Abstract
Many catchments in Nepal are affected by intensive agricultural activities, leading to extensive pesticide usages. This study aimed to assess pesticide abundance in concurrently collected water, sediment and fish samples for the first time in intensively cultivated catchment (Indra Sarowar) located in the mid-hill region of Nepal during the rice and vegetables growing season. A total of 75 pesticides were analysed, of which 4 pesticides (alachlor, diuron, metalaxyl and pyrimethanil) were present in water with detection frequency (DF) > 40%, with alachlor (0.62 – 2.68 µg L−1) being ubiquitous. In the sediment of tributaries, the pesticides p,p′-DDT, β-HCH, alachlor and diuron were detected with DF exceeding 40%, where β-HCH was commonly observed (DF = 92%) with concentration ranging from 6.29 – 99.22 µg kg−1. The ecotoxicological risk indicated that herbicides (alachlor and diuron) posed a high risk to aquatic organisms in both tributaries and reservoir water. Such risk in sediment was even more pronounced, with alachlor and diuron showing up to 2.3 and 53.7 times higher risk respectively compared to water samples. However, none of these herbicides were detected in fish muscles. Among the fish species studied, pyrimethanil was the only quantified pesticide in edible tissue of both cage cultured (0.35 – 1.80 µg g−1 ww) and open stock fishes (0.06 – 1.12 µg g−1 ww). The consumer risk assessment showed very low human health risk associated with fish consumption (HQ < 0.2). Nonetheless, long-term consumption of contaminated fish may pose some risk that cannot be ignored. Overall, this study generated the benchmark data highlighting pervasive presence of banned (DDT, endosulfan, HCH) and unapproved (alachlor, diuron, pyrimethanil) pesticides in the environmental compartments in the mid-hill’s streams of Nepal.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The inputs of non-point source pesticides to streams and rivers, which continue to rise in developing countries such as Nepal, are regulated by land use activities and hydrology. Vegetable cultivation, both seasonal and off-season, has emerged as a crucial source of income and effective means of poverty reduction in Nepal (GoN, 2011). However, it often involves intensive pesticides usage to mitigate losses caused by pests and diseases. While the average pesticides use is low in Nepal (0.4 kg pesticides ha−1), it can escalate significantly upto 2.9 kg pesticides ha−1 crop−1 season−1 in specific vegetable production pocket areas (Bhandari et al., 2018). Notably, approximately 89% of pesticides used in Nepal are dedicated to vegetable production (GoN, 2018). The real concern lies in the extensive use of pesticides including banned (highly persistent) and counterfeit ones, across the country due to weak regulation of pesticide use and their residue monitoring in soil and water (Pokhrel et al., 2018; Yadav et al., 2016). The assessment and monitoring of pesticide residues in environmental compartments (water, sediment and biota) have long been recognised as essential to address ecological threats and human health concerns in developing countries (FAO/UNEP, 1976). Yet, in Nepal, the evaluation of pesticide impact, whether individually or cocktails, remain insufficient to ensure their use does not harm aquatic life and the environment at the catchment scale. Therefore, a comprehensive understanding of the consequences of pesticide usage is imperative to safeguard the aquatic ecosystem as well as achieving United Nation’s Sustainable Development Goals.
Pesticides presence in water and sediment, even at sublethal concentration, pose extreme toxicity to aquatic organisms, including micro/macro organisms, crustaceans and fish, impacting different stages of their life cycle and leading to a decline taxa in the streams, rivers and lakes (Beketov et al., 2013) For instance, common pesticides like cypermethrin have been shown to cause embryo death, inhibit hatching rate and induce deformities during embryonic development in fish like Rohu (Dawar et al., 2016). Similarly, studies have demonstrated the negative effects of glyphosate on rainbow trout populations, damaging vital organs such as liver, kidney and brain (Meshkini et al., 2019) as well as significantly affecting the gill microbiome (Bellec et al., 2022). These examples represent just the fraction of larger problem posed by pesticides in aquatic ecosystems. Hydrophobic pesticides such as organochlorines and pyrethroids tend to strongly adsorb to sediment, impacting sediment-dwelling organisms and acting as a secondary source of pesticides pollution in aquatic environments (Katagi, 2010). Apart from their direct effect on individual aquatic organisms, pesticides in freshwater have the potential to disrupt the entire aquatic food chain, affecting the structure of the biotic community and the functioning of aquatic ecosystem (Beketov et al., 2013). Despite the uncertainty surrounding the extent of the adverse effects on various levels of biological organisation and aquatic ecosystem functions, freshwater ecosystems in Nepal continue to face increasing pesticides pollution due to agriculture intensification and pesticides resistance beyond the planetary boundary (Varah et al., 2020).
Over the past two decades, numerous studies have primarily focused on investigating the knowledge and practices of pesticide use in Nepal (Bhandari et al., 2018 and references therein), whereas only limited number of studies quantified the presence of pesticides in water and soil (Bhandari et al., 2021; Shah & Devkota, 2009; Yadav et al., 2016), atmosphere (Pokhrel et al., 2018) and fish muscle (Dahal et al., 2012). The evaluation of ecotoxicological risk associated with pesticides in the mountainous streams of Nepal has largely overlooked, despite the fact that these hydro-systems harbour the greatest biodiversity in the Himalaya (Gurung et al., 2019) and generate a significant portion of freshwater for downstream communities.
This study aims (i) to quantify pesticide residues in water, sediment and fish continuum and (ii) to evaluate toxicological risk of pesticides for aquatic organisms and human health in an intensively vegetable cultivated catchment in the mid-hill region of Nepal. This is accomplished through assessment of pesticides concentration in water and sediment from tributaries of the Indra Sarowar reservoir during high pesticide application season. Subsequently, we analaysed pesticide residues in the Indra Sarowar reservoir water to understand the impact of environmentally relevant concentrations of pesticides on both open water and cage-cultured fishes. The livelihoods of the local communities residing around the reservoir heavily rely on cage and open stock fisheries, making them a significant source of income. Unfortuantely, the slow growth of fish and the high mortality rate within the cages have severely affected the economic situations of resource-poor farmers (Husen et al., 2018). This study represents the first and preliminary attempt to establish a link between agricultural pesticide usage and the degradation of freshwater quality in Nepal. It also provides valuable insights into the off-site impacts of pesticides under natural conditions within the evolving agricultural context.
2 Materials and Methods
2.1 Catchment Characteristic
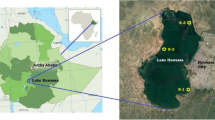
Indra Sarowar catchment (126 km2) is one of the intensively cultivated (seasonal and off-season vegetable) catchment in the mid-hill of central Nepal (Fig. 1). This is composed of rugged terrain, comprising steep hills and narrow valleys with elevation ranging from 1,430 to 2,621 m above sea level. The land use types are forest (43%), upland agriculture terraces (Bari) (34%), shrub (9%), lowland valley terraces (Khet) (7%) and others (7%). The wide and relatively flat land spreads throughout the middle part of the catchment and is densely populated by farmers (80%) who practise patchy cropping systems (Supplementary material, Fig. S1a). In Khet, rice and off-season vegetables are dominant crops during June to October while maize and off-season vegetables are dominant in Bari. The main crops growing and pesticides application period in this catchment occurs between April to October (Fig. 2). The frequency of pesticides applied in off-season vegetables was about 3 – 4 times per cropping season.
This catchment drains into the Indra Sarowar (also known as Kulekhani) hydroelectric reservoir (2.2 km2) that stores water at least 6 – 7 months each year (Fig. 2) and has been used for energy production during dry periods and fish farming. The reservoir is ~ 7 km long, 380 m wide and 105 m deep at its full capacity. This reservoir is well known for indigenous fish Katle (Neolissocheilus hexagonolepis) and production of exotic planktivorous fish namely Silver carp and Bighead carp in the cage. Both cage fish culture and open stocking of fish were initiated almost three decades ago. Fish stocking usually constitutes a 3:2 Bighead and Silver carp ratio with 10 – 12 fingerlings m−3 in the cage. Fish are grown in a cage up to 0.5 – 1 kg before they are harvested, which takes almost 12 – 18 months.
2.2 Samples Collection and Pesticide Extraction
Small streams within agricultural catchment are diverse and ecologically relevant. Therefore, sampling sites (a total of 12) were identified in the tributaries of the Indra Sarowar based on the agricultural area and stream size (Fig. 1 and Table S1). Agricultural land was situated on both sides of the stream, so the potential for pesticides transport from agricultural lands to the sampling site was very likely. Sampling was carried out in mid-September 2017 to evaluate the pesticides concentration in the streams and to assess the impact of environmentally realistic concentration of pesticides in reservoir water and fish. September represents peak month for rice and commercial vegetables growing in Palung, Tistung, Bishenkhel and Chitlang sub-catchments while reservoir water level reaches close to the maximum during this time (Fig. 2). Water and streambed sediment samples (4 replicates of each) were collected from each site in stream and reservoir (no sediment sample in reservoir). In addition, fish samples were collected only from the reservoir that includes both open stock and cage cultured fish. Open stock fish were sampled from the reservoir in the morning using multi-panel gillnets of 40 – 50 mm mesh size with the help of local fishermen. In total, six fish species popular in the region were sampled from open water, namely Rahu (Labeo rohita; n = 4), Silver carp (Hypophthalmichthys molitrix, n = 4), Naini (Cirrhinus mrigala; n = 5), Bighead carp (Hypophthalmichthys nobilis; n = 2), Katle (Acrossocheilus hexagonolepis; n = 2) and Grass carp (Ctenopharyngodon idella; n = 2). Additionally, Silver carp (n = 4) and Bighead carp (n = 4) were collected from a nylon net (mesh size ~ 5 mm) cage (~ 50 m3) mounted on a bamboo frame. Edible muscle (fillet) was separated from each fish and immediately frozen.
Each water sample was a grab water collected by filling 1 L of clean amber glass bottles from the middle of the streams and the reservoir (close to fish cage in R–C site; Fig. 1). Activated solid phase extraction cartridges (Sep-Pak C18 cartridges) were used to extract pesticides from filtered water (pore size 45 µm) samples. The filtrate was pumped through a cartridge to retain pesticides on the column within 2 h of sample collection. In case of sediment, each sample was a composite of grab 6 – 8 sub-samples of bed sediments (top 0 – 3 cm) from multiple depositional areas at the stream close to water sample collection point. Sediment was collected by using a plastic spoon (Fig. S1b). All samples were transported to Belgium in frozen condition using dry ice and subsequently stored in ultra-freezer (− 80 °C) until the pesticide analyses.
Ten grams of sediment were placed into a clean centrifuge tube and 20 mL of acetonitrile was added. Samples were shaken for 1 h on a horizontal shaker at 150 rpm, followed by centrifugation at 10,000 rpm for 5 min. The liquid phase was then separated from the solid phase and evaporated, and 6 mL of acetonitrile was added to dissolve pesticides. Then, 1 mL of acetonitrile-based extract was diluted with Milli-Q water for LC–MS/MS analysis. Remaining (5 mL) acetonitrile solution was evaporated and residue was dissolved in 5 ml hexane for GC-ECD analysis. Additionally, pesticides were desorbed from cartridges with 10 mL acetonitrile. Two separate 4 mL acetonitrile-based extract were evaporated and 4 mL acetonitrile:water (10:90) and hexane was added to dissolve pesticides for LC–MS/MS and GC-ECD analysis respectively.
In case of fish, QuEChERS method was used to extract pesticides from edible muscle parts. Ten grams of muscle sample was placed in a centrifuge tube and 15 mL of acetonitrile was added. Samples were homogenized by an Ultra-Turax mixer for 2 min. After that 1.5 g of sodium chloride (NaCl), 1.5 g of Na3 Citrate dihydrate, 0.75 g of Na2H citrate and 6 g of magnesium sulphate (MgSO4) were added to remove all excess water. The samples were shaken with hands for 2 min, followed by centrifugation for 5 min at 10,000 rpm. Then, 8 mL of supernatant was transferred to a PSA tube to remove lipids and other covariates (interferences). The PSA tube was shaken by hand for 1 min and centrifuged at 3000 rpm for 5 min. Again, 1 mL of supernatant was diluted with Milli-Q water for LC–MS/MS analysis. For GC-ECD analysis, 4 mL acetonitrile-based supernatant was evaporated using a rotavapor and 4 mL hexane was added to dissolve pesticides.
2.3 Pesticide Residues Analytical Procedure
Overall, 75 pesticides were monitored using a multiresidue method. Most of the pesticides (n = 60) in water, sediment and fish samples were analysed by LC–MS/MS system, consisting of a Waters ACQUITY UPLC™ equipped with a quaternary pump and membrane degasser. Approximately 10 µL of sample was injected and pesticides were separated using an Acquity UPLC BEH C18 column (130 Å, 1.7 µm, 2.1 mm × 50 mm) in triple quadrupole system with electrospray ionisation (Waters Xevo® TQD mass spectrometer detection; Waters, Zellik, Belgium), Whereas heavily chlorinated and fluorinated pesticides (n = 15) were analysed using GC-ECD, consisted of an Agilent Technologies 6890N gas chromatograph equipped with an Agilent Technologies 7683 Series autosampler injector and coupled to an electron capture detector. Separation was performed on a HP-5MS (5% phenyl methyl siloxane) capillary column (30 m × 0.25 mm, 0.25-μm film thickness). Operational parameters of both analytical devices (LC–MS/MS and GC-ECD) are given in Table S2.
For validation, recovery of each pesticide was evaluated by the spike-placebo recovery method. Blank samples (8 for water and 4 for sediment and fish) were spiked and analysed under the same conditions with the same extraction procedure as described above. The obtained recoveries for 75 pesticides in different environmental matrices are presented in Table S3.
2.4 Risk Assessment
2.4.1 Ecotoxicological Risk Assessment
Ecotoxicological risk to aquatic organisms was evaluated based on risk quotient (RQ) method (Eq. 1) (Papadakis et al., 2018).
where MEC was the measured environmental concentration (median and maximum detected concentration used to represent general and worst-case scenario respectively) of each pesticide in water samples or the converted pore-water concentration in sediment samples. PNEC was the predicted no effect value calculated from lowest acute toxicity value (EC50 or LC50) using an assessment factor (AF) of 100, as proposed by Lepper (2005) and applied in recent publications (Chen et al., 2020; Zeng et al., 2018). The PNEC values are shown in Table S4. Pore-water concentrations from sediment samples were calculated by Eq. 2 (Carazo-Rojas et al., 2018).
where Cpw was the pore-water concentration, Cs was the sediment median/maximum concentration and Kd was the adsorption partition coefficient of each pesticide.
As agricultural practices can result different types of pesticides mixture in the environment, we calculated mixture risk quotient (MRQ) based on concentration addition concept (Backhaus & Faust, 2012). MRQ was expressed by two parameters, i.e., MRQMEC/PNEC and MRQSTU. MRQMEC/PNEC (Eq. 3) was obtained by summing up the RQ of each pesticide present in the mixture and MRQSTU (Eqs. 4 and 5) was calculated by the sum of toxic units (STU) of most sensitive organism group for each trophic level and the corresponding AF of 100. Generally, MRQMEC/PNEC overestimates the ecological risks compared to MRQSTU (Backhaus & Faust, 2012; Chen et al., 2020); therefore, a later parameter was used to evaluate the risk of pesticide mixture in this study.
where n is the number of individual pesticides in mixture.
where E(L)C50i is the half effect/lethal concentrations of green algae, daphnid or fish for individual pesticide.
Both RQ/MRQSTU values > 1 indicates high risk, 0.1 to 1 indicates moderate risk and < 0.1 indicates low risk to aquatic organisms (Chen et al., 2020). The ecotoxicological data for this study were obtained from the Pesticides Properties Database from Lewis et al. (2016).
2.4.2 Human Health Risk Assessment
Potential human health risk via consumption of contaminated fish was evaluated by calculating estimated daily intake (EDI) of pesticide as given in Eq. 6.
where C is the median concentration of pesticide measured in fish (fillet) (µg g−1), CR is the average daily fish consumption rate (g person−1 day−1) and BW is the average body weight (kg). Although the Government of Nepal has recommended to have at least 30 g of fish (or animal protein) per day, consumption was far below than the recommended amount. The study done by Thapa et al. (2014) to understand the fish-eating habits of people living around lake/reservoir in Nepal revealed that consumption rate differed among different groups of people (e.g., hotel owners, fishermen and locals). As hotel owners (107.14 g day−1) and locals (71.43 g day−1) were the highest and lowest consumers of fish respectively, we calculated EDI for these two groups and average body weight was considered as 60 kg for adults (WHO, 2012).
Then, potential non-cancer and carcinogenic risks were carried out as per USEPA (1991) and Tyohemba et al. (2021). Briefly, hazard quotient (HQ) for non-cancer risk was estimated by dividing EDI to acceptable daily intake values (ADI). HQ values ≤ 0.2 indicate negligible adverse health effects, whereas HQ values exceeding this threshold require detailed risk assessment (Health Canada, 2012). For carcinogenic risk, lifetime cancer risk (LCR) was calculated by multiplying EDI with cancer slope factor. LCR below 10−6 is considered acceptable, between 10−6 and 10−4 is considered as an area of concern and greater than 10−4 is considered a high cancer risk (USEPA, 1991).
3 Results and Discussion
3.1 Prevalence of Pesticide Residues in Different Environmental Compartments
3.1.1 Pesticide Residues in Water
Pesticides chemical characteristics and catchment hydrological conditions play a significant role in the delivery of pesticides from the agricultural lands to different compartments of receiving water bodies. Among analysed pesticides, 6 were detected in water at concentrations high enough to be quantified including banned and unregistered pesticides in Nepal (Table 1). Two herbicides namely alachlor (DF = 100%) and diuron (DF = 63%) were observed in tributary water with median concentrations of 1.11 µg L−1 and 0.02 µg L−1 respectively. The alachlor concentration was higher in Palung stream (2.68 µg L−1) compared to Bishenkhel (1.12 µg L−1) (p < 0.001, Bonferroni-adjusted) and other streams remained similar (2.44 µg L−1 in Tistung and 2.05 µg L−1 in Chitlang). Despite low persistence of alachlor in water (DT50 = 1 – 2 day) and dilution in reservoir, slightly higher mean alachlor concentration observed in reservoir (1.8 ± 0.6 µg L−1; p = 0.04) compared to streams water (1.3 ± 0.6 µg L−1) suggested its recent intensive and repeated use in upstream agricultural lands, even though this herbicide (including other pesticides, Table 1) was not authorised to use and registered by the Plant Quarantine and Pesticide Management Centre, Nepal (GoN, 2018). Alachlor concentration in this catchment was higher than the concentration observed in the Tejo river in Portugal (0.1 µg L−1) (Silva et al., 2015) and Satrymonas and Netos river in Greek (0.86 µg L−1) (Papadakis et al., 2018) and further suggests that farmers apply relatively high quantity of alachlor in this catchment. Despite high spatial variability of alachlor concentration in tributaries (0.6 – 2.7 µg L−1) and reservoir (0.7 – 2.5 µg L−1) water (Table 1), its concentration in the tributaries is of great concern due to water supply vulnerability since its concentration is 13 orders of magnitude larger than the drinking water limit (0.1 µg L−1).
Most of the pesticides quantified in tributaries water were fungicides and higher detection frequency was found for metalaxyl (94%) followed by boscalid (77%), iprodione (52%) and pyrimethanil (40%) (Table 1). The presence of metalaxyl and pyrimethanil were found in all water samples from the reservoir. The observed concentration of metalaxyl (Cmax = 0.11 µg L−1) in the water samples of studied catchment was higher than the concentration reported in Strymonas river basin (Cmax = 0.06 µg L−1) and Nestos river basin (Cmax = 0.10 µg L−1) of Greek (Papadakis et al., 2018). Pyrimethanil concentration in the reservoir water was higher (0.8 ± 0.09 µg L−1, p < 0.01) compared to its tributaries (except Chitlang) which is up to 7 order of magnitude higher than the concentration reported in surface water draining vineyard in France (Gregoire et al., 2010). Additionally, boscalid and iprodione were detected in stream water (but not in reservoir) regardless of their lower solubility (Sboscalid = 4.6 mg L−1and Siprodione = 6.8 mg L−1) (Table 1). This finding was contradictory to other studies which suggested that boscalid has low potential for leaching from soil and rapidly transfer from the water phase to sediment (DT50 < 2 weeks) (USEPA, 2012). Quantification of boscalid and other pesticides in the flowing stream water during late monsoon season clearly highlighted that the point sources cannot be disregarded. During field visit, authors observed inappropriate pesticide container disposal (Fig. S1c) and directly washing pesticides spray equipment in the streams. Unfortunately, this catchment lacks a community-level pesticides container disposal programme (personal communication with farmers) which might be equally responsible for pesticide contamination in the aquatic system. Literature showed that farmers in this region lack adequate awareness and training on the pesticides handling practices (Atreya et al., 2022).
Pyrimethanil is a broad-spectrum fungicide with wide application against tomato and potato blight in this catchment (personal communication with farmers). Although pyrimethanil application rate and quantity are not available for this catchment, irregular presence of pyrimethanil in the stream but high concentration in reservoir is likely to correspond to its stability against photolysis and hydrolysis in water (Lewis et al., 2016) as well as incapability of aquatic microflora to degrade it. Accordingly, persistence of pesticides and longer water residence time in reservoir potentially exacerbate their impact on non-target organisms including fish in the reservoir (Sect. 3.1.3).
3.1.2 Pesticide Residues in Sediment
Sediment is one of the major off-site sinks of diffused pesticides from agricultural land. Seven pesticides were quantified in stream sediment samples (Table 1) of which two herbicides (alachlor and diuron) were common in water and sediment. The accumulation dynamics of pesticides in sediment is highly complex and mostly driven by hydrophobic interactions of pesticides with sediment and total suspended sediment concentration (Boithias et al., 2014). Therefore, high concentrations of cypermethrin and deltamethrin in sediment (Table 1) were not surprising due to their strong binding capacity with organic carbon in sediment (Lewis et al., 2016). Sediment organic carbon was reported high in various sites (2 ± 0.7% for T-1 and 4.6 ± 1.5% for C-2) in this catchment (Upadhayay et al., 2018). Concentrations of cypermethrin and deltamethrin reported in this study (19 ± 9 and 6 ± 4 µg kg−1 respectively) were lower than the Indus river sediment (190 and 250 µg kg−1 respectively) in Pakistan (Jabeen et al., 2015) but comparable to global average sediment concentration (Agarwal et al., 2015; Li et al., 2017). Overall, accumulation of these pesticides and their persistency in sediment pose long-term exposure threat to benthic communities, thereby degrading aquatic ecosystems.
Abundance of banned pesticides metabolites like β-HCH (DF = 92%), p,p′-DDT (DF = 63%) and endosulfan-α (DF = 21%) as well as un-registered pesticides such as alachlor (DF = 46%) and diuron (DF = 71%) in sediment samples (Table 1) are particularly very concerning. The abundance of endosulfan-α (0.001 – 1.00 µg kg−1) and β-HCH (Cmax = 14.88 µg kg−1) were much lower in the sediment compared to the Gomti River, India (Malik et al., 2009). Additionally, presence of diuron (DF = 71%) and alachlor (DF = 46%) in the sediment of fast flowing mountainous streams strongly suggests their repeated application in the agriculture. Finding of banned pesticides metabolites highlighted the presence of aged legacy contaminants and potential long-term impact on water quality and aquatic ecosystem. The binding of pesticides and their metabolites in sediment not only facilitates their reductive degradation but also increases exposure time to aquatic organisms. Lamoureux & Brownawell (1999) reported 16 – 50% of sediment associated pesticides are bioavailable depending on the compound and characteristics of the sediment.
3.1.3 Pesticide Residues in Fish from the Reservoir
Pesticide concentration in fish is highly public concern due to the popularity of fish from the reservoir (Indra Sarowar). Fish are most likely exposed to pesticide through consumption of pesticide contaminated food stuffs and/or through passive diffusion from contaminated water/sediment (Dureja & Rathore, 2012; Katagi, 2010). Among 75 analysed pesticides, alachlor, o,p′-DDT, p,p′-DDT, iprodione and pyrimethanil were detected in fish muscle mainly in Bighead carp however, pyrimethanil concentration was high enough to be quantified except in open stock Grass carp (Table 2). Pyrimethanil concentration was observed higher in Bighead carp (0.7 – 2.1 µg g−1 ww) compared to Silver carp (0.008 – 0.8 µg g−1 ww) collected from the cage. Despite similar growing conditions in the cage and their body weight, higher pyrimethanil concentration in Bighead carp than Silver carp might be associated with differences in feeding habits and pesticides bioaccumulation factor. Silver carp was reported slightly lower in tropic position with higher contribution of fine particulate organic matter to their diet than Bighead carp (Zhang et al., 2019). In a productive aquatic ecosystem, Bighead carp consumed more zooplankton than phytoplankton while the converse was true for Silver carp (Rutherford et al., 2021). The reservoir was reported highly productive with significant spatio-temporal variability of phytoplankton and zooplankton density (Adhikari et al., 2017). Notwithstanding, Dahal et al. (2012) did not observe such contrast in pesticides concentration in cage cultured Bighead and Silver carp from this reservoir during 2006 – 2007. We conjecture that pesticide contaminated detritus and sediment may settle in the cages that likely serve as an important source of dietary exposure of agricultural pesticides to cage cultured fish.
Overall, higher pyrimethanil concentration and variability in cage cultured compared to open stock fish (Table 2) can be related to their limited movement in the reservoir. We anticipated that exposure of open stock fish to pyrimethanil would have low impact, as these can exploit wider habitat for their feeding. In fact, pyrimethanil is reported as habitat disruptor as a result it can trigger the avoidance behaviour of fish (or other aquatic organisms) before toxic effects are visible (Araujo et al., 2014; Tierney, 2016). Open stock fish can utilise less contaminated zone in the reservoir where they might eliminate accumulated toxic pollutants (Araújo et al., 2015). Nevertheless, pyrimethanil present in fish clearly indicated persistence in water and sediment with the high risk of its transfer to other organisms including human through the food web (Sect. 3.2.2). Prolonged exposure of fish and other aquatic organisms to cocktail of pesticides (Table 1) is inevitable in the reservoir due to slow hydrolysis of different pesticides like pyrimethanil (DT50 = 2 – 3 years) and long water residence time (6 – 7 months) which coincides with breeding season of many fishes (April – October) in the reservoir. Such pesticides exposure is responsible for changes in biochemical/pathological reaction in fish and other aquatic organisms which are cumulative, causing lethality even at the sublethal concentration (Weisner et al., 2021).
3.2 Risk Assessment
3.2.1 Ecotoxicological Risk Assessment
The consequences of pesticides transfer from agricultural soils to waters and sediments are of great environmental concerns as they may pose risk to many non-target aquatic organisms and/or their entire ecosystem (Liu et al., 2013). Herbicides, both alachlor and diuron, individually posed high to medium risk to aquatic organisms and their risk level differs in between tributaries and reservoir (Table 3) as well as environmental compartments (Table 4). Even at median concentration, diuron was found to have high risk (RQ > 1) in reservoir water compared to alachlor. The risk level remained medium at highest concentration for alachlor both in stream and reservoir water. In contrast, diuron showed high risk at its maximum concentration both in streams and reservoir. Although several fungicides were found in both streams and reservoir water but showed negligible risk except for pyrimethanil at its maximum concentration in the streams (RQmax = 0.109) (Table 3).
Sediment associated pesticides can have long-lasting impact directly on periphyton and benthic invertebrates and indirectly on higher trophic levels and important ecosystem processes. This study showed that sediment-associated herbicides namely alachlor and diuron risk to aquatic biota increased by upto a factor of 2.3 and 53.7 respectively (Table 4) compared to water samples. Sediment-associated insecticides i.e., cypermethrin, op′-DDT, endosulfan-α and β-HCH showed moderate to high risk. Both type of pesticides can adsorb in the periphyton during each pulse of pesticides runoff in the stream and later may desorb into the water column. Therefore, ecotoxicological risk assessment of pesticides present in the sediment is very important for understanding the dynamics of pesticides exposure routes and their controlling factors (Vonk & Kraak, 2020). Unquestionably, occurrence of single pesticide in water/sediment is rather an exception than the rule. The number of combinations of pesticides in water/sediment is infinite and complex interactions and associated multiple modes of actions of pesticides mixture i.e., cocktail is often unpredictable and underestimated (Rizzati et al., 2016). Almost all sites were at high risk due to pesticides mixture in the streams and reservoir (Fig. 3).
Risk quotients corresponding to pesticide mixture in (a) water and (b) sediment samples in each sampling sites (see Fig. 1 for sites location). Blue, yellow and red filled circle and shaded area represent low (RQ < 0.1), medium (RQ = 0.1 – 1) and high (RQ > 1) risk respectively
Independent impact of pesticide to aquatic biota depends on its mode of action which can be modified in the pesticide mixture. Alachlor exert genotoxic effect that is highly harmful to aquatic organisms and humans and therefore, banned by many European countries in 2006 (Ghani et al., 2021). Diuron is a selective urea based herbicide for broadleaf weed and can modify the function (marked decrease in photosynthetic efficiency) and the structure (decrease biovolume of diatom) of biofilm community at environmentally realistic concentration (0.07 – 7 µg L) (Ricart et al., 2009). Among pesticides, cypermethrin and pyrimethanil are also highly concerning due to their harmful effect. Cypermethrin (belongs to pyrethroid synthetic insecticide) is neurotoxic and alters the biochemical, hematological parameters and exerts stress in Labeo rohita (a very common fish in the study reservoir) even at sub-lethal concentration (Das & Mukherjee, 2003). Similarly, pyrimethanil is considered as endocrine disrupting chemicals and affects the life of fish and other aquatic organisms including amphibians (Bernabò et al., 2017; Ghani et al., 2021). Müller et al. (2019) reported long-lasting/delayed (after nine months of application) direct and indirect effects of pyrimethanil on filamentous microalgae and rooted macrophytes creating imbalance in aquatic community structure. Most of these pesticides can jointly exert more harmful/toxic effect i.e., pesticides cocktail additive and synergistic effect (Relyea, 2009); therefore, catchment-wide ecological risk assessment of pesticide mixture can help to evaluate whether agricultural pest management strategies minimise contamination to a level that protects aquatic ecosystem and human health. This study demonstrated that the pesticides mixture exerts medium to high risk in most of the sites in Indra Sarowar and its tributaries (Fig. 3).
There is no information about the quantity and intensity of pesticides used in the study catchment but labor shortage due to out-migration (Jaquet et al., 2015; Ojha et al., 2017) is the primary driver of farmer dependency on herbicides for weed control in Nepal. Such systemic herbicides may be linked to the death of aquatic plants/algae, loss of habitat and decrease in dissolved oxygen thereby changing the entire aquatic food webs structure (Knight & Hauxwell, 2010). Admittedly, higher water residence time in the reservoir (7 – 8 months of a year) raises real concerns about chronic exposure of biota to the pesticide mixture. Given the potential cocktail effects between pesticides and their degradation products to aquatic biota (Daam & Rico, 2018), future research should focus on the effect of pesticides and their degradation products in species and at community level for long-term and high concentration pulse exposure context.
3.2.2 Human Health Risk Assessment
The preliminary human health risk assessment revealed that all HQ values in all fish species were ≤ 0.2 (Table 2), thus the probability of humans getting non-carcinogenic diseases with the consumption of fish from Indra Sarowar are very unlikely. However, the LCR values calculated using chronic population adjusted dose (cPAD, protective of non-cancer and cancer end points, 0.17 mg kg−1 day−1, since CSF was not available) were considerably higher than the recommended threshold, indicating chances of developing cancer. The United States Environmental Protection Agency classifies pyrimethanil as a possible human carcinogen, we strongly recommend regular monitoring of pesticide residues in fish to reduce pesticides exposure via dietary routes and to improve public health safety. Long-term dietary exposure through consumption of pesticides contaminated food (water, fish) may also result in neurological dysfunction, detrimental reproductive changes and several chronic disorders in human health (Mamane et al., 2015; Requena et al., 2018).
3.3 Implications and Limits
This study represents a first step towards understanding of the pesticides use footprint in water-sediment-biota (fish) continuum in the intensively cultivated catchment of Nepal. Pesticides use is expected to increase in agriculture as a result of labour shortage (Jaquet et al., 2015; Ojha et al., 2017), spread and emergence of pests and diseases due to climate change and implementation impacts of prime minister agriculture modernisation project in Nepal. This catchment-scale study demonstrated that current pesticides regulation mechanism is not efficiently functioning to control import, distribution and use of pesticides. Local and central governments should effectively promote integrated pest management approaches and strengthen pesticides regulatory processes to discourage sale and use of banned and unregistered pesticides in order to address further aggravating water quality and biodiversity loss due to pesticides pollution.
Various factors including timing of pesticides application and the time elapses before the next major runoff event are equally important for accumulation of pesticides in run-off water and sediments (Ccanccapa et al., 2016). It should be noted that our interpretation is based on the single time sampling in September, therefore our data might not fully capture the numbers and concentrations of pesticides in the stream water and sediment throughout the year. Flux of pesticides can vary several orders of magnitude during rainfall events (Weisner et al., 2022). Therefore, aquatic organisms exposed to variable concentration of pesticides over time could be more stressful than steady exposure conditions (Weisner et al., 2021). Therefore, we acknowledge that our sampling approach (grab sampling and disconnected with rainfall event) might incur underestimation of number and concentration of pesticides and thereby RQ especially in the streams. Additionally, pesticides abundance in the water and sediments generally derive from agriculture lands, here, actual pesticide concentrations could be confounded by the dilution of water and sediment originated from forest (forest contributed > 60% sediment in Chitlang stream, Table S1) (Upadhayay et al., 2018). Therefore, we expect field-edge small streams, irrigation ditches and ponds can be highly impacted by pesticides pollution. Seasonal information of pesticide occurrence in those hydro-systems is therefore, very crucial in optimising pesticide use in agriculture.
4 Conclusion
This initial and exploratory study presented compelling and comprehensive evidence of the widespread presence of pesticides in the water-sediment-fish continuum, along with their potential risk to aquatic biota in the intensively vegetable-cultivated catchment in Nepal. Among the detected pesticides, alachlor, boscalid, p,p′-DDT, diuron, β-HCH, iprodione, metalaxyl and pyrimethanil were found to be widely distributed in water and sediment (DF > 40%). Notably, the prevalence of banned pesticides like p,p′-DDT and endosulfan-α as well as unregistered ones like alachlor and pyrimethanil, points to inadequate legislative implementation mechanism for pesticides monitoring and control. The injudicious use of pesticides due to a lack of ecological literacy among farmers also contributed to this concerning scenario. The ecotoxicological risk assessment revealed that two herbicides, alachlor and diuron, posed significant threats to aquatic organisms, and human health risk cannot be ignored, especially with the long-term consumption of fish exposed to pesticides from the reservoir. The high concentration of illegal pesticides in water and sediment highlights the urgent need for systematic monitoring activities and risk assessment based on simultaneous pesticides presence in both water and sediment compartments throughout the country. To address this issue effectively, both federal and local governments should actively promote integrated pest management practices, ensure the safe disposal of empty pesticides containers, implement robust water protection policies and conduct biomonitoring activities.
Data Availability
The datasets used and analysed in this study are available from the corresponding author on request.
References
Adhikari, P. L., Shrestha, S., Bam, W., Xie, L., & Perschbacher, P. (2017). Evaluation of spatial-temporal variations of water quality and plankton assemblages and its relationship to water use in Kulekhani Multipurpose Reservoir, Nepal. Journal of Environmental Protection, 8(11), 1270.
Agarwal, A., Prajapati, R., Singh, O. P., Raza, S. K., & Thakur, L. K. (2015). Pesticide residue in water-a challenging task in India. Environmental Monitoring and Assessment, 187(2), 54.
Araujo, C. V. M., Shinn, C., Mendes, L. B., Delello-Schneider, D., Sanchez, A. L., & Espindola, E. L. G. (2014). Avoidance response of Danio rerio to a fungicide in a linear contamination gradient. Science of the Total Environment, 484, 36–42.
Araújo, C. V. M., Shinn, C., Müller, R., Moreira-Santos, M., Espíndola, E. L., & Ribeiro, R. (2015). The ecotoxicity of Pyrimethanil for aquatic biota. In M. L. Larramendy (Ed.), Toxicity and Hazard of Agrochemicals (pp. 127–141). InTech: Rijeka, Croatia.
Atreya, K., Kattel, K., Pandit, S., Chaudhary, P., & Sipkhan, P. (2022). Understanding farmers’ knowledge, attitudes and practices of pesticide use in Nepal: Synthesis of a systematic literature review. Archives of Agriculture and Environmental Science, 7(2), 278–287.
Backhaus, T., & Faust, M. (2012). Predictive Environmental risk assessment of chemical mixtures: A conceptual framework. Environmental Science and Technology, 46, 2564–2573.
Beketov, M. A., Kefford, B. J., Schäfer, R. B., & Liess, M. (2013). Pesticides reduce regional biodiversity of stream invertebrates. Proceedings of the National Academy of Sciences, 110, 11039–11043.
Bellec, L., Le Du-Carré, J., Almeras, F., Durand, L., Cambon-Bonavita, M. A., Danion, M., & Morin, T. (2022). Glyphosate-based herbicide exposure: Effects on gill microbiota of rainbow trout (Oncorhynchus mykiss) and the aquatic bacterial ecosystem. FEMS Microbiology Ecology, 98, 35749560.
Bernabò, I., Guardia, A., Macirella, R., Tripepi, S., & Brunelli, E. (2017). Chronic exposures to fungicide pyrimethanil: Multi-organ effects on Italian tree frog (Hyla intermedia). Science and Reports, 7(1), 6869.
Bhandari, G., Atreya, K., Yang, X., Fan, L., & Geissen, V. (2018). Factors affecting pesticide safety behaviour: The perceptions of Nepalese farmers and retailers. Science of the Total Environment, 631–632, 1560–1571.
Bhandari, G., Atreya, K., Vašíčková, J., Yang, X., & Geissen, V. (2021). Ecological risk assessment of pesticide residues in soils from vegetable production areas: A case study in S-Nepal. Science of the Total Environment, 788, 147921.
Boithias, L., Sauvage, S., Merlina, G., Jean, S., Probst, J.-L., & Sánchez Pérez, J. M. (2014). New insight into pesticide partition coefficient Kd for modelling pesticide fluvial transport: Application to an agricultural catchment in south-western France. Chemosphere, 99, 134–142.
Carazo-Rojas, E., Pérez-Rojas, G., Pérez-Villanueva, M., Chinchilla-Soto, C., Chin-Pampillo, J. S., Aguilar-Mora, P., Alpízar-Marín, M., Masís-Mora, M., Rodríguez-Rodríguez, C. E., & Vryzas, Z. (2018). Pesticide monitoring and ecotoxicological risk assessment in surface water bodies and sediments of a tropical agro-ecosystem. Environmental Pollution, 241, 800–809.
Ccanccapa, A., Masiá, A., Navarro-Ortega, A., Picó, Y., & Barceló, D. (2016). Pesticides in the Ebro River basin: Occurrence and risk assessment. Environmental Pollution, 211, 414–424.
Chen, C., Zou, W., Cui, G., Tian, J., Wang, Y., & Ma, L. (2020). Ecological risk assessment of current-use pesticides in an aquatic system of Shanghai, China. Chemosphere, 257, 127222.
Daam, M. A., & Rico, A. (2018). Freshwater shrimps as sensitive test species for the risk assessment of pesticides in the tropics. Environmental Science and Pollution Research, 25(14), 13235–13243.
Dahal, S. P., Shrestha, M. K., Wagle, S. K., Bista, J. D., Pandit, N. P., KC, K. & Prasad, S. (2012). Investigation into fish mortality in cages in Kulekhani Reservoir, Nepal, In M. K. Shrestha & J. Pant (Eds.), Small-scale Aquaculture for Rural Livelihoods: Proceedings of the National Symposium on Small-scale Aquaculture for Increasing Resilience of Rural Livelihoods in Nepal (pp. 114–117). Institute of Agriculture and Animal Science, Tribuvan University , Rampur, Chitwan, Nepal and The WorldFish Center, Penang, Malaysia.
Das, B. K., & Mukherjee, S. C. (2003). Toxicity of cypermethrin in Labeo rohita fingerlings: Biochemical, enzymatic and haematological consequences. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 134(1), 109–121.
Dawar, F. U., Zuberi, A., Azizullah, A., & Khan Khattak, M. N. (2016). Effects of cypermethrin on survival, morphological and biochemical aspects of rohu (Labeo rohita) during early development. Chemosphere, 144, 697–705.
Dureja, P., & Rathore, H. S. (2012). Pesticide residues in fish. In H. S. Rathore & L. M. L. Nollet (Eds.), Pesticides: Evaluation of environmental pollution (pp. 361–392). UK CRC Press.
FAO/UNEP. (1976). Impact monitoring of agricultural pesticides: Proceedings of the FAO/UNEP expert consultation on impact monitoring of residues from the use of agricultural pesticides in developing countries held in Rome , 29 September to 3 October 1975.
Ghani, M. U., Asghar, H. N., Nadeem, H., Shahid, M., Zeshan, M. A., Niaz, A., Hussain, S., & Hussain, S. (2021). Processes governing the environmental fates of alachlor in soil and aqueous media: A critical review. International Journal of Environmental Science and Technology, 19(8043), 8060.
GoN. (2011). A report on value chain analysis of off-season vegetables (OSV), Government of Nepal, Ministry of Agricultural Development, High Value Agriculture Project in Hill and Mountain Areas (HVAP) pp. 58.
GoN. (2018). List of registered pesticides (in Nepali), Harihar bhawan, Lalitpur, Government of Nepal, Ministry of Agricultural and Livestock Development, Department of Plant Quarantine and Pesticide Registration Centre. https://www.npponepal.gov.np/downloadsdetail/2/2018/39799637/. Accessed 08/09/2021.
Gregoire, C., Payraudeau, S., & Domange, N. (2010). Use and fate of 17 pesticides applied on a vineyard catchment. International Journal of Environmental Analytical Chemistry, 90(3–6), 406–420.
Gurung, J., Chettri, N., Sharma, E., Ning, W., Chaudhary, R. P., Badola, H. K., Wangchuk, S., Uprety, Y., Gaira, K. S., Bidha, N., Phuntsho, K., Uddin, K. & Shah, G. M. (2019). Evolution of a transboundary landscape approach in the Hindu Kush Himalaya: Key learnings from the Kangchenjunga Landscape. Global Ecology and Conservation, 17, e00599.
Health Canada. (2012). Federal contaminated site risk assessment in Canada: PART 1: Guidance on human health Preliminary Quantitative Risk Assessment (PQRA). https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/contaminated-sites/federal-contaminated-site-risk-assessment-canada-part-guidance-human-health-preliminary-quantitative-risk-assessment-pqra-version-2-0.html. Accessed 23 Aug 2021.
Husen, M. A., Nepal, A. P., Gurung, T. B., & Wagle, S. K. (2018). Cage fish farming in lakes and reservoirs of Nepal: A mini review and update. Nepalese Journal of Aquaculture and Fisheries, 5, 34–41.
Jabeen, F., Chaudhry, A. S., Manzoor, S., & Shaheen, T. (2015). Examining pyrethroids, carbamates and neonicotenoids in fish, water and sediments from the Indus River for potential health risks. Environmental Monitoring and Assessment, 187(2), 29.
Jaquet, S., Schwilch, G., Hartung-Hofmann, F., Adhikari, A., Sudmeier-Rieux, K., Shrestha, G., Liniger, H., & Kohler, T. (2015). Does outmigration lead to land degradation? Labour shortage and land management in a western Nepal watershed. Applied Geography, 62, 157–170.
Katagi, T. (2010). Bioconcentration, bioaccumulation, and metabolism of pesticides in aquatic organisms. Reviews of Environmental Contamination and Toxicology, 204, 1–132.
Knight, S., & Hauxwell, J. (2010). Distribution and abundance of aquatic plants–Human impacts. In G. E. Likens (Ed.), Biogeochemistry of Inland Waters (pp. 601–610). Elsevier.
Lamoureux, E. M., & Brownawell, B. J. (1999). Chemical and biological availability of sediment-sorbed hydrophobic organic contaminants. Environmental Toxicology and Chemistry, 18(8), 1733–1741.
Lepper, P. (2005). Manual on the methodological framework to derive environmental quality standards for priority substances in accordance with Article 16 of the Water Framework Directive (2000/60/EC) (p. 51). Germany: Fraunhofer-Institute Molecular Biology and Applied Ecology, Schmallenberg.
Lewis, K. A., Tzilivakis, J., Warner, D. J., & Green, A. (2016). An international database for pesticide risk assessments and management. Human and Ecological Risk Assessment, 22(4), 1050–1064.
Li, H. Z., Cheng, F., Wei, Y. L., Lydy, M. J., & You, J. (2017). Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: An overview. Journal of Hazardous Materials, 324, 258–271.
Liu, S. S., Wang, C. L., Zhang, J., Zhu, X. W., & Li, W. Y. (2013). Combined toxicity of pesticide mixtures on green algae and photobacteria. Ecotoxicology and Environmental Safety, 95, 98–103.
Malik, A., Ojha, P., & Singh, K. P. (2009). Levels and distribution of persistent organochlorine pesticide residues in water and sediments of Gomti River (India)-a tributary of the Ganges River. Environmental Monitoring and Assessment, 148(1–4), 421–435.
Mamane, A., Raherison, C., Tessier, J. F., Baldi, I., & Bouvier, G. (2015). Environmental exposure to pesticides and respiratory health. European Respiratory Review, 24(137), 462–473.
Meshkini, S., Rahimi-Arnaei, M., & Tafi, A. A. (2019). The acute and chronic effect of Roundup herbicide on histopathology and enzymatic antioxidant system of Oncorhynchus mykiss. International Journal of Environmental Science and Technology, 16, 6847–6856.
Müller, R., Shinn, C., Waldvogel, A.-M., Oehlmann, J., Ribeiro, R., & Moreira-Santos, M. (2019). Long-term effects of the fungicide pyrimethanil on aquatic primary producers in macrophyte-dominated outdoor mesocosms in two European ecoregions. Science of the Total Environment, 665, 982–994.
Ojha, H. R., Shrestha, K. K., Subedi, Y. R., Shah, R., Nuberg, I., Heyojoo, B., Cedamon, E., Rigg, J., Tamang, S., & Paudel, K. P. (2017). Agricultural land underutilisation in the hills of Nepal: Investigating socio-environmental pathways of change. Journal of Rural Studies, 53, 156–172.
Papadakis, E. N., Tsaboula, A., Vryzas, Z., Kotopoulou, A., Kintzikoglou, K., & Papadopoulou-Mourkidou, E. (2018). Pesticides in the rivers and streams of two river basins in northern Greece. Science of the Total Environment, 624, 732–743.
Pokhrel, B., Gong, P., Wang, X., Khanal, S. N., Ren, J., Wang, C., Gao, S., & Yao, T. (2018). Atmospheric organochlorine pesticides and polychlorinated biphenyls in urban areas of Nepal: Spatial variation, sources, temporal trends and long range transport potential. Atmospheric Chemistry and Physics, 18, 1325–13336.
Relyea, R. A. (2009). A cocktail of contaminants: How mixtures of pesticides at low concentrations affect aquatic communities. Oecologia, 159(2), 363–376.
Requena, M., Parron, T., Navarro, A., Garcia, J., Ventura, M. I., Hernandez, A. F., & Alarcon, R. (2018). Association between environmental exposure to pesticides and epilepsy. Neurotoxicology, 68, 13–18.
Ricart, M., Barceló, D., Geiszinger, A., Guasch, H., d Alda, M. L., Romaní, A. M., Vidal, G., Villagrasa, M., & Sabater, S. (2009). Effects of low concentrations of the phenylurea herbicide diuron on biofilm algae and bacteria. Chemosphere, 76(10), 1392–1401.
Rizzati, V., Briand, O., Guillou, H., & Gamet-Payrastre, L. (2016). Effects of pesticide mixtures in human and animal models: An update of the recent literature. Chemico-Biological Interactions, 254, 231–246.
Rutherford, E. S., Zhang, H., Kao, Y.-C., Mason, D. M., Shakoor, A., Bouma-Gregson, K., Breck, J. T., Lodge, D. M., & Chadderton, W. L. (2021). Potential effects of bigheaded carps on four Laurentian Great Lakes food webs. North American Journal of Fisheries Management, 41(4), 999–1019.
Shah, B. P., & Devkota, B. (2009). Obsolete pesticides: Their environmental and human health hazards. Journal of Agriculture and Environment, 10, 60–66.
Silva, E., Daam, M. A., & Cerejeira, M. J. (2015). Aquatic risk assessment of priority and other river basin specific pesticides in surface waters of Mediterranean River Basins. Chemosphere, 135, 394–402.
Thapa, D. S., Sharma, C. M., Kang, S., & Sillanpää, M. (2014). The risk of mercury exposure to the people consuming fish from lake Phewa, Nepal. International Journal of Environmental Research and Public Health, 11(7), 6771–6779.
Tierney, K. B. (2016). Chemical avoidance responses of fishes. Aquatic Toxicology, 174, 228–241.
Tyohemba, R. L., Pillay, L., & Humphries, M. S. (2021). Bioaccumulation of current-use herbicides in fish from a global biodiversity hotspot: Lake St Lucia, South Africa. Chemosphere, 284, 131407.
Upadhayay, H. R., Smith, H. G., Griepentrog, M., Bodé, S., Bajracharya, R. M., Blake, W., Cornelis, W., & Boeckx, P. (2018). Community managed forests dominate the catchment sediment cascade in the mid-hills of Nepal: A compound-specific stable isotope analysis. Science of the Total Environment, 637–638, 306–317.
USEPA. (1991). Risk Assessment Guidance for Superfund (RAGS): Part B, Chapter 3: Calculation of risk-based preliminary remediation goals. https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part-b. Accessed 22 Mar 2021.
USEPA. (2012). Pesticide product information System (PPIS). https://www.epa.gov/ingredients-used-pesticide-products/pesticide-product-information-system-ppis. Accessed 15 May 2018.
Varah, A., Ahodo, K., Coutts, S. R., Hicks, H. L., Comont, D., Crook, L., Hull, R., Neve, P., Childs, D. Z., Freckleton, R. P., & Norris, K. (2020). The costs of human-induced evolution in an agricultural system. Nature Sustainability, 3(1), 63–71.
Vonk, J. A., & Kraak, M. H. S. (2020). Herbicide exposure and toxicity to aquatic primary producers. In P. de Voogt (Ed.), Reviews of Environmental Contamination and Toxicology (Vol. 250, pp. 119–171). Springer International Publishing.
Weisner, O., Frische, T., Liebmann, L., Reemtsma, T., Roß-Nickoll, M., Schäfer, R. B., Schäffer, A., Scholz-Starke, B., Vormeier, P., Knillmann, S., & Liess, M. (2021). Risk from pesticide mixtures – The gap between risk assessment and reality. Science of the Total Environment, 796, 149017.
Weisner, O., Arle, J., Liebmann, L., Link, M., Schäfer, R. B., Schneeweiss, A., Schreiner, V. C., Vormeier, P., & Liess, M. (2022). Three reasons why the Water Framework Directive (WFD) fails to identify pesticide risks. Water Research, 208, 117848.
WHO. (2012). Inventory of evaluations performed by the Joint Meeting on Pesticide Residues (JMPR). https://apps.who.int/pesticide-residues-jmpr-database. Accessed 21 Mar 2021.
Yadav, I. C., Devi, N. L., Li, J., Zhang, G., & Shakya, P. R. (2016). Occurrence, profile and spatial distribution of organochlorines pesticides in soil of Nepal: Implication for source apportionment and health risk assessment. Science of The Total Environment, 573(Supplement C), 1598–1606.
Zeng, H., Fu, X., Liang, Y., Qin, L., & Mo, L. (2018). Risk assessment of an organochlorine pesticide mixture in the surface waters of Qingshitan Reservoir in Southwest China. RSC Advances, 8(32), 17797–17805.
Zhang, M., Wang, Y., Gu, B., Li, Y., Zhu, W., Zhang, L., Yang, L., & Li, X. (2019). Resources utilization and trophic niche between silver carp and bighead carp in two mesotrophic deep reservoirs. Journal of Freshwater Ecology, 34(1), 199–212.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This research was financially supported by Vlaamse Inter-universitaire Raad (VLIR) Belgium as a part of an ICP-Master grant (Master of Environmental Sanitation) for S. Acharya. She is also supported by La Trobe University Postgraduate Research Scholarship (LTUPRS), La Trobe University Full Fee Research Scholarship (LTUFFRS) and PhD Top-Up Scholarship supported by the Murray-Darling Basin Joint Government in association with the Centre for Freshwater Ecosystems (CFE). Rothamsted Research receives strategic funding from the UK Biotechnology and Biological Sciences Research Council (BBSRC) and contributions to this manuscript by H.R. Upadhayay was supported by the institute strategic programme Soil to Nutrition funded by grant award BBS/E/C/000I0330.
Author information
Authors and Affiliations
Contributions
S. Acharya: experimental design, sample collection and processing, data curation and analysis, writing – original draft, reviewing and editing. H.R. Upadhayay: experimental design, sample collection, writing – reviewing and editing. M. Houbraken: methodology, writing – reviewing and editing. R.M. Bajracharya: writing – reviewing and editing. P. Spanoghe: supervision, writing – reviewing and editing.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(1.65 MB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Acharya, S., Upadhayay, H.R., Houbraken, M. et al. Occurrence of Unapproved Pesticides and their Ecotoxicological Significance for an Agriculturally Influenced Reservoir and its Tributaries in Nepal. Water Air Soil Pollut 234, 565 (2023). https://doi.org/10.1007/s11270-023-06570-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06570-8