe2(SO4)3 results in a modest improvement, potentially attributable to the augmented conductivity of the solution after their addition.
Comparison of using EC only, EC-CC, and CC-EC for removal of COD from domestic wastewater (COD=560 mg/L, time=60 min, pH=7.80). a COD removal using EC, EC-CC, and CC-EC (coagulant is FeSO4). b COD removal using EC, EC-CC, and CC-COD removal using EC, EC-CC, and CC-EC after 60-min treatment.EC (coagulant is Fe2(SO4)3). c COD removal using EC, EC-CC, and CC-EC (coagulant is FeCl3)
The highest achieved efficiency for COD elimination was 90.18% while employing a CC with a concentration of 0.5 g/L of ferric chloride prior to a 60-min electrocoagulation process. The COD elimination performance in the CC-EC studies exhibited a notable enhancement when compared to the earlier experiments with EC and EC-CC. This demonstration aligns with prior research endeavors that aimed to improve the removal of COD from brewery wastewater (Swain et al., 2020).
3.9 Cost Analysis (Consumption of Electricity, and Electrodes)
The importance of cost analysis is significant in the implementation of wastewater treatment procedures. The total expenditure associated with EC operations is mostly dependent on the utilization of electrode material and the amount of electrical energy needed for the treatment procedure (Khadir et al., 2021; Özyonar et al., 2020). The equations Eq (1) and (2) can be used to calculate the energy consumption and electrode material usage.
where V = average cell voltage (V), i = applied current (A), t = electrolysis time (h), ∀ = volume of wastewater in EC units (L), M is the molar mass of the iron electrode (55.845 g/mol), n is the number of electrons (2 for iron), and f is the Faraday constant (96,485 °C/mol).
where α is the cost of the electricity unit ($/kWh), β is the price of the iron electrode ($/kg), γ is the coagulant cost ($/kg), and Qenergy, Qelectrode, and Qcoagulant are the quantities consumed during the process (per m3 of treated wastewater). Equation (3) was utilized to determine the treatment’s operating costs.
Fig. 13 illustrates that the operational expenses associated with EC and EC-CC exhibit a notable degree of similarity. The utilization of CC-EC as a treatment method resulted in a maximum urea removal rate of 59.40% for synthetic wastewater and 39.10% for domestic wastewater. The cost associated with the CC-EC method is marginally lower compared to the EC procedure, while also exhibiting superior efficacy in terms of urea removal. These results indicate that the CC-EC method, when employing ferric chloride as a coagulant, is the most effective and economical approach for eliminating urea, particularly in terms of domestic wastewater treatment.
4 Research Limitations
One of the limitations of this work is the absence of data regarding the concentrations of iron ions in the effluent. The quantification of ion concentrations in the effluent was not conducted in this investigation, as the primary objective of the study was to assess the efficacy of the EC, EC-CC, and CC-EC methods in eliminating urea from both synthetic and domestic wastewaters.
5 Conclusions
This research conducted a comparative analysis of the efficacy of electrocoagulation, chemical coagulation, and sequential electrocoagulation and chemical coagulation methods in eliminating urea from both synthetic and domestic wastewater samples. The results of this investigation indicate that the utilization of CC-EC resulted in the most optimal efficiency for the removal of urea from both synthetic and domestic wastewater, with FeCl3 being employed as a coagulant. The maximum urea removal rate using CC-EC as a treatment procedure was 59.40% for synthetic wastewater and 39.10% for domestic wastewater. The CC-EC approach has a somewhat lower cost in comparison to the EC procedure. Based on the results, the CC-EC is the most effective and economical approach for eliminating urea, particularly in terms of domestic wastewater treatment. It is advisable to augment the coagulant dose in the CC-EC system to get enhanced effectiveness in urea removal. However, it is imperative to thoroughly examine the metal content present in the effluent. Future research may focus on the assessment of the synergistic effects achieved by integrating electrocoagulation and adsorption techniques, with the aim of optimizing the efficiency of urea removal.
Data Availability
Data will be made available on request.
References
Acharya, S., Khandegar, V., Sharma, S. K., & Kumar, A. (2022). Nitrate removal from synthetic and real groundwater by electrocoagulation: Effect of operating parameters and electrolytes. International Journal of Environmental Analytical Chemistry, 00(00), 1–19. https://doi.org/10.1080/03067319.2021.2023513
Arambarri, J., Abbassi, B., & Zytner, P. (2019). Enhanced removal of phosphorus from wastewater using sequential electrocoagulation and chemical coagulation. Water, Air, and Soil Pollution, 230(12). https://doi.org/10.1007/s11270-019-4367-7
Attour, A., Touati, M., Tlili, M., Ben Amor, M., Lapicque, F., & Leclerc, J. P. (2014). Influence of operating parameters on phosphate removal from water by electrocoagulation using aluminum electrodes. Separation and Purification Technology, 123, 124–129. https://doi.org/10.1016/j.seppur.2013.12.030
Azerrad, S. P., Isaacs, M., & Dosoretz, C. G. (2019). Integrated treatment of reverse osmosis brines coupling electrocoagulation with advanced oxidation processes. Chemical Engineering Journal, 356, 771–780. https://doi.org/10.1016/j.cej.2018.09.068
Bajpai, M., Singh, S., Abudukeremu, K., & Adarsh, K. (2022). A review on electrocoagulation process for the removal of emerging contaminants: Theory, fundamentals , and applications. Environmental Science and Pollution Research, 0123456789. https://doi.org/10.1007/s11356-021-18348-8
Cataldo Hernández, M., Russo, N., Panizza, M., Spinelli, P., & Fino, D. (2014). Electrochemical oxidation of urea in aqueous solutions using a boron-doped thin-film diamond electrode. Diamond and Related Materials, 44, 109–116. https://doi.org/10.1016/j.diamond.2014.02.006
Cho, K., Qu, Y., Kwon, D., Zhang, H., Cid, C. A., Aryanfar, A., & Hoffmann, M. R. (2014). Effects of anodic potential and chloride ion on overall reactivity in electrochemical reactors designed for solar-powered wastewater treatment. Environmental Science and Technology, 48(4), 2377–2384. https://doi.org/10.1021/es404137u
Deveci, E. Ü., Akarsu, C., Gönen, Ç., & Özay, Y. (2019). Enhancing treatability of tannery wastewater by integrated process of electrocoagulation and fungal via using RSM in an economic perspective. Process Biochemistry, 84(May), 124–133. https://doi.org/10.1016/j.procbio.2019.06.016
Dolatabadi, M., & Ahmadzadeh, S. (2020). Microplastics pollution in the aquatic environment: problems and challenges. Journal of Environmental Health and Sustainable Development, 5(2), 980–981. https://doi.org/10.18502/jehsd.v5i2.3383
Dolatabadi, M., Akbarpour, R., & Ahmadzadeh, S. (2022). Catalytic ozonation process using ZnO/Fe2 O3 nanocomposite for efficient removal of captopril from aqueous solution. Analytical Methods in Environmental Chemistry Journal, 5(3), 31–39. https://doi.org/10.24200/amecj.v5.i03.197
Dolatabadi, M., Ehrampoush, M. H., Pournamdari, M., Ebrahimi, A. A., Fallahzadeh, H., & Ahmadzadeh, S. (2023). Simultaneous electrochemical degradation of pesticides from the aqueous environment using Ti/SnO2–Sb2O3/PbO2/Bi electrode; process modeling and mechanism insight. Chemosphere, 311. https://doi.org/10.1016/j.chemosphere.2022.137001
Dolatabadi, M., Kheirieh, A., Yoosefian, M., & Ahmadzadeh, S. (2022). Hydroxyzine removal from the polluted aqueous solution using the hybrid treatment process of electrocoagulation and adsorption: Optimization, and modeling. Applied Water Science, 12(11). https://doi.org/10.1007/s13201-022-01780-7
Dolatabadi, M., Malekahmadi, R., Ghorbanian, A., & Ahmadzadeh, S. (2021). Investigation of electrocoagulation process for efficient removal of bisphenol a from the aqueous environment: Promising treatment strategy. Journal of Environmental Health and Sustainable Development, 6(2), 1275–1283. https://doi.org/10.18502/jehsd.v6i2.6539
Dolatabadi, M., Świergosz, T., Wang, C., & Ahmadzadeh, S. (2023). Accelerated degradation of groundwater-containing malathion using persulfate activated magnetic Fe3O4/graphene oxide nanocomposite for advanced water treatment. Arabian Journal of Chemistry, 16(1). https://doi.org/10.1016/j.arabjc.2022.104424
El Gheriany, I., Abdel-Aziz, M. H., El-Ashtoukhy, E. S. Z., & Sedahmed, G. H. (2022). Electrochemical removal of urea from wastewater by anodic oxidation using a new cell design: An experimental and modeling study. Process Safety and Environmental Protection, 159, 133–145. https://doi.org/10.1016/j.psep.2021.12.055
Elazzouzi, M., Haboubi, K., & Elyoubi, M. S. (2019). Enhancement of electrocoagulation-flotation process for urban wastewater treatment using Al and Fe electrodes: Techno-economic study. Materials Today: Proceedings, 13, 549–555. https://doi.org/10.1016/j.matpr.2019.04.012
Gafoor, A., Ali, N., Kumar, S., Begum, S., & Rahman, Z. (2020). Materials Today: Proceedings applicability and new trends of different electrode materials and its combinations in electro coagulation process: A brief review. Materials Today: Proceedings, xxxx. https://doi.org/10.1016/j.matpr.2020.05.379
Hakizimana, J. N., Gourich, B., Chafi, M., Stiriba, Y., Vial, C., Drogui, P., & Naja, J. (2017). Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination. Elsevier B.V. https://doi.org/10.1016/j.desal.2016.10.011
Hernlem, B. J. (2005). Electrolytic destruction of urea in dilute chloride solution using DSA electrodes in a recycled batch cell. Water Research, 39(11), 2245–2252. https://doi.org/10.1016/j.watres.2005.04.018
Hussin, F., Aroua, M. K., & Szlachtac, M. (2019). Combined solar electrocoagulation and adsorption processes for Pb(II) removal from aqueous solution. Chemical Engineering and Processing Process Intensification, 143(August), 107619. https://doi.org/10.1016/j.cep.2019.107619
Jing, G., Ren, S., Pooley, S., Sun, W., Kowalczuk, P. B., & Gao, Z. (2021). Electrocoagulation for industrial wastewater treatment: An updated review. Environmental Science: Water Research and Technology. Royal Society of Chemistry. https://doi.org/10.1039/d1ew00158b
Khadir, A., Negarestani, M., & Motamedi, M. (2021). Optimization of an electrocoagulation unit for purification of ibuprofen from drinking water: Effect of conditions and linear/non-linear isotherm study. Separation Science and Technology (Philadelphia), 56(8), 1431–1449. https://doi.org/10.1080/01496395.2020.1770795
Mahmoud, M. S., Farah, J. Y., & Farrag, T. E. (2013). Enhanced removal of Methylene Blue by electrocoagulation using iron electrodes. Egyptian Journal of Petroleum, 22(1), 211–216. https://doi.org/10.1016/j.ejpe.2012.09.013
Mamdouh, M., Safwat, S. M., Abd-Elhalim, H., & Rozaik, E. (2021). Urea removal using electrocoagulation process with copper and iron electrodes. Desalination and Water Treatment, 213, 259–268. https://doi.org/10.5004/dwt.2021.26690
Millán, M., García-Orozco, V. M., Lobato, J., Fernández-Marchante, C. M., Roa-Morales, G., Linares-Hernández, I., et al. (2021). Toward more sustainable photovoltaic solar electrochemical oxidation treatments: Influence of hydraulic and electrical distribution. Journal of Environmental Management, 285. https://doi.org/10.1016/j.jenvman.2021.112064
Mollah, M. Y. A., Morkovsky, P., Gomes, J. A. G., Kesmez, M., Parga, J., & Cocke, D. L. (2004). Fundamentals, present and future perspectives of electrocoagulation. Journal of Hazardous Materials, 114(1–3), 199–210. https://doi.org/10.1016/j.jhazmat.2004.08.009
Moussa, D. T., El-Naas, M. H., Nasser, M., & Al-Marri, M. J. (2017). A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. Journal of Environmental Management. Academic Press. https://doi.org/10.1016/j.jenvman.2016.10.032
Naje, A. S., Chelliapan, S., Zakaria, Z., Ajeel, M. A., & Alaba, P. A. (2017). A review of electrocoagulation technology for the treatment of textile wastewater. In Reviews in Chemical Engineering. Walter de Gruyter GmbH. https://doi.org/10.1515/revce-2016-0019
Nandi, B. K., & Patel, S. (2017). Effects of operational parameters on the removal of brilliant green dye from aqueous solutions by electrocoagulation. Arabian Journal of Chemistry, 10, S2961–S2968. https://doi.org/10.1016/j.arabjc.2013.11.032
Nicolau, E., Fonseca, J. J., Rodríguez-Martínez, J. A., Richardson, T. M. J., Flynn, M., Griebenow, K., & Cabrera, C. R. (2014). Evaluation of a urea bioelectrochemical system for wastewater treatment processes. ACS Sustainable Chemistry & Engineering, 2(4), 749–754. https://doi.org/10.1021/sc400342x
Özyonar, F., Gökkuş, Ö., & Sabuni, M. (2020). Removal of disperse and reactive dyes from aqueous solutions using ultrasound-assisted electrocoagulation. Chemosphere, 258. https://doi.org/10.1016/j.chemosphere.2020.127325
Perez-Rodriguez, P., Maqueira Gonzalez, C., Bennani, Y., Rietveld, L. C., Zeman, M., & Smets, A. H. M. (2018). Electrochemical oxidation of organic pollutants powered by a silicon-based solar cell. ACS Omega, 3(10), 14392–14398. https://doi.org/10.1021/acsomega.8b02502
Safwat, S. M., Hamed, A., & Rozaik, E. (2019). Electrocoagulation/electroflotation of real printing wastewater using copper electrodes: A comparative study with aluminum electrodes. Separation Science and Technology (Philadelphia), 54(1), 183–194. https://doi.org/10.1080/01496395.2018.1494744
Safwat, S. M., Mamdouh, M., Rozaik, E., & Abd-Elhalim, H. (2020). Performance evaluation of electrocoagulation process using aluminum and titanium electrodes for removal of urea. Desalination and Water Treatment, 191, 239–249. https://doi.org/10.5004/dwt.2020.25616
Safwat, S. M., & Matta, M. E. (2020). Performance evaluation of electrocoagulation process using zinc electrodes for removal of urea. Separation Science and Technology (Philadelphia), 55(14), 2500–2509. https://doi.org/10.1080/01496395.2019.1636067
Shahedi, A., Darban, A. K., Taghipour, F., & Jamshidi-Zanjani, A. (2020). A review on industrial wastewater treatment via electrocoagulation processes. Current Opinion in Electrochemistry, 22, 154–169. https://doi.org/10.1016/j.coelec.2020.05.009
Shamaei, L., Khorshidi, B., Perdicakis, B., & Sadrzadeh, M. (2018). Treatment of oil sands produced water using combined electrocoagulation and chemical coagulation techniques. Science of the Total Environment, 645, 560–572. https://doi.org/10.1016/j.scitotenv.2018.06.387
Singla, J., Sangal, V. K., & Verma, A. (2019). Evaluation and optimization of the process parameters for the photo-electrochemical treatment of urea using mixed metal oxide anodes. Process Safety and Environmental Protection, 130, 197–208. https://doi.org/10.1016/j.psep.2019.08.017
Singla, J., Verma, A., & Sangal, V. K. (2017). Performance and evaluation of electro-oxidation treatment of human urine metabolite uric acid using response surface methodology. Journal of the Electrochemical Society, 164(12), E312–E320. https://doi.org/10.1149/2.0681712jes
Singla, J., Verma, A., & Sangal, V. K. (2018). Parametric optimization for the treatment of human urine metabolite, creatinine using electro-oxidation. Journal of Electroanalytical Chemistry, 809, 136–146. https://doi.org/10.1016/j.jelechem.2017.12.061
Swain, K., Abbassi, B., & Kinsley, C. (2020). Combined electrocoagulation and chemical coagulation in treating brewery wastewater. Water (Switzerland), 12(3), 1–12. https://doi.org/10.3390/w12030726
Tahreen, A., Jami, M. S., & Ali, F. (2020). Role of electrocoagulation in wastewater treatment: A developmental review. Journal of Water Process Engineering, 37(May), 101440. https://doi.org/10.1016/j.jwpe.2020.101440
Urbańczyk, E., Sowa, M., & Simka, W. (2016). Urea removal from aqueous solutions—a review. Journal of Applied Electrochemistry. Springer Netherlands. https://doi.org/10.1007/s10800-016-0993-6
Yılmaz Nayır, T., & Kara, S. (2018). Container washing wastewater treatment by combined electrocoagulation–electrooxidation. Separation Science and Technology (Philadelphia), 53(10), 1592–1603. https://doi.org/10.1080/01496395.2017.1411365
Zaher, A., & Shehata, N. (2021). Recent advances and challenges in management of urea wastewater: A mini review. IOP Conference Series: Materials Science and Engineering, 1046(1), 012021. https://doi.org/10.1088/1757-899x/1046/1/012021
Zhang, C., He, D., Ma, J., & Waite, T. D. (2018). Active chlorine mediated ammonia oxidation revisited: Reaction mechanism, kinetic modelling and implications. Water Research, 145, 220–230. https://doi.org/10.1016/j.watres.2018.08.025
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaban, A., Basiouny, M.E. & AboSiada, O.A. Evaluation of Using Sequential Electrocoagulation and Chemical Coagulation for Urea Removal from Synthetic and Domestic Wastewater. Water Air Soil Pollut 234, 723 (2023). https://doi.org/10.1007/s11270-023-06743-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06743-5