Abstract
Among pollutants, petroleum hydrocarbons are recognized as the priority pollutants of the environment. Petroleum hydrocarbons can cause changes in the physical and chemical properties of soils, leading to a decrease in the functional activity of the microbiota of soil biocenoses. The aim of the study was to develop ways and methods of oil-contaminated soil remediation with the new generation sorbents based on organomineral biofertilizer “Kazuglegumus” and aluminum alloys activated by indium, gallium, and tin. The structure and composition of the organomineral reagents, as well as soils with different degrees of contamination were proved by Fourier transform infrared spectroscopy, scanning electron microscopy, energy dispersive X-ray spectroscopy, differential scanning calorimetry, and thermogravimetry. As a working hypothesis, it was accepted that the aluminum alloy activated by gallium, indium, and tin forms complex compounds with humic or fulvic acids, which are low-toxic or non-toxic for plants. The efficiency of cleaning oil-contaminated soils with organomineral sorbents was evaluated. The reduction of oil concentration in soil samples by 12–22% depending on the concentration of reagents and oil content in soil was revealed. The character of oil pollution impact on plants of legume families alfalfa, melilot, and sainfoin, which are characterized by the ability to accumulate and then give nitrogen to the soil, was studied. The phytotoxicity of uncontaminated and non-oil-contaminated soils was studied in laboratory and field conditions. Significantly accelerated plant growth was observed in samples of oil-contaminated soil after treatment with sorbent based on activated aluminum alloy Rau-85 and fertilizer “Kazuglegumus”. The plants had stronger root systems. These experiments are explained by the effect of Rau-85 alloys in favor of reducing the oil concentration to the normalized limits, as well as the transformation of natural humic substances and additionally applied fertilizer “Kazuglegumus” (potassium humates), which increased their biological activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Spills, discharges, and leaks of petroleum hydrocarbons are an environmental problem. Oil hydrocarbons can cause changes in the physical and chemical properties of soils, leading to a decrease in the functional activity of the microbiota of soil biocenoses. The most negative consequences are caused by polycyclic aromatic hydrocarbons with two or more condensed aromatic rings (PAHs) contained in oil, representing a large group of mutagenic and carcinogenic compounds. It has been revealed (Dai et al., 2022, Pryanichnikova, 2018) that natural self-purification of natural objects from oil pollution is a long process. Studies (Ambaye et al., 2022, Wei et al., 2020, Liu et al., 2021) found that the recovery of the number of microorganisms is observed in 6 months. At this time, the components of oil are used by microorganisms as foodstuffs.
Currently, various physical, chemical and biological technologies are used to restore soils contaminated with oil (Zhang et al., 2017).
It is noted in Robles-Mora et al., (2021) that an effective method of soil remediation is washing away oil hydrocarbons adsorbed by soil particles with extracting agents, organic surfactants or microbial biosurfactants, which have advantages over synthetic ones due to their better decomposition and the possibility of recycling. It was also found that the addition of surfactants to contaminated soils enhances the desorption and solubilization of organic pollutants and, accelerates the bioremediation process (Robles-Mora et al., 2021). It is noted that usually, the choice of surfactants for application in the field is determined by the cost, efficiency and toxicity (Befkadu et al., 2018; Zheng et al., 2022; Chowdhury et al., 2021).
Non-toxic surfactants derived from natural materials promote both an increase in biomass on washed soils and further natural soil regeneration (Kulikova et al., 2021; Piccolo et al., 2021).
Humic acids (HAs) or fulvic acids (FAs) are recognized as possible means of biological treatment of soil in a natural way. They are supramolecular structures of natural origin, ubiquitous in waters, soils, and sediments, which are responsible for many biotic and abiotic processes in the environment (Lipczynska-Kochany et al., 2018). It is noted that humic substances are a good source of energy and minerals for soil microorganisms.
A number of researchers point out that the surface-active activity of humic substances contributes to the reduction of organic pollutants in soils and can become a relatively inexpensive alternative to soil remediation (Stancampiano et al., 2023, Ossai et al., 2020). Humic acids have another remarkable property: they serve as ligands in complex metal compounds (Nardi et al., 2021). The high content of complexing groups (carboxyl and phenolic) in humic acids allows researchers to claim the participation of humic compounds, especially in soils, in binding reactions of heavy metals and organic toxicants into strong complexes. In the bound form, pollutants have less migration ability, bioavailability and toxicity, on the basis of which humic acids are considered as natural detoxicants.
According to the authors Perminova, (2000), Shah et al., (2018), the compounds formed can be represented by monodentant and polydentant complexes; metal cations can be in the inner and outer sphere. In Wang et al., (2023), the possibility of formation of complex aluminous-iron-humine compounds of complex nature was described.
The authors in a number of works (Shen et al., 2022; Klavins et al., 2020; Yang et al., 2019; Grechishcheva et al., 2019; Kumar, et al., 2021; Mayans et al., 2019) note that HAs in comparison with synthetic surfactants in the treatment of soils contaminated with hydrocarbons have a higher efficiency. In general, based on the analysis of the literature, it is concluded that the use of surfactants for washing the soil contaminated with crude oil is promising, but in practice not fully mastered.
This paper presents the results on research of possibility of oil-contaminated soils remediation by new generation sorbents based on organomineral biofertilizer “Kazuglegumus” and aluminum alloys activated by indium, gallium, and tin. New methods of soil treatment will give promising description of humic substances application for washing of soils polluted with crude oil.
2 Materials and Methods
2.1 Reagents
The organomineral (humic) fertilizer “Kazuglegumus” is presented by “Kaztechnougol” Research and Production Association LLP. Casuglegumus is a natural high-quality biofertilizer with high content of humic acids of prolonging effect with stable consumer properties (Ermagambet et al., 2016). Technical and consumer characteristics of the Kazuglegumus humic fertilizer are presented in the electronic source of the Institute of Chemistry of Coal and Technology (Yermagambet et al., 2021). The biofertilizer “Kazuglegumus” consists of the following elements: C — 33 (wt%), N — 0.82 (wt%), O — 14 (wt%), Na — 0.18 (wt%), Si — 26.12 (wt%), K — 25.88 (wt%). Mass fraction of free humic acids is 87.12%, hydrogen pH is 11.6.
Biofertilizer was characterized by FT-IR absorption spectra taken on Brucker ALPHA spectrometer, thermogravimetric analysis was performed in an air atmosphere when heated from 0 to 1000°C at a heating rate of 10°C/min on a TGA/DSC1 STAR device (MettlerToledo).
Activated aluminum alloy Rau-85 containing metals-activators: gallium, indium, tin (5 wt% each) was produced by melting method (Sarmurzina et al., 2015; He et al., 2016). Powder with given particle sizes 80–1250 μkm was produced from ingots by changing the gap between crusher jaws (mm): 1, 2, 5 and 10.
2.2 Soil Sampling
Point soil samples were taken in accordance with state standard (STST) 17.4.4.02-2017. To control soil contamination by surface-distributing substances — oil, petroleum products, heavy metals, etc. — point samples were taken in layers from the depth of 0–5 and 5–20 cm with the weight not more than 200 g each. A combined sample was compiled by mixing the point samples taken on the same sampling site.
2.3 Sample Preparation for Analysis
For analysis, a combined sample was made by mixing at least five-point samples taken from the same sample site. Mass of the combined sample is not less than 1 kg and the sample is dried at room temperature to air-dry. The sample is distributed evenly on a sheet of paper, tweezers remove mechanical impurities, crushed in a porcelain mortar, sifted through a sieve with a mesh diameter of 1 mm. The sample is divided into squares with sides 3–4 cm. A small amount of soil is taken from each square to the full depth of the layer with a spatula and these portions are analytical soil samples, two parallel samples are taken for analysis.
2.4 Soil Chemical Analysis
To assess soil salinity, an aqueous extract from the soil was used.
2.4.1 Preparation of Aqueous Extract from Soil
Preparation of aqueous extract from the soil was carried out on the basis of STST 26423-85. Soil samples weighing 30 g and weighed with an accuracy of no more than 0.1 g were placed in conical flasks. A cylinder of 150 cm3 of distilled water was added to the samples. The soil and water were stirred for 3 min with a stirrer, left to stand for 5 min, and then filtered.
2.4.2 Determination of Elements in Aqueous Extracts of Soil
The content of elements in aqueous extracts of a soil sample from the bottom of the 2020 evaporation pond (soil sample B) was determined on an atomic emission spectrometer with inductively coupled plasma ICP-7400. The method allows simultaneous multi-element analysis and determination of the elemental composition in a wide range of determinable concentrations.
2.4.3 Determination of Oil and Oil Products in Soil by Gravimetric Method
Oil and oil products content was determined in accordance with environmental regulatory documents 16.1.41-04 (ERD). Soil samples, dried at room temperature, were weighed in two weights of 30–100 g, depending on the estimated content of petroleum products. Soil samples were extracted with 10–15 cm3 of chloroform until a colorless extract was obtained in the last portion. The time of each extraction was 5–10 min. The extracts were filtered into a conical flask through a “red band” filter. The remaining soil in the flask where the extraction was performed was washed with 5 cm3 of chloroform. The combined chloroform extract was evaporated in a fume hood using a water bath or the chloroform was removed by distillation. The residue left in the beaker after evaporation of chloroform was dissolved with 5–10 cm3 of hexane, which was passed through a chromatographic column to get rid of polar compounds. The hexane was evaporated in a current of air at room temperature. After complete removal of the hexane, the beaker was weighed on an analytical scale, incubated for half an hour in the laboratory, and reweighed. The weighing was repeated until a constant weight was reached. The analysis was carried out at least three times and the average value was taken. The accuracy limit of the relative error of the methodology was ±δ 40%. The limiting value of the relative error of the results of the analysis at confidence probability P = 0.95 does not exceed 28% [STST R ISO 5725-1-2002].
2.4.4 Determination of Oil Content in Soil by Infrared Spectroscopy
The analysis was performed on a Brucker ALPHA Fourier transform infrared spectrometer with the use of a Platinum ATR diamond module in the form of pressed tablets. Crushed soil samples were placed on the clamping mechanism of the Platinum ATR diamond module. The analyses were performed at 22 °C (FT-IR spectra).
2.4.5 Research of Morphological and Structural Features of the Soil Surface
This was carried out on a scanning electron microscope (SEM) JEOL model JSM-6490LV in combination with energy dispersive X-ray spectroscopy (EDX) on soil samples not contaminated with oil (soil sample E), oil-contaminated soil (soil sample B) and after treatment of oil-contaminated soil with reagents (soil sample B-3).
2.4.6 Determination of Humus in Soil
Humus content was determined in accordance with STST 23740-2016. Clean, dry, numbered crucibles were calcined in a muffle furnace at (525 + 25) °C, cooled in a desiccator with calcium chloride and weighed with an error of not more than 0.001 g.
Thoroughly dried and mixed samples of oil-contaminated soil were washed from chlorides with distilled water, acidified with a few drops of sulfuric acid (1n), then by decantation were transferred to a filter. After that, air-dry soil samples were placed in pre-weighed porcelain crucibles, then in a cold desiccator, heated to 105°С and samples were dried to constant weight. Crucibles with soil samples dried at (105 + 2) °C to constant weight were placed in a cold muffle furnace and gradually brought to a temperature of 525 + 25 °C, and crucibles were calcined for 3 h. After cooling and weighing, the change in ash residue mass was evaluated and the humus content in the soil was calculated. The analysis was performed at least three times and the average value was taken.
2.5 Methodology of Soil Treatment with Reagents
The soil sample was dried at room temperature until air-dry. Then, the soil was spread on paper or tracing paper and mechanical inclusions (undecomposed roots, plant residues, stones, etc.) were removed with tweezers, crushed using a porcelain mortar and rubbed through a sieve with a diameter less than 1 mm. A soil sample weighing 900 ± 1 g was taken from the sample, air-dried to a constant weight, and divided into 3 equal parts.
The first part of an air-dry soil sample weighing 100 g was treated with 0.1 to 1.0 g of Rau-85 alloy, the mixture was stirred with an electric stirrer for 1–2 min, 40 g of water was added, stirred for 30 min and left in the air at room temperature for 24 h (distributing in a thin layer). At least three soil samples were prepared for analysis.
The second part of the sample weighing 100 g was treated with 40 g of carbon humus solution. The mixture was stirred with an electric stirrer until homogeneous and left in the air at room temperature for 24 h (distributing a thin layer).
The solution of carbon humus was prepared as follows: 0.1–0.5 g of 87% solution of carbon humus was diluted with water in an amount of 39.5–39.9 g.
At least three soil samples were prepared for analysis.
The third part of the 100 g soil sample was treated with complex organomineral reagent. For this purpose, a sample of 0.1–1.0 g of Rau-85 alloy was introduced into the soil, the mixture was stirred with an electric stirrer for 1–2 min and then 40 g of prepared solution of carbon humus was added. The mixture was stirred with an electric stirrer until homogeneous and left in the air at room temperature for 24 h (distributing a thin layer). At least three soil samples were prepared for analysis.
3 Results and Discussion
3.1 Study of Chemical and Structural Properties of Organomineral (Humic) Fertilizer “Kazuglegumus”
Humic organomineral fertilizers (humus, humic substances) are characterized by the high content of humic acids and fulvic acids (89.5–93.1%) and are a complex object of analysis. To increase the reliability of the results of the chemical analysis of humic acid samples, the correlation of the data obtained using different chemical and spectroscopic methods is very relevant (Abbt-Braun et al., 2004). Since humic substances in many natural sources are presented in solid form, solid-phase methods of investigation play an important role in their study (Ronald J Smernik, 2005).
The most commonly used solid-phase method for studying humic substances is infrared spectroscopy. Early works on the study of humic substances (Schnitzer M, 1978; Giovanela et al., 2004; Stevenson et al., 1974) using infrared transmission spectroscopy allowed us to describe sets of functional groups of humic substances, which formed the basis for the most popular models of humic molecules. The development of Fourier transforms infrared spectroscopy made it possible to significantly improve the picture of the obtained spectra and led to the widespread application of this method for the analysis, comparison, and even quantitative description of the composition of the functional groups of humic substances (Davis et al., 1999). Fig. 1 shows the IR spectra of the organomineral fertilizer “Kazuglegumus”.
In the infrared spectrum (Figure 1) observed absorption bands in the region of 3600–3300 cm−1 due to the presence of hydroxyl groups. The bands in the region of about 1700–1600 cm−1, refer to the valence vibration of C=O carboxyl groups. The bands in the region of 1556 cm−1 can be mainly related to the C=C of aromatic rings (Pizzeghello et al., 2015; Tinti et al., 2015) and aromatic carboxylic acids. The region between 1440 and 1300 cm−1 was attributed to CH2- and CH3- groups, and to the C-OH deformation of COOH as well as to the symmetrical carboxylate-ion vibration of COO−. The region between 1100 and 1000 cm−1 was attributed to the C-O functional groups of alcohols and carbohydrates.
DSC analysis of humic substances was used to determine endothermic and exothermic peaks, as well as to study structural changes in humic substances. Fig. 2 shows the results of DSC analysis on the TGA/DSC1 STAR instrument (MettlerToledo) in the temperature range of 0–500°C.
DSC analysis of the organomineral fertilizer showed several endothermic peaks up to 200 °C, which are associated with dehydration processes, thermal decarboxylation of acid groups and/or loss of peripheral polysaccharide chains (Mayans et al., 2019; De Oliveira et al., 2002; Provenzano et al., 1998, Plante et al., 2009). In the image, one can also observe small exothermic shoulders at about 339 °C (labile organic matter), as well as another shoulder at 467 °C, which is associated with the oxidation and polycondensation of the aromatic nuclei of the humic molecule, corresponding to the highly thermo-stable and intractable lignin structures (Mayans et al., 2019; De Oliveira et al., 2002; Provenzano et al., 2000; Plante et al., 2009; Pérez-Cruzado et al., 2014).
Thermogravimetric analysis (Picollo and Stevenson 1994; Giovanela et al., 2010, Esteves et al., 1999) is usually used to quantify the moisture and ash content (Campanella et al., 1990), as well as to characterize the structural changes of humic substances. The thermal decomposition curves of humic substances samples depend on the degree of humification, biological and chemical method of production, which can affect the structure of humic substances.
In the analysis of the organomineral fertilizer “Kazuglegumus”, thermogravimetry was used to obtain information about its thermal stability. Fig. 3 shows the thermogram of the loss in mass of the substance during heating, taken on a TGA/DSC1 STAR thermogravimetric analyzer (MettlerToledo) in the temperature range of 0–1000 °C in a nitrogen atmosphere.
As shown in Figure 3, the organomineral fertilizer has four stages of decomposition. The first one proceeds at 130–140°C, at which time 15% of the sample decomposes. The second and third stages involve the loss of 29% and 46% at 520–530 °C and 790–800 °C, respectively. The fourth stage is 870–880 °C, with a weight loss of 58%. Accordingly, the ash content of the sample is 42%.
3.2 Research of Physical and Chemical Properties of Soil
The research was carried out on soils from the bottom of the wastewater evaporation pond in the left-bank part of Atyrau city, collected in 2019–2021 (soil samples A, B and C, respectively). Samples were collected from the shoreline (soil sample D) and 10 m from the shoreline of the evaporation pond (soil sample E).
Acid-base properties of soils, content of salts — chloride, sulfate, carbonate, hydrocarbonate of water extract from soil samples, are presented in Table 1. Humus and oil content is presented in Table 2.
Characteristics of the aqueous extract from soil samples are shown in Table 1.
Data in Table 1 show that the рН of aqueous extracts from soil samples varies from 7.40 to 7.77, i.e., the reaction of soil is slightly alkaline. The degree of salinity changes depending on the sampling location from 1620 mg/L for soil sample B to 6525 mg/L for soil sample D from the shoreline.
Soil samples taken in 2019 compared to soil samples from 2021 are more mineralized, there is an increased content of chloride and sulfate, and as a result, higher electrical conductivity values. The highest value of electric conductivity 7.3 mS/cm is observed for the water extract from the soil (sample D) from the shoreline, which has a higher degree of salinity.
The results of the analysis for determining the content of humus and oil in the soil samples of the evaporation pond are shown in Table 2.
Humus is an important indicator that characterizes the potential fertility of the soil (Vorobyev, 2009). The physical and physicochemical properties of the soil, its water, air, and thermal regimes, the nitrogen nutrition regime, and the biological activity of the soil largely depend on its content. At the same time, with the increase of soil humus content, not only the gross nitrogen content, but also the biological activity grows in the soil.
The analysis of Table 2 allows us to conclude that the humus content of the soils from the evaporator pond bottom (soil samples A, B, C) is characterized by a higher value than that from the shoreline (sample D). The highest oil content of 26.9 g/kg is noted for soil sample D from the shoreline, the lowest for soil sample C taken from the bottom of the evaporator pond in 2021.
The results of elemental analysis of aqueous extracts of soil samples from the bottom of the evaporation pond are shown in Table 3.
Data in Table 3 indicate that the soil contains nutritional elements: cations of calcium, magnesium, iron, zinc, copper, etc., which affect processes in plant cells (photosynthesis, respiration, glycolysis). Magnesium content is 4.28 times less than calcium (3.06 mg/L calcium and 0.715 mg/L magnesium). It is noted (Pryanichnikova, 2018) that calcium and magnesium cations contribute to the formation of water-resistant aggregates, aluminum - to the acidic destruction of minerals. Iron is a part of enzymes and also participates in chlorophyll synthesis and metabolism. Calcium also activates enzymes, enhances metabolism, participates in the creation of cell membranes, maintains acid-base balance (buffering) in plant organisms, and is necessary for normal growth of aboveground parts and roots of plants. Magnesium is involved in the synthesis of carbohydrates, provides their transport to the underground part of the plant, thanks to which a well-developed root system is formed. To increase the fertility of soils of the evaporation pond, it is necessary to enrich them with calcium and magnesium by liming and gypsumisation. Analysis of aqueous extracts of samples A, B, D shows similar data on the elemental composition of soils.
For comparative characterization of soil samples not containing oil (soil sample E), soil containing 17.9 g/kg of oil (soil sample B) and soil after treatment with a complex organomineral reagent (soil sample B-3), data from FT-IR absorption spectra were used. Fourier transform infrared spectra of soil sample E, soil sample B, and soil sample B-3 are shown in Fig. 4.
For FT-IR spectra of soil contaminated with crude oil, peaks in the region of 2921 and 2852 cm−1 related to asymmetric and symmetric valence vibrations of CH2 and CH3 groups, as well as bands in the region of 1453 cm−1 and 1376 cm−1 characteristic of the C-C bond in aliphatic hydrocarbons are identified. The bands at 900 cm−1 to 700 cm−1 refer to the characteristic peaks of the aromatic structure.
In soil samples without oil content and soil after treatment with the reagent, bands in these regions are absent. The band at 1428 cm−1 observed for these samples refers to the CO32− (Khang et al., 2016) vibration, the band at 999 cm−1 to the Si-O-Si bond; the bands at 780, 688, 533, and 469 cm−1 to the Mg-O and Al-O vibrations. The absence of bands in the region of 2921 and 2852 cm−1, as well as a decrease in the intensity of vibrations in the region of bands ~1630 cm−1, indicates a decrease in oil content in soil samples after treatment.
SEM (scanning electron microscope) analysis was involved to characterize the morphological and structural features of soil sample E, soil sample B, and soil sample (B-3) (Fig. 5).
The SEM image (Fig. 5(a)) of soil sample E shows that the soil sample has a massive and agglomerated morphology with sizes ranging from 5 to 20 mm. According to the SEM image (Fig. 5(b)), the crude oil is firmly bound to the soil particles due to Van der Waals forces and hydrogen bonds. Consequently, the morphology of crude oil-contaminated soil has a “dense-packet” flake structure. As shown in Figure 5(c), soil remediation shows a significant improvement in the degree of dispersion.
The surface elemental composition of soil sample E, soil sample B, and soil sample B-3 was analyzed by energy dispersive X-ray spectroscopy (EDX) (Fig. 6).
As shown in Fig. 6(a), quantitative EDX microanalysis showed the presence of Si, Ti, Mg, Al, Ca, Fe, K, O, and C in soil not contaminated with crude oil. The results of EDX analysis showed that C, O, S, Si, Al, Mg, Ca, and K were evenly distributed in crude oil contaminated soil (Fig. 6b).
The EDX of the B-3 soil sample treated with a complex organomineral reagent confirmed the reduction of oil in the soil, with Si, Al, C, and O as the major components (Fig. 6c). The effectiveness of the soil treatment was also characterized by a change in carbon content, since the vast majority of crude oil consists of hydrocarbons. The carbon content was 12.46%, which may be the organic matter of soil not contaminated with oil (Fig. 6a). In Fig. 6(b), the percentage carbon content was higher and was 25.67%, which is explained by the presence of crude oil in the contaminated soil. The carbon content of the soil after treatment was 15.59%, indicating removal of the oil. The residual carbon content may be due to the treatment with organomineral fertilizers, the main element of which is carbon.
3.3 Evaluation of the Effectiveness of Oil-contaminated Soils Treatment with Organomineral Reagents of complex Action
The oil content in soil sample B is 17.9 g/kg, which exceeds the maximum allowable concentration of oil in the soil and refers to a very high level of pollution.
Efficiency of soil neutralization from oil by treatment with complex organomineral composition containing reagent Rau-85 was carried out on soil samples (soil sample A), (soil sample B), (soil sample C). Soil before and after treatment was investigated by analyses like IR spectroscopy, SEM and EDX analyses. The results of the above methods of soil investigation before and after treatment are shown in Figures 4, 5, 6, respectively.
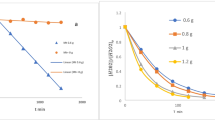
The results of research on determination of residual oil content in soil from the bottom of evaporation pond (soil samples A, B, D) after treatment with reagent Rau-85 acting as sorbent (solid products of interaction of Rau-85 with water), and with organomineral fertilizer “Kazuglegumus” and its compositions with Rau-85 depending on component content are shown in Figs. 7, 8, 9. Accuracy index of the relative error boundary of the method ±δ 40%.
Residual oil content in soil from the bottom of the evaporation pond (2019, soil sample A) before and after treatment:(a) oil-contaminated soil treated with 0.1–1 wt% Rau-85; (b) oil-contaminated soil treated with 0.1–0.5 wt% organomineral fertilizer; (c) oil-contaminated soil treated with 0.1–1 wt% Rau-85 + 0.1–0.5 wt% organomineral fertilizer
Residual oil content in soil from the bottom of the evaporation pond (2020, soil sample B) before and after treatment:(a) oil-contaminated soil treated with 0.1–1 wt% Rau-85; (b) oil-contaminated soil treated with 0.1–0.5 wt% organomineral fertilizer; (c) oil-contaminated soil treated with 0.1–1 wt% Rau-85 + 0.1–0.5% wt% organomineral fertilizer
Residual content in soil from the bottom of the evaporation pond (2021, soil sample C) before and after treatment:(a) oil-contaminated soil treated with 0.1–1 wt% Rau-85; (b) oil-contaminated soil treated with 0.1–0.5 wt% organomineral fertilizer; (c) oil-contaminated soil treated with 0.1–1 wt% Rau-85 + 0.1–0.5 wt% organomineral fertilizer
Analysis of the results suggests that the content of the organic component in soil sample A after treatment decreases with an increase in Rau-85 consumption from 0.1 to 1 wt% from 18.6 to 14.5 mg/g and is 22%. The results are repeated for soil samples B and C, the organic component in soil sample B decreases from 17.9 to 13.85 mg/g and is 22.6% and in soil sample C from 15.4 to 11.8 mg/g and is 22.8%.
When treating oil-contaminated wet soil with the reagent Rau -85 in the soil was observed, a significant increase in temperature, due to the reaction of activated aluminum with soil moisture, the appearance of hydrocarbon gases in the gas mixture with hydrogen, indicating the course of complex processes of destruction of heavy oil hydrocarbons and the formation of lighter (Baigaziyev et al., 2018; Baigaziyev et al., 2018), which lead to a decrease in oil content in the soil.
The dependence of the content of the organic component of the consumption of carbon humus is less pronounced, and with increasing consumption of the reagent in a sample of soil B decreases from 17.9 to 16.3 g/kg, which is 9.16%.
When soil sample A was treated together with reagent Rau-85 (1 wt.%) and 0.5% solution of carbon humus at a ratio of 1:2, the oil content decreased from 18.6 to 16.3 g/kg and was 12.1%, in sample B the oil content decreased from 17.9 to 15.2 g/kg and was 12.18%, and in sample D from 15.4 to 13.4 g/kg and was 12.9%.
Due to the high content of oxygen-containing functional groups such as carboxyl, hydroxyl, and carbonyl (Elkins et al., 2002) in humic acid, humic acid interacts with aluminum ions. One of the methods for studying complex compounds formed as a result of the interaction of humic substances with aluminum is Fourier transform infrared spectroscopy (FT-IR). IR spectrum of the reaction product of humic organomineral fertilizer “Kazuglegumus” and reagent Rau-85 is shown in Figure 10, organomineral fertilizer “Kazuglegumus” (Fig. 1)
Comparative analysis of IR spectra showed the presence in the complex compound of absorption band in the region of 1577 cm−1, which can be attributed to the absorption of COO- (carboxylate ion). Particular attention should be paid to the appearance of a new absorption band in the region of 2116 cm−1 (Fig. 10), which is absent in the IR spectrum of organomineral fertilizer (Fig. 1). As the studies (Elkins et al., 2002) show, the peak in the region from 2100 to 2400 is typical for the complex compound of aluminum with humic substances.
Peaks in the region of 1000–1100 cm−1 refer to deformation OH vibrations and C-O stretching of phenolic and alcoholic OH groups.
On the basis of spectral analysis, it was assumed as a working hypothesis that aluminum alloy activated, gallium, indium, tin, forms with humic or fulvic acids in Kazuglegumus fertilizer, complex compounds of low or non-toxic for plants.
3.4 Toxicity Study of Oil-Contaminated Soils to Herbaceous Plants
The nature of the impact of oil pollution on plants was studied in order to identify species suitable for restoration of disturbed lands and subsequently formulate recommendations for their use in phytoremediation of disturbed lands in arid zones (Shulaev et al., 2009).
Seeds of legume family plants such as alfalfa, melilot, and Hungarian sainfoin were chosen as plants. The plants are characterized by rapid germination and the ability to build up green mass in a fairly short period of time. An undoubted advantage of leguminous green manure crops is their ability to accumulate and then release a certain amount of nitrogen into the soil due to symbiosis with nodule bacteria, which accumulate nitrogen. In addition, legumes convert hard-to-reach phosphorus compounds into easily accessible ones.
Study of phytotoxicity of uncontaminated and oil-polluted soils was carried out in laboratory and field conditions with sowing of seeds of alfalfa, Hungarian sainfoin and melil. The first experiments were laid soil sample A in Petri dishes with seeding depth of seeds of herbaceous plants 0.5 cm. Then, studies were conducted on soil samples B in plastic cups 10 cm high (Fig. 11). Planting seeds in the field was performed on soil sample C (Fig. 12).
In a study for phytotoxicity, germination of legume crops is not observed in the treatment of oil-contaminated soil separately with Rau-85 alloys. This phenomenon is explained by the high concentration of aluminum in the soil. The solubility of Al in soils and the degree of its toxicity to plants depend on a number of factors such as soil pH; soil type, salt and cation concentration; buffer capacity; and organic matter content. The authors Canellas et al., (2014) indicated that 0.033 mmol Al/100 g caused toxicity in a Norfolk soil with pH 5.5 and kaolinite as the predominant clay mineral. They noted that it takes 0.83 mmol Al/100 g to cause toxicity in the Bladen soil, which is characterized by smectite clay and a pH of 4.9. Humic substances are among the most important Al3+ complexing agents, which plays an important role in maintaining aluminum bioavailability in the soils studied.
As seen in the figure, germination of legume crops is not observed in oil-polluted soil treated separately with Rau-85 alloys or fertilizer. In the oil-polluted soil treated only with organomineral fertilizer, there was a lag in growth and sharply pronounced suppression. In samples of oil-contaminated soil after treatment with sorbent on the basis of activated aluminum alloy Rau-85 and fertilizer “Kazuglegumus”, a significant accelerated growth of plants was observed. Plants had stronger root system. These experiments are explained by the effect of Rau-85 alloys in favor of reducing the concentration of oil to normalized limits, as well as the conversion of natural humic substances and additionally introduced fertilizer “Kazuglegumus” (potassium humates), which increased their biological activity.
4 Conclusions
Organomineral reagent consisting of indium, gallium, tin-activated aluminum and humic fertilizer “Kazuglegumus” was developed and experimentally tested for the first time for cleaning of oil-contaminated soil. The organomineral reagents were characterized by FT-IR absorption spectra as well as by thermogravimetric analysis methods. The structure and composition of soils with different degrees of pollution were studied by chemical, physico-chemical methods.
The degree of cleaning of oil-contaminated soils was analyzed. The reduction of oil concentration in soil samples by 12–22% depending on the concentration of introduced complex organomineral reagents and oil content in soil was revealed.
Phytotoxicity of uncontaminated and oil-contaminated soils was studied in laboratory and field conditions with sowing of alfalfa, sainfoin and melil seeds. In the samples of oil-contaminated soil after treatment with sorbent based on activated aluminum alloy Rau-85 and fertilizer “Kazuglegumus”, a significant accelerated plant growth was observed. The plants had stronger root system. These experiments are explained by the effect of Rau-85 alloys in favor of reducing the oil concentration to the normalized limits, as well as the transformation of natural humic substances and additionally applied fertilizer “Kazuglegumus” (potassium humates), which increased their biological activity.
The results indicate that the combined use of Rau-85 alloy and organomineral fertilizer can be used as an effective means to wash the soil from crude oil components, which is an effective solution for the remediation of oil-contaminated soil.
Data Availability
All data that support the findings of this study are included in the article, as well as any supplementary files.
References
Abbt-Braun, G., Lankes, U., & Frimmel, F. H. (2004). Structural characterization of aquatic humic substances– The need for a multiple method approach. Aquatic sciences, 66(2), 151–170. https://doi.org/10.1007/s00027-004-0711-z
Ambaye, T. G., Chebbi, A., Formicola, F., Prasad, S., Gomez, F. H., Franzetti, A., & Vaccari, M. (2022). Remediation of soil polluted with petroleum hydrocarbons and its reuse for agriculture: Recent progress, challenges, and perspectives. Chemosphere, 293. https://doi.org/10.1016/j.chemosphere.2022.133572
Baigaziyev M.T. New generation of energy accumulating substances on the basis of activated aluminum R.G. Sarmurzina, G.I. Boyko, M.T. Baigaziyev, U.S. Karabalin, N.P. Lubchenko Journal of chemical technology metallurgy 2018 119-124. https://journal.uctm.edu/node/j2018-1/16_17-117_p_119-124.pdf
Baigaziyev, M. T., Sarsenbekov, N. D., Boyko, G. I., Sarmurzina, R. G., Lubchenko, N. P., Karabalin, U. S., & Akchulakov, B. U. (2018). Research of influence of activated aluminum alloys for the cores saturated with oil of Kazakhstan fields (Russian). Oil industry, 07, 86–89. https://doi.org/10.24887/0028-2448-2018-7-86-89
Befkadu, A. A., & Chen, Q. (2018). Surfactant-enhanced soil washing for removal of petroleum hydrocarbons from contaminated soils: A review. Pedosphere, 28(3), 383–410. https://doi.org/10.1016/S1002-0160(18)60027-X
Campanella, L., & Tomassetti, M. (1990). Thermogravimetric and IR analysis of different extracts of humic substances. Thermochimica Acta, 170, 67–80. https://doi.org/10.1016/0040-6031(90)80525-4
Canellas, L. P., & Olivares, F. L. (2014). Physiological responses to humic substances as plant growth promoter. Chemical and Biological Technologies in Agriculture, 1, 3. https://doi.org/10.1186/2196-5641-1-3
Chowdhury, S., Rakshit, A., Acharjee, A., & Saha, B. (2021). Biodegradability and biocompatibility: Advancements in synthetic surfactants. Journal of Molecular Liquids, 324. https://doi.org/10.1016/j.molliq.2020.115105
Dai, C., Han, Y., Ya, D., Lai, X., Fu, R., Liu, S., Leong, K. H., Ya, T., & Zhou, L. (2022). Review on the contamination and remediation of polycyclic aromatic hydrocarbons (PAHs) in coastal soil and sediments. Environmental Research, 205. https://doi.org/10.1016/j.envres.2021.112423
Davis, W. M., Erickson, C. L., Johnston, C. T., Delfino, J. J., & Porter, J. E. (1999). Quantitative Fourier transform infrared spectroscopic investigation humic substance functional group composition. Chemosphere, 38(12), 2913–2928. https://doi.org/10.1016/S0045-6535(98)00486-X
De Oliveira, S. C., Provenzano, M. R., Santiago Silva, M. R., & Senesi, N. (2002). Maturity degree of composts from municipal solid wastes evaluated by differential scanning calorimetry. Environmental technology, 23, 1099–1105. https://doi.org/10.1080/09593332308618340
Elkins, K. M., & Nelson, D. J. (2002). Spectroscopic approaches to the study of the interaction of aluminum with humic substances. Coordination chemistry reviews, 228, 205–225. https://doi.org/10.1016/S0010-8545(02)00040-1
Ermagambet, B. T. (2016). Patent of the Republic of Kazakhstan No. 32562. In B. T. Ermagambet, N. U. Nurgaliev, K. Zh. M, A. V. Kholod, L. N. Bizhanova, & L. D. Abylgazina (Eds.), Method for obtaining humic organo-mineral biofertilizers from oxidized coals IPC: C05F 11/02 (2006.01), C05F 11/06 (2006.01). App. 2016/0520.1 dated 06/17/2016. Published 12/20/2017. Bull. №25 https://gosreestr.kazpatent.kz/Trademark/Details?docNumber=144267
Esteves, V. I., & Duarte, A. C. (1999). Thermogravimetric properties of aquatic humic substances. Marine Chemistry, 63, 225–233. https://doi.org/10.1016/S0304-4203(98)00064-4
Giovanela, M., Crespo, J. S., Antunes, M., Adametti, D. S., Fernandes, A. N., Barison, A., da Silva, C. W. P., Guégan, R., & Motelica-Heino, M. (2010). Chemical and spectroscopic characterization of humic acids extracted from the bottom sediments of a Brazilian subtropical microbasin. Journal of Molecular Structure, 981(1-3), 111–119. https://doi.org/10.1016/j.molstruc.2010.07.038
Giovanela, M., Parlanti, E., Soriano-Sierra, E. J., Soldi, M. S., & Sierra, M. M. D. (2004). Elemental compositions, FT-IR spectra and thermal behavior of sedimentary fulvic and humic acids from aquatic and terrestrial environments. Geochemical journal, 38. https://doi.org/10.2343/geochemj.38.255
Grechishcheva, N. Y., Fekhretdinova, D. R., Murygina, V. P., & Gaydamaka, S. N. (2019). Evaluation of the effectiveness of humic substances use as washing agents of oil-contaminated soils. Environmental protection in the oil and gas industry, 6(291), 22–26. https://doi.org/10.33285/2411-7013-2019-6(291)-22-26
He, T., Wang, W., Chen, W., Chen, D. M., & Yang, K. (2016). (2016) Influence of In and Sn compositions on the reactivity of Al-Ga-In-Sn alloys with water. International Journal of Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2016.11.112
Khang, V. C., Korovkin, M. V., & Ananyeva, L. G. (2016). Identification of clay minerals in reservoir rocks by FTIR spectroscopy. IOP Conf. Series: Earth and environmental science, 43. https://doi.org/10.1088/1755-1315/43/1/012004
Klavins, M., & Krumins, J. (2020). Characterisation of humic acids in fen peat. International Journal of Agricultural Resources Governance and Ecology, 16(1), 74. https://doi.org/10.1504/IJARGE.2020.10029073
Kulikova, N. A., & Perminova, I. V. (2021). Interactions between humic substances and microorganisms and their implications for nature-like bioremediation technologies. Molecules, 26(9). https://doi.org/10.3390/molecules26092706
Kumar, M., Bolan, N. S., Hoang, S. A., Sawarkar, A. D., Jasemizad, T., Gao, B., Keerthanan, S., Padhye, L. P., Singh, L., Kumar, S., Vithanage, M., Li, Y., Zhang, M., Kirkham, M. B., Vinu, A., & Rinklebe, J. (2021). Remediation of soils and sediments polluted with polycyclic aromatic hydrocarbons: To immobilize, mobilize, or degrade? Journal of Hazardous Materials, 420. https://doi.org/10.1016/j.jhazmat.2021.126534
Lipczynska-Kochany, E. (2018). Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere, 202, 420–437. https://doi.org/10.1016/j.chemosphere.2018.03.104
Liu, J.-W., Wei, K.-H., Xu, S.-W., Cui, J., Ma, J., Xiao, X.-L., Xi, B.-D., & He, X.-S. (2021). Surfactant-enhanced remediation of oil-contaminated soil and groundwater: A review. Science of The Total Environment, 756. https://doi.org/10.1016/j.scitotenv.2020.144142
Mayans, B., Pérez-Esteban, J., Escolástico, C., Eymar, E., & Masaguer, A. (2019). Evaluation of commercial humic substances and other organic amendments for the immobilization of copper through 13C CPMAS NMR, FT-IR, and DSC analyses. Agronomy, 9, 762. https://doi.org/10.3390/agronomy9110762
Nardi, S., Schiavon, M., & Francioso, O. (2021). Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules, 26(8). https://doi.org/10.3390/molecules26082256
Ossai, I. C., Ahmed, A., Hassan, A., & Hamid, F. S. (2020). Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environmental Technology & Innovation, 17. https://doi.org/10.1016/j.eti.2019.100526
Pérez-Cruzado, C., Sande, B., Ormil, B., Rovira, P., Martin-Pastor, M., Barros, N., Salgado, J., & Merino, A. (2014). Organic matter properties in soils afforested with Pinus radiata. Plant Soil, 374, 381–398. https://doi.org/10.1007/s11104-013-1896-5
Perminova, I. V. (2000). Analysis, classification and prediction of the properties of humic acids: Dis. In doc. chem. Sciences: 02.00.02 / I.V. Perminov (p. 359). Moscow State University https://www.dissercat.com/content/analiz-klassifikatsiya-i-prognoz-svoistv-gumusovykh-kislot
Piccolo, A., De Martino, A., Scognamiglio, F., Ricci, R., & Spaccini, R. (2021). Efficient simultaneous removal of heavy metals and polychlorobiphenyls from a polluted industrial site by washing the soil with natural humic surfactants. Environmental Science and Pollution Research, 28. https://doi.org/10.1007/s11356-021-12484-x
Picollo, A., & Stevenson, F. J. (1994). Infrared evidence of thermal decarboxylation in potassium salts of humic substances. In N. Senesi & T. M. Miano (Eds.), Humic substances in the global environment and implications on human health (pp. 329–334). Elsevier. https://doi.org/10.2343/geochemj.38.255
Pizzeghello, D., Cocco, S., Francioso, O., Ferrari, E., Cardinali, A., Nardi, S., Agnelli, A., & Corti, G. (2015). Snow vole (Chionomys Nivalis martins) affects the redistribution of soil organic matter and hormone-like activity in the alpine ecosystem: Ecological implications. Ecology and evolution, 5, 4542–4554. https://doi.org/10.1002/ece3.1727
Plante, A. F., Fernández, J. M., & Leifeld, J. (2009). Application of thermal analysis techniques in soil science. Geoderma, 153, 1–10. https://doi.org/10.1016/j.geoderma.2009.08.016
Provenzano, M. R., Senesi, N., & Miikki, V. (1998). Characterization of composts and humic acids from pulp and paper mill biosludges by DSC in association with FT-IR spectroscopy. Journal of thermal analysis and calorimetry, 52, 1037–1046. https://doi.org/10.1023/A:1010149009749
Provenzano, M. R., Ouatmane, A., Hafidi, M., & Senesi, N. (2000). Differential scanning calorimetric analysis of composted materials from different sources. Journal of Thermal Analysis and Calorimetry, 61, 607–614. https://doi.org/10.1023/A:1010146207459
Pryanichnikova, V. V. (2018). Electrochemical method of liquidation of consequences of oil pollution of soils discand techn (p. 162). Sciences Pryanichnikova Valeria Valerievna https://www.dissercat.com/content/elektrokhimicheskii-sposob-likvidatsii-posledstvii-neftyanogo-zagryazneniya-gruntov
Robles-Mora, G., Barrera-Cortés, J., Valdez-Castro, L., Solorza-Feria, O., & García-Díaz, C. (2021). Polycyclic aromatic hydrocarbon sorption by functionalized humic acids immobilized in micro- and nano-zeolites. Sustainability, 3(18). https://doi.org/10.3390/su131810391
Smernik, R. J. (2005). A new way to use solid-state carbon-13 nuclear magnetic resonance spectroscopy to study the sorption of organic compounds to soil organic matter. Journal of environmental quality, 34(4), 1194–1204. https://doi.org/10.2134/jeq2004.0371
Sarmurzina R.G., Kurapov G.G., Karabalin U.S., Boyko G.I. (2015). Method for obtaining activated aluminum powders [Sposob polucheniya poroshkov aktivirovannogo alyuminiya] Innovative Patent of the Republic of Kazakhstan №30429 [Innovaczionny`j Patent Respubliiki Kazakhstan №30429] (in Russian) https://kzpatents.com/3-ip30429-sposob-polucheniya-poroshkov-aktivirovannogo-alyuminiya.html
Schnitzer, M. (1978). Chapter 1 Humic substances: Chemistry and reactions. Developments in soil science, 8(1978), 1–64. https://doi.org/10.1016/S0166-2481(08)70016-3
Shah, Z. H., Rehman, H. M., Akhtar, T., Alsamadany, H., Hamooh, B. T., Mujtaba, T., Daur, I., Al Zahrani, Y., Alzahrani, H. A. S., Ali, S., Yang, S. H., & Chung, G. (2018). Humic substances: Determining potential molecular regulatory processes in plants. Frontiers in plant science, 9, 263. https://doi.org/10.3389/fpls.2018.00263
Shen, X., Zhang, J., Xie, H., Liang, S., Ngo, H. H., & Guo, W. (2022). Effect of humic acid on phenanthrene removal by constructed wetlands using birnessite as a substrate. RSC Advances, 12(24), 15231–15239. https://doi.org/10.1039/d1ra06927f
Shulaev, M. V. (2009). RF Patent No. 2355488. IPC B09C1/10, B09C1/08. In M. V. Shulaev, S. G. Fattakhov, K. A. Zakharova, M. M. Shulaeva, V. S. Reznik, O. G. Sinyashin, & A. I. Konovalov (Eds.), The method of biological cleaning of soils from oil pollution filing: 2007130925/13, 13.08.2007, publ. 05/20/2009. https://patents.google.com/patent/RU2355488C1/ru
Stancampiano, L. M., Verrillo, M., Cangemi, S., et al. (2023). The molecular composition of humic substances extracted from green composts and their potential for soil remediation. Environmental Chemistry Letters. https://doi.org/10.1007/s10311-023-01619-w
Stevenson, F. J., & Goh, K. M. (1974). Infrared spectra of humic acids: Elimination of interference due to hygroscopic moisture and structural changes accompanying heating with KBR. Soil science, 117(1), 34–41. https://doi.org/10.1016/0016-7037(71)90044-5
Tinti, A., Tugnoli, V., Bonora, S., & Francioso, O. (2015). Recent applications of vibrational mid-infrared (IR) spectroscopy for studying soil components: A review. Journal of Central European Agriculture, 16, 1–22. https://doi.org/10.5513/JCEA01/16.1.1535
Vorobyev, V. B. (2009). On the optimal level of humus content of sod-podzolic light loamy soil at different doses of nitrogen fertilizer. In V. B. Vorobyev & I. Y. Grishchenko (Eds.), Modern technology of agricultural production (pp. 171–173). Materials XII International Scientific and Practical Conference https://cyberleninka.ru/article/n/vliyanie-soderzhaniya-gumusa-na-urozhaynost-zerna-ozimoy-pshenitsy-vozdelyvaemoy-na-dernovo-podzolistoy-legkosuglinistoy-pochve-pri
Wang, N., Li, W., Wang, N., Li, M., & Wang, H. (2023). Influence of humic acids on the removal of arsenic and antimony by potassium ferrate. International Journal of Environmental Research and Public Health, 20(5), 4317. https://doi.org/10.3390/ijerph20054317
Wei, W., Ran, Z., He, H., Zhou, K., Huangfu, Z., & Yu, J. (2020). Desorption process and morphological analysis of real polycyclic aromatic hydrocarbons contaminated soil by the heterogemini surfactant and its mixed systems. Chemosphere, 254, 126854. https://doi.org/10.1016/j.chemosphere.2020.126854
Yang, F., Zhang, S., Cheng, K., & Antonietti, M. (2019). A hydrothermal process to turn waste biomass into artificial fulvic and humic acids for soil remediation. Science of The Total Environment, 686, 1140–1151.
Yermagambet, B. T., Kasenov, B. K., Kazankapova, M. K., Kassenova, Z. M., Kuanyshbekov, E. E., & Nauryzbaeva, A. T. (2021). Physicochemical and electrophysical properties of carbon materials based on humic acids. Solid Fuel Chemistry, 55(1), 41–46. https://doi.org/10.3103/S036152192101002X
Zhang, S., Mao, G., Crittenden, J., Liu, X., & Du, H. (2017). Groundwater remediation from the past to the future: a bibliometric analysis. Water Research, 119, 114–125. https://doi.org/10.1016/j.watres.2017.01.029
Zheng, X.-J., Li, Q., Peng, H., Zhang, J.-X., Chen, W.-J., Zhou, B.-C., & Chen, M. (2022). Remediation of heavy metal-contaminated soils with soil washing: a review. Sustainability, 14(20). https://doi.org/10.3390/su142013058
Funding
This work was financially supported by a grant funding for scientific research from the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan for 2021-2023 №AP09260008
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akhanova , T.R., Lyubchenko, N.P., Sarmurzina, R.G. et al. Complex Restoration of Oil-Contaminated Soils with New Organomineral Reagents. Water Air Soil Pollut 234, 686 (2023). https://doi.org/10.1007/s11270-023-06689-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06689-8