Abstract
Response surface methodology (RSM) and Box–Behnken design (BBD) were investigated to optimize the coagulation–flocculation process (CF) of cardboard industry effluent using polyaluminum chloride (PAC) and cationic polyacrylamides (c-PAM). The aim is to identify the optimum combination of coagulant dose, pH, and flocculant dosage for the highest removal efficacy of chemical oxygen demand (COD) and anionic surfactant removal. According to the results, COD reduction was principally controlled by PAC and c-PAM, while anionic surfactant was affected by pH and PAC. In addition, COD and anionic surfactant removal models fitted well with the experimental results (R2 > 0.85). Coagulation/flocculation using optimum conditions of pH, PAC, and c-PAM led to the total removal of anionic surfactant and the reduction of more than 95% of COD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The discharge of various pollutants, including heavy metals, dyes, surfactants, pharmaceuticals, pesticides, and personal care products, from both industrial and municipal sources has led to the contamination of the world’s water resources (Siyal et al., 2020). This pollution is a significant environmental concern, as it not only impacts the health of aquatic ecosystems, but also poses a risk to human health through the consumption of contaminated water or aquatic organisms (Bani-Melhem et al., 2023). Additionally, the degradation of water quality can have economic consequences, as it can limit the availability of clean water for drinking, irrigation, and industrial purposes (Harif et al., 2022a). As such, it is critical to implement effective strategies to mitigate the discharge of pollutants into water resources and promote the sustainable use of this precious natural resource.
Surfactants, also known as surface-active agents, are widely recognized for their cleaning and solubility properties. They are used in daily life application and various industries, and most are dispersed in diverse environmental compartments. Surfactants consist of amphiphilic molecules that have a hydrophilic group and a lipophilic group (Yuan et al., 2014). They are compounds that alter energy relationships at interfaces, often by changing either the surface or interfacial tension (Glassman, 1948). When used in low concentrations, surfactants decrease the interfacial free energy required to modify the interfacial or surface area, leading to the expansion of one of the interfaces in the system (Rosen, 1972). This property enhances the efficiency of various processes (Hoffmann & Ebert, 1988), and surfactants are commonly employed as wetting agents, detergents, and emulsifying agents (Glassman, 1948). Some surfactants, such as quaternary ammonium compounds (QACs), can damage the outer membrane of gram-negative bacteria and hence are used as disinfectants (Palmer & Hatley, 2018).
The world production of surfactants has increased since the 1980s, from 1.7 million tons utilized in 1984 to 15.93 million tons used in 2014 and the 24.19 million tons forecast used in 2022 (Chen et al., 2020). In 2019, the global market for surfactants was esteemed at $41.3 billion, and it is predicted to grow at $58.5 billion by 2027, with a CAGR of 5.3% from 2020 to 2027 (Allied Market Research, 2020; Saurabh et al., 2023). This anticipated growth is now believed to be underestimated because of the rising demand for products containing surfactants resulting from the COVID-19 pandemic (Johnson et al., 2021). A wide range of these products are currently employed in many fields, including pulp paper, textiles, food, polymers, industries, cosmetics, and pharmaceuticals (Aboulhassan et al., 2006). Surfactants are important categories of pollutants in industrial wastewater. After utilization, residual surfactants are discharged directly into surface waters or into wastewater and accumulate in wastewater treatment plants. One of the main challenges in wastewater treatment is the treatment of surfactants. This is related to surfactants’ wide use and variety, as well as their negative effects on the wastewater treatment process and, more significantly, on the environment (Boethling, 2019; Mousavi et al., 2011; Pettersson et al., 2000; Yuan et al., 2014).
According to the literature, anionic surfactants are the most common type found in wastewater (Al-Qodah et al., 2020a; Johnson et al., 2021; Rashid et al., 2020). Surfactants, both cationic and non-ionic, were also regularly discovered, but at lower concentrations (AGRICE, 2001; Palmer & Hatley, 2018; Rashid et al., 2020). Nevertheless, there is a relative deficiency of wastewater characterizations that draw attention to the presence of various surfactant classes (Palmer & Hatley, 2018). Removal methods of surfactants require processes such as electrochemical and chemical oxidation, biological systems, membrane technology, chemical precipitation, adsorption, and photocatalytic degradation (Abd El-Lateef et al., 2018; Aboulhassan et al., 2006; Adesina et al., 2019; Al-Qodah et al., 2020b; Bankole et al., 2017; Bolong et al., 2009; Fernández et al., 2005; Khosravanipour Mostafazadeh et al., 2019; Kong et al., 2006; Kowalska et al., 2005; Lin et al., 1999; Lissens et al., 2003; Ono et al., 2012; Park et al., 2021; Pham et al., 2016; Wei et al., 2015). The method used to treat wastewater differs depending on the type of surfactant. According to previous works, surfactants are not efficiently eliminated by traditional biological degradation processes, requiring additional chemical treatment, particularly with industrial effluent (Arslan-Alaton et al., 2007; Ikehata & El-Din, 2004; Renault et al., 2009). Hence, coagulation–flocculation process has received significant regard because of its high pollution removal efficacy (Mahvi & Maleki, 2004; Aboulhassan et al., 2006; Adesina et al., 2019; Beltrán-Heredia et al., 2009; Çırak & Hoşten, 2017; Park et al., 2021). This process involves the neutralization of cationic hydrolysis products by negatively charged colloids, which then leads to the amalgamation of contamination in amorphous hydroxide precipitation (Azimi & Shirini, 2020).
Coagulation is mostly performed using inorganic metal salts, such as ferric and aluminum chlorides and sulfates. Polyelectrolytes of different structures, such as polyacrylamides, polysaccharides, polyvinyl, and chitosan, are generally employed as flocculants to rise the floc density aiming to enhance the sedimentation rate. As mentioned by Nandy et al. (Nandy et al., 2002), adding cationic polyelectrolyte to polyaluminium chloride (PAC) improved separation efficiency and made the flocs stronger than those created by simple salts. In the previous studies, polyacrylamide has been found to be efficient for the coagulation-flocculation of cardboard industry effluent (Ahmad et al., 2008; Gao et al., 2005; Harif et al., 2022b). PAC is generally used, in surface water and wastewater treatment, as coagulant. The action mechanism is mostly explained by the bridge formation mechanism and the charge neutralization of negatively charges colloids via cationic hydrolysates (Gao et al., 2005; Hennecke et al., 2018). Many parameters, such as coagulant dose and pH, influence the relative importance of these mechanisms.
Box–Behnken design (BBD), central composite design, and three-level factorial design are the most general designs used by the researchers. In the ensuing optimization studies, the BBD was utilized to characterize the effects of the factors on their responses (Garg & Prasad, 2016). The aim of this work is (a) the performance of coagulation–flocculation process with the application of polyaluminum chloride (PAC) as coagulant and cationic polyacrylamides (c-PAM) as flocculant, (b) optimize CF process by using the response surface methodology (RSM) and Box–Behnken design (BBD), and (c) study the effectiveness of parameters PAC concentration, c-PAM dosage, and pH in CF process for COD and surfactant removal and cardboard wastewater treatment.
A response surface methodology is a valuable statistical tool that allows for the efficient and cost-effective assessment of the interactive effects of various factors (Dehghani et al., 2020; Harif et al., 2022a). To our knowledge, this study is the first of its kind to investigate the improvement of anionic surfactant removal from cardboard industry wastewater using coagulation/flocculation.
2 Material and Methods
2.1 Samples Handling and Characterization
The cardboard wastewater was collected from a cardboard industry situated in Agadir City, Morocco. The samples were stored until analysis according to the Standard Methods for the Examination of Water and Wastewater (APHA, 1930). The characterization of the cardboard wastewater is summarized in Table 1.
The closed reflux colorimetric method (Method 5220 C) was used to evaluate the COD analysis, the WTW InoLab pH 7110 was used to test pH, and the concentration of anionic surfactant (AS) was performed via solvent extraction-spectrophotometric with ethyl violet method (Motomlzu et al., 1982).
A spectrophotometer was used to obtain Fourier-transform infrared (FTIR) spectroscopy of the products in KBr pellets (2 mg/200 mg). At a resolution of 4 cm−1,128 scans were evaluated.
2.2 Coagulant and Flocculants Used
Coagulation–flocculation process was performed by a commercial-grade polyaluminum chloride (PAC) and cationic polyacrylamides (c-PAM). Polyaluminum chloride (PAC) has grown to be the most widely used coagulant because of the benefits over traditional aluminum or ferric-based coagulants, such as less aluminum residuals, better performance at low temperature, less effect on the pH value of water, rapid flocculation, and less sludge volume (Wei et al., 2015). Cationic polyacrylamides (c-PAM) used as flocculants are characterized as a high molecular product (pH: 3–4, density: approx. 1.2 g/m3, viscosity in tap water: < 600 cp, and cationicity: 35%).
The structure of c-PAM was studied using infrared spectroscopy (Fig. 1). The stretching vibration of the -NH2 group refers to the absorption peak shown at wave-length 3450 cm1, and the one at 2900 cm1 indicates the existence of -CH2- group. The presence of the -C = O bond, NH2 group, C-N bond, and -C–C- group was also demonstrated by peaks at 1650 cm1, 1600 cm−1, 1450 cm−1, and 1100 cm−1, respectively.
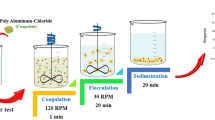
2.3 Procedure in the Jar Tests
A jar test method comprising six beakers was installed at room temperature for each test. The samples were removed into 1-L beakers for each run. The beakers were put in the jar test setup after the pH was adjusted by applying 1 mol/l NaOH or 1 mol/l HCl, and various coagulant dosages were added.
After being agitated at 200 rpm for 5 min, the mixture was then slowly agitated for 15 min at 30 rpm. After 1 h settling period, anionic surfactant and COD determinations were performed by taking samples around 2 cm below the water level. The anionic surfactant and COD removal can be determined as in Eq. (1).
where Ci and Cf represent, respectively, the initial and final concentrations.
2.4 Experimental Design
2.4.1 Box Behnken Design
With a limited number of experiments, RSM was analyzed to identify the interaction between factors and responses. In this study, the model coefficients are evaluated using a Box–Behnken design (BBD) with 14 experiments (Table 2). The experimental points are located in the center of the cube’s ridges (12 experiments) and in the cube’s center (2 experiments). Replicates are performed at the center point to establish a nearly orthogonal design and evaluate the pure error variance(Box et al., 2005; Mazerolles et al., 1989; Pereira et al., 2007; Singh & Ahuja, 1999). Coagulant concentration (X1), initial pH (X2), and flocculant concentration (X3) were the variables. The coding method of the variables has been reported by Gunst et al. (Gunst & Mason, 2009). Table 2 presents the range and the level of variables.
COD and anionic surfactant removals were chosen as responses in the experiment (Y). As given in Eq. (2), a second-order polynomial equation represents the model (Box et al., 2005; Douahem et al., 2016; Huzir et al., 2019; Mason et al., 2003; Sridhar et al., 2015):
where Y is the theoretical response function; Xj is coded variables of the system; and b0, bj, bjk, and bjj are model coefficients.
The regression coefficients and their effects were investigated using analysis of variance (ANOVA) (Akhnazarova & Kafarov, 1984). The efficiency of the fit polynomial model was indicated by the coefficient of determination, R2.
The statistically significant experimental design and analysis were carried out using the NEMRODW software (Mathieu et al., 2000). The optimum values of chosen variables were determined by applying the desirability function available in NEMRODW and evaluating the response surface contour plots.
3 Results and Discussion
3.1 Physicochemical Characteristics of the Cardboard Effluent
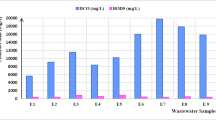
The cardboard industry generates a huge volume of wastewater; the average flow rate of wastewater is about 6000 m3/day. The cardboard wastewater characteristics are presented in Table 1. It should be noted that values do not conform to Moroccan environmental regulations. In fact, the cardboard wastewater presents the dark gray color due to the mixture of several inks as well as other residual products used during the cardboard processing. Low BOD5 (880 g O2/l) and high COD concentration (11,983 g O2/l) conclude the presence of huge amounts of non-biodegradable organic matter. Indeed, the BOD5/COD ratio was 0.073 (< 0.4) (Soloman et al., 2009), indicating that the wastewater is not easily biodegradable. The cardboard wastewater is characterized by including a high number of suspended solids, and the TSS content was about 12,825 mg/l. The average anionic surfactant content was about 0.72 mg/l. Therefore, the persistent foams may appear in the water and may create an insulating layer leading to the weakness of the exchange between the gas atmosphere and water body, then leading to the reduction of dissolved oxygen.
3.2 Box–Behnken Design
Experiments were performed to analyze the effect of PAC (X1), pH (X2), and c-PAM (X3) on the responses Y1 (COD removal) and Y2 (anionic surfactant removal) by the design matrix. The results (Table 3) indicate that the highest COD removal of 95.69% was achieved using 300 mg/l of PAC, pH = 7, and 4.5 mg/l of c-PAM (experiment 12). However, 1 and 11 experiments with 200 and 300 mg/l of PAC, pH = 5, and 3.5 and 4.5 mg/l of c-PAM, respectively, demonstrated the total anionic surfactant removal.
3.3 Model Equations
Results of the BBD experiments are applied to evaluate the model coefficients. Equations (3) and (4) represent the fitted models expressed in coded variables:
-
COD removal:
$${Y}_{1}=93.185+{1.495X}_{1}+{0.105X}_{2}+{1.877X}_{3}-{3.356X}_{1}^{2}+{0.554X}_{2}^{2}+{0.209X}_{3}^{2}+{2.367X}_{1}{X}_{2}-{0.207X}_{1}{X}_{3}+{0.488X}_{2}{X}_{3}$$(3) -
Anionic surfactant removal:
$${Y}_{2}=93.03+{18.266X}_{1}-{47.129X}_{2}+{1.635X}_{3}-{26.226X}_{1}^{2}-{14.956X}_{2}^{2}-{28.574X}_{3}^{2}+{5.243X}_{1}{X}_{2}+{6.315X}_{1}{X}_{3}-{0.5X}_{2}{X}_{3}$$(4)
3.4 Model Validation and Statistical Analysis
The variance analysis results for the fitted models are summarized in Tables 4 and 5. The regression sum of squares for Y1 and Y2 reaches statistical significance at levels of 99 and 95%, respectively. The models were found to be significant at more than 95% confidence level as illustrated in Table 4, with all p-values of regression less than 0.05. It can be concluded that all three variables were significant for the Y1 response, but X3 was not significant for Y2 response (Table 5). In addition, all factor interactions were significant for Y1, but the interaction between X2 and X3 was not significant for Y2.
Moreover, R2 must be at least 0.80 for the good fit of a model (Guan & Yao, 2008). The R2 results were 0.852 and 0.918 for Y1 and Y2, respectively, and demonstrate that the second-order polynomial model fitted well with the experimental results (Table 6). In fact, Figs. 2 and 3 demonstrated that the measured versus anticipated plot values were evenly distributed near the straight line.
This showed the high effectiveness and correct fitting of the obtained model for COD removal and surfactant removal. However, as shown in Table 4, the variance associated with lack of fit and pure error were compared, highlighting the significance of lack of fit. Applying the obtained model, iso-response lines and response surfaces are constructed, and the response value in the research region is predicted.
3.5 Effect of Variables on Responses
Figures 3 and 4 describe the surface design for the coagulation–flocculation process of cardboard wastewater using PAC and c-PAM. The flexure of the figures illustrates how the variables and reactions interact.
3.5.1 Effects of PAC Dosage and Initial pH
At a constant c-PAM concentration of 3.5 mg/l, Figs. 3a and 4a illustrate the effects of increasing the PAC dosage (X1) and initial pH (X2) on COD and anionic surfactant removal, respectively. The p-value for PAC dosage (0.559) compared to pH (1.65) in ANOVA (Table 5) showed that PAC dosage had a more significant impact on COD removal. However, for anionic surfactant removal, the effect of pH (p = 0.775) was more significant than the effect of PAC dosage (p = 1.22).
Figure 3a shows a moderate interaction between pH and COD removal, because any variation in its value along its axis will not result in any significant change in COD removal. Figure 3a also reveals a significant interaction between the PAC dosage and COD removal as any change in PAC dosage affects the COD removal. The two-dimensional contour plot in Fig. 4a shows that the PAC dosage has minimal effect on the anionic surfactant removal. The anionic surfactant is affected mostly by pH, which suggested that anionic surfactant was more easily coagulated in acidic medium.
Figure 4a also demonstrated that the optimal conditions for surfactant removal were achieved at a PAC concentration between 200 and 350 mg/l and a pH of less than 6. These conditions resulted in remarkable performance in terms of anionic surfactant removal, with average removal efficiencies of 99%. Similar conditions were also effective in removing more than 93% of COD, as shown in Fig. 4a. PAC works by neutralizing the negative charges on the anionic surfactants and the organic particles contributing to COD levels, forming larger particles that can be removed by settling.
Deng et al. (Deng et al., 2011) previously demonstrated that the use of PAC in the coagulation process can increase the efficiency of anionic surfactant removal. The authors hypothesized that the anionic surfactant molecules interacted primarily with the surface of the PAC particles through hydrophobic interactions, leading to a progressive increase in the hydrophilicity of the surface. To achieve an increase in adsorption through this approach, a contact between the hydroxyl group and the polarized group of the anionic surfactant was necessary.
3.5.2 Effect of PAC Dosage and c-PAM Dosage
At a constant pH of 6, Figs. 3b and 4b demonstrate the effects of PAC dosage (X1) and c-PAM dosage (X3), respectively, and their reciprocal interactions on COD and anionic surfactant removal. The p-value of PAC dosage (0.559) was higher than the p-value for c-PAM dosage (0.539) demonstrates that c-PAM could explain why c-PAM was the significant variable for achieving high COD removal on CF process. Furthermore, the p-value for PAC dose of 1.22 was significantly lower than the p-value for c-PAM dosage of 11.2 for anionic surfactant removal. This result concluded that c-PAM dosage wielded the most important effect on COD removal. However, PAC dosage had a more significant effect on anionic surfactant removal than c-PAM dosage.
Figures 3b and 4b illustrate that COD and Surfactant were influenced either by PAC and c-PAM dosages. The results revealed that the high COD removal (94%) could be reached at a PAC dosage between 250 and 350 mg/l with a c-PAM concentration of over 3.5 mg/l. The highest anionic surfactant removal condition is substantially inside the design boundary, as seen by the response surface of anionic surfactant removal, which illustrates a clear peak (Fig. 4b). According to Fig. 4b, the high anionic surfactant removal of more than 82% is reached at PAC dosage of 250–350 mg/l and c-PAM dosage 3–4 mg/l. The removal of anionic surfactant increased with a further increase of the PAC and c-PAM dosage beyond the optimum conditions.
Recent scientific publications that support the findings of this study include the research by Li et al. (Li et al., 2019) which studied the effects of PAC and c-PAM dosages on the removal of heavy metals. They reported that increasing PAC and c-PAM dosages led to increased heavy metal removal, but excessive dosages resulted in reduced removal efficiency.
In addition to these studies, there are several possible explanations for the observed effects of PAC and c-PAM dosages on COD and anionic surfactant removal. PAC is a coagulant that can neutralize the negatively charged particles in wastewater by adsorbing them onto its positively charged surface. This process can enhance the removal of organic matter, such as COD, by forming larger particles that are easier to settle or filter out. However, excessive dosages of PAC can result in the destabilization of the flocs and reduce the removal efficiency.
On the other hand, c-PAM is a flocculant that can bridge the destabilized particles together to form larger flocs, which can also enhance the removal of organic matter and anionic surfactants. The positive charges of c-PAM can neutralize the negative charges of the anionic surfactants, thereby reducing their solubility and facilitating their removal. However, excessive dosages of c-PAM can result in the formation of smaller, weaker flocs, which can reduce the removal efficiency.
3.5.3 Effect of Initial pH and c-PAM Dosage
Figures 3c and 4c demonstrate the effect of variable pH (X2) and c-PAM dosage (X3) on anionic surfactant and COD removals at constant conditions of 300 mg/l of PAC concentration. The molecular structure of c-PAM, which did not vary with pH, had no effect on its charge neutralization and adsorption bridging effect. c-PAM is a polyacrylamide cationic that contains NH2 which could yield NH3+ in acid media. It can thus conceivably hypothesize that C-PAM works better in acid medium, as shown in the results, especially with anion surfactants. As a result, introducing c-PAM increased the COD removal but not for surfactant removal. From Table 5, it can be seen that the pH (1.65) affected significantly COD removal and was less significant than the c-PAM dosage (0.539). However, c-PAM dosage was not significant on anionic surfactant removal. This is due to the higher p-value obtained for c-PAM in anionic surfactant removal (11.2) as compared to initial pH (0.775).
The result showed that total removal of anionic surfactant could be obtained at a low pH using 3 to 4.5 mg/l of c-PAM dosages. The highest COD removal (95%) can be reached at similar conditions. It can be concluded that the anionic surfactant and COD reduction were favored by the wastewater’s acidic condition. According to Yongabi (Garg & Prasad, 2016), the more efficiently the suspended solids can coagulate depends on the pH of the wastewater. This can be explained by the charge neutralization mechanism, in which cationic particles are attracted to colloidal particles strongly.
3.6 Optimization
The optimizations of the two individual responses, COD and surfactant removals, were reached according to different optimal conditions. The optimum COD and surfactant removals may have a mutual effect. As a result, a compromise between the two responses’ optimal conditions is required. The desirability of the two responses was established by determining the desirable limits of 95% COD removal and 100% surfactant removal. Figure 5 shows the results of the NEMROD-W software’s estimate of the global desirability function D as a contour graph and a three-dimensional graph. The relatively flat zone relating to optimum conditions could be noted (D = 1).
Based on the shaded area of overlain contour in Fig. 5, a compromise for 95.02% COD removal and 100% anionic surfactant removal can be met at 311 mg/l PAC, pH 5, and 4.5 mg/l c-PAM. The results were superior to previous studies of surfactant removal by coagulation–flocculation process: Mohan (Dehghani et al., 2020) found 96.3% anionic surfactant removal from laundry wastewater with the naturalized coagulants. Aboulhassan et al. (Aboulhassan et al., 2006) have previously stated 99% surfactant removal from microelectronic factory wastewater. It has been also shown in Kaleta and Elektorowicz’s (Kaleta & Elektorowicz, 2013) work the similar result (100% anionic surfactant removal) using powdered activated carbon before introducing basic coagulant to the coagulation process. The use of PAC and c-PAM in the treatment of pulp and paper industry effluents might be deemed cost-effective (Wong et al., 2006). In this work, the cost of wastewater treatment using PAC and c-PAM is around 791,29 $ per m3.
The biological processes may provide an alternative for surfactant treatment due to their cheaper cost. However, several disadvantages must be considered when using these processes (Palmer & Hatley, 2018). For instance, the reaction time can be slow, and high surfactant concentrations can lead to biomass death. Additionally, foam formation and high sewage sludge generation can be problematic (Bering et al., 2018; Collivignarelli et al., 2019; Jardak et al., 2016; Palmer & Hatley, 2018). Nevertheless, operational costs of physicochemical and chemical treatments can be important due to the requirement on regeneration/disposing of exhausted adsorbent materials in physicochemical treatments and the oxidant value in chemical treatments (Bering et al., 2018; Collivignarelli et al., 2019; Jardak et al., 2016; Palmer & Hatley, 2018). The physicochemical processes such as coagulation/flocculation had been attracted considerable attention due to its high removal efficacy of surfactant (Deng et al., 2011; Park et al., 2021).
4 Conclusion
This study has studied the efficiency of the coagulation–flocculation process applied to the cardboard industry wastewater treatment. Parameters affecting coagulation–flocculation process were optimized using jar-test methods and RSM, including the dosage of coagulant and initial pH flocculant dosage. Based on preliminary experiments, polyaluminum chloride (PAC) was used as coagulant and cationic polyacrylamides (c-PAM) as flocculant. Our work has led us to conclude that CF treatment reaches a fast and effective removal of surfactant and chemical oxygen demand.
A response surface methodology-based BBD model was employed to investigate the effect of coagulant dosage, pH, and flocculant dosage on COD and surfactant removals and to determine the optimum experimental conditions for these three control factors. This RSM has revealed a high interaction between experimental and predicted values. A high coefficient of determination value was revealed by variance analysis, demonstrating that the model was properly tuned to the experimental design. The results indicate that PAC, c-PAM, and pH were important factors on the CF of cardboard industry wastewater. COD reduction was principally controlled by PAC and c-PAM and was less influenced by pH. However, surfactant was principally controlled first by pH and secondly by PAC, while c-PAM has no significant effect on surfactant removal. The treatment of cardboard industry wastewater using optimum dosages of PAC and C-PAM at acidic conditions allows more than 95% of COD reduction and a total removal of surfactant.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abd El-Lateef, H. M., Khalaf Ali, M. M., & Saleh, M. M. (2018). Adsorption and removal of cationic and anionic surfactants using zero-valent iron nanoparticles. Journal of Molecular Liquids, 268, 497–505. https://doi.org/10.1016/j.molliq.2018.07.093.
Aboulhassan, M. A., Souabi, S., Yaacoubi, A., & Baudu, M. (2006). Removal of surfactant from industrial wastewaters by coagulation flocculation process. International Journal of Environmental Science & Technology, 3, 327–332. https://doi.org/10.1007/BF03325941.
AGRICE. (2001). Tensioactifs et oléagineux: Étude sur les matières premières oléagineuses disponibles sur le marché européen. ADEME, 60.
Adesina, O. A., Abdulkareem, F., Yusuff, A. S., Lala, M., & Okewale, A. (2019). Response surface methodology approach to optimization of process parameter for coagulation process of surface water using Moringa oleifera seed. South African Journal of Chemical Engineering, 28, 46–51. https://doi.org/10.1016/j.sajce.2019.02.002.
Ahmad, A., Wong, S., Teng, T., & Zuhairi, A. (2008). Improvement of alum and PACl coagulation by polyacrylamides (PAMs) for the treatment of pulp and paper mill wastewater. Chemical Engineering Journal, 137, 510–517. https://doi.org/10.1016/j.cej.2007.03.088.
Akhnazarova, S., & Kafarov, V. (1984). Experiment optimization in chemistry and chemical engineering. Journal of Polymer Science: Polymer Letters Edition, 22, 372–372. https://doi.org/10.1002/pol.1984.130220614.
Allied Market Research. (2020). Surfactants market by type : Global opportunity analysis and industry forecast, 2020–2027, p 5134088.
Al-Qodah, Z., Tawalbeh, M., Al-Shannag, M., Al-Anber, Z., & Bani-Melhem, K. (2020a). Combined electrocoagulation processes as a novel approach for enhanced pollutants removal: A state-of-the-art review. Science of the Total Environment, 744. https://doi.org/10.1016/j.scitotenv.2020.140806.
Al-Qodah, Z., Tawalbeh, M., Al-Shannag, M., Al-Anber, Z., & Bani-Melhem, K. (2020b). Combined electrocoagulation processes as a novel approach for enhanced pollutants removal: A state-of-the-art review. Science of The Total Environment, 744, 140806. https://doi.org/10.1016/j.scitotenv.2020.140806.
APHA. (1930). Library Catalogue. Journal of the Textile Institute Proceedings, 21, P145–P145. https://doi.org/10.1080/19447013008687143.
Arslan-Alaton, I., Cokgor, E. U., & Koban, B. (2007). Integrated photochemical and biological treatment of a commercial textile surfactant: Process optimization, process kinetics and COD fractionation. Journal of Hazardous Materials, 146, 453–458. https://doi.org/10.1016/j.jhazmat.2007.04.069.
Azimi, S. C., Shirini, F., & Pendashteh A. (2020). Treatment of wood industry wastewater by combined coagulation – flocculation – decantation and fenton process. Water Environment Research pp. 33–444. https://doi.org/10.1002/wer.1441.
Bani-Melhem, K., Al-Kilani, M. R., & Tawalbeh, M. (2023). Evaluation of scrap metallic waste electrode materials for the application in electrocoagulation treatment of wastewater. Chemosphere, 310. https://doi.org/10.1016/j.chemosphere.2022.136668.
Bankole, M. T., Abdulkareem, A. S., Tijani, J. O., Ochigbo, S. S., Afolabi, A. S., & Roos, W. D. (2017). Chemical oxygen demand removal from electroplating wastewater by purified and polymer functionalized carbon nanotubes adsorbents. Water Resources and Industry, 18, 33–50. https://doi.org/10.1016/j.wri.2017.07.001.
Beltrán-Heredia, J., Sánchez-Martín, J., & Solera-Hernández, C. (2009). Anionic surfactants removal by natural coagulant/flocculant products. Industrial and Engineering Chemistry Research, 48, 5085–5092. https://doi.org/10.1021/ie801913y.
Bering, S., Mazur, J., Tarnowski, K., Janus, M., Mozia, S., & Morawski, A. W. (2018). The application of moving bed bio-reactor (MBBR) in commercial laundry wastewater treatment. Science of the Total Environment, 627, 1638–1643. https://doi.org/10.1016/j.scitotenv.2018.02.029.
Boethling, R. S. (2019). Catonic Surfactants. CRC Press. https://doi.org/10.1201/9780429270376.
Bolong, N., Ismail, A. F., Salim, M. R., & Matsuura, T. (2009). A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination, 239, 229–246. https://doi.org/10.1016/j.desal.2008.03.020.
Box, G. E. P., Hunter, J. S., & Hunter, W. G. (2005). Statistics for experimenters. JOHN WILEY & SONS, INC.
Chen, Q., Tian, M., Kasomo, R. M., Li, H., Zheng, H., Song, S., Luo, H., & He, D. (2020). Depression effect of Al(III) and Fe(III) on rutile flotation using dodecylamine polyxyethylene ether as collector. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 603, 125269. https://doi.org/10.1016/j.colsurfa.2020.125269.
Çırak, M., & Hoşten, Ç. (2017). Optimization of coagulation-flocculation process for treatment of a colloidal suspension containing dolomite/clay/borax. International Journal of Mineral Processing, 159, 30–41. https://doi.org/10.1016/j.minpro.2016.12.007.
Collivignarelli, M. C., CarnevaleMiino, M., Baldi, M., Manzi, S., Abbà, A., & Bertanza, G. (2019). Removal of non-ionic and anionic surfactants from real laundry wastewater by means of a full-scale treatment system. Process Safety and Environmental Protection, 132, 105–115. https://doi.org/10.1016/j.psep.2019.10.022.
Dehghani, M. H., Karri, R. R., Yeganeh, Z. T., Mahvi, A. H., Nourmoradi, H., Salari, M., et al. (2020). Statistical modelling of endocrine disrupting compounds adsorption onto activated carbon prepared from wood using CCD-RSM and DE hybrid evolutionary optimization framework: Comparison of linear vs non-linear isotherm and kinetic parameters. Journal of Molecular Liquids, 302. https://doi.org/10.1016/j.molliq.2020.112526.
Deng, S., Zhou, Q., Yu, G., Huang, J., & Fan, Q. (2011). Removal of perfluorooctanoate from surface water by polyaluminium chloride coagulation. Water Research, 45, 1774–1780. https://doi.org/10.1016/j.watres.2010.11.029.
Douahem, H., Hammi, H., Hamzaoui, A. H., & Adel, M. (2016). Modeling and optimization of phosphogypsum transformation into calcium fluoride using experimental design methodology. Journal of the Tunisian Chemical Society, 106–113.
Fernández, E., Benito, J. M., Pazos, C., & Coca, J. (2005). Ceramic membrane ultrafiltration of anionic and nonionic surfactant solutions. Journal of Membrane Science, 246, 1–6. https://doi.org/10.1016/j.memsci.2004.04.007.
Gao, B., Chu, Y., Yue, Q., Wang, B.-J., & Wang, S. (2005). Characterization and coagulation of a polyaluminum chloride (PAC) coagulant with high Al13 content. Journal of Environmental Management, 76, 143–147. https://doi.org/10.1016/j.jenvman.2004.12.006.
Garg, K. K., & Prasad, B. (2016). Development of Box Behnken design for treatment of terephthalic acid wastewater by electrocoagulation process: Optimization of process and analysis of sludge. Journal of Environmental Chemical Engineering, 4, 178–190. https://doi.org/10.1016/j.jece.2015.11.012.
Glassman, H. N. (1948). Surface active agents and their application in bacteriology. Bacteriological Reviews, 12, 105–148. https://doi.org/10.1128/BR.12.2.105-148.1948.
Guan, X., & Yao, H. (2008). Optimization of Viscozyme L-assisted extraction of oat bran protein using response surface methodology. Food Chemistry, 106, 345–351. https://doi.org/10.1016/j.foodchem.2007.05.041.
Gunst, R. F., & Mason, R. L. (2009). Fractional factorial design. WIREs Computational Statistics, 1, 234–244. https://doi.org/10.1002/wics.27.
Harif, S., Aboulhassan, M. A., & Bammou, L. (2022). Multi-response optimization for color removal from cardboard wastewater using polyaluminum chloride and cationic polyacrylamide. International Journal of Environmental Science and Technology, 4281–4292. https://doi.org/10.1007/s13762-022-04267-9.
Harif, S., Aboulhassan, M. A., & Bammou, L. (2022a). Water consumption optimization in cardboard industry by experimental design. Water and Environment Journal, pp. 1–12. https://doi.org/10.1111/wej.12772.
Hennecke, D., Bauer, A., Herrchen, M., Wischerhoff, E., & Gores, F. (2018). Cationic polyacrylamide copolymers (PAMs): Environmental half life determination in sludge-treated soil. Environmental Sciences Europe, 30, 16. https://doi.org/10.1186/s12302-018-0143-3.
Hoffmann, H., & Ebert, G. (1988). Surfactants, micelles and fascinating phenomena. Angewandte Chemie International Edition, 27, 902–912.
Huzir, N. M., Aziz, M. M. A., Ismail, S. B., Mahmood, N. A. N., Umor, N. A., & Faua’ad Syed Muhammad, S. A. (2019). Optimization of coagulation-flocculation process for the palm oil mill effluent treatment by using rice husk ash. Industrial Crops and Products, 139, 111482. https://doi.org/10.1016/j.indcrop.2019.111482.
Ikehata, K., & El-Din, M. G. (2004). Degradation of recalcitrant surfactants in wastewater by ozonation and advanced oxidation processes: A review. Ozone Science and Engineering, 26, 327–343. https://doi.org/10.1080/01919510490482160.
Jardak, K., Drogui, P., & Daghrir, R. (2016). Surfactants in aquatic and terrestrial environment: Occurrence, behavior, and treatment processes. Environmental Science and Pollution Research, 23, 3195–3216. https://doi.org/10.1007/s11356-015-5803-x.
Johnson, P., Trybala, A., Starov, V., & Pinfield, V. J. (2021). Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Advances in Colloid and Interface Science, 288, 102340. https://doi.org/10.1016/j.cis.2020.102340.
Kaleta, J., & Elektorowicz, M. (2013). The removal of anionic surfactants from water in coagulation process. Environmental Technology (United Kingdom), 34, 999–1005. https://doi.org/10.1080/09593330.2012.733415.
KhosravanipourMostafazadeh, A., Benguit, A. T., Carabin, A., Drogui, P., & Brien, E. (2019). Development of combined membrane filtration, electrochemical technologies, and adsorption processes for treatment and reuse of laundry wastewater and removal of nonylphenol ethoxylates as surfactants. Journal of Water Process Engineering, 28, 277–292. https://doi.org/10.1016/j.jwpe.2019.02.014.
Kong, W., Wang, B., Ma, H., & Gu, L. (2006). Electrochemical treatment of anionic surfactants in synthetic wastewater with three-dimensional electrodes. Journal of Hazardous Materials, 137, 1532–1537. https://doi.org/10.1016/j.jhazmat.2006.04.037.
Kowalska, I., Kabsch-Korbutowicz, M., Majewska-Nowak, K., & Pietraszek, M. (2005). Removal of detergents from industrial wastewater in ultrafiltration process. Environment Protection Engineering, 31, 207–219.
Li, Y., Li, L., Farouk, R. Y., & Wang, Y. (2019). Optimization of polyaluminum chloride-chitosan flocculant for treating pig biogas slurry using the Box–Behnken response surface method. International Journal of Environmental Research and Public Health, 16, 996. https://doi.org/10.3390/ijerph16060996.
Lin, S. H., Lin, C. M., & Leu, H. G. (1999). Operating characteristics and kinetic studies of surfactant wastewater treatment by fenton oxidation. Water Research, 33, 1735–1741. https://doi.org/10.1016/S0043-1354(98)00403-5.
Lissens, G., Pieters, J., Verhaege, M., Pinoy, L., & Verstraete, W. (2003). Electrochemical degradation of surfactants by intermediates of water discharge at carbon-based electrodes. Electrochimica Acta, 48, 1655–1663. https://doi.org/10.1016/S0013-4686(03)00084-7.
Mahvi, A. H., & Maleki, A. (2004). Removal of anionic surfactants in detergent wastewater by chemical coagulation. Pakistan Journal of Biological Sciences, 2222-2226.
Mason, R. L., Gunst, R. F., & Hess, J. L. (2003). Statistical design and analysis of experiments.https://doi.org/10.2307/2289624.
Mathieu, D., Nony, J., & Luu, R. P. T. (2000). Software for the conception and analysis of experimental designs.
Mazerolles, G., Mathieu, D., Aix-marseille, U., & Siouffi, A.M. (1989). Computer-assisted optimization with NEMROD software. Journal of Chromatography A, 485, 433–451.
Mohan, S. M. (2014). Use of naturalized coagulants in removing laundry waste surfactant using various unit processes in lab-scale. Journal of Environmental Management, 136, 103–111. https://doi.org/10.1016/j.jenvman.2014.02.004.
Motomlzu, S., Fujlwara, S., Fujiwara, A., & Toei, K. (1982). Solvent extraction-spectrophotometric determination of anionic surfactants with ethyl violet. Analytical Chemistry, 54, 392–397. https://doi.org/10.1021/ac00240a011.
Mousavi, S. A. R., Mahvi, A. H., & Nasseri, S. (2011). Effect of fenton process (H2O2/Fe2+) on removal linear alkylbenzene sulfonate using central composite. Iranian Journal of Environmental Health Science and Engineering, 8, 129–138.
Nandy, T., Vyas, R. D., Shastry, S., & Kaul, S. N. (2002). Optimization of coagulants for pretreatment of printing ink wastewater. Environmental Engineering Sciences, 19, 1–7. https://doi.org/10.1089/109287502753590197.
Ono, E., Tokumura, M., & Kawase, Y. (2012). Photo-Fenton degradation of non-ionic surfactant and its mixture with cationic or anionic surfactant. Journal of Environmental Science and Health, Part A, 47, 1087–1095. https://doi.org/10.1080/10934529.2012.668034.
Palmer, M., & Hatley, H. (2018). The role of surfactants in wastewater treatment: Impact, removal and future techniques: A critical review. Water Research, 147, 60–72. https://doi.org/10.1016/j.watres.2018.09.039.
Park, B. H., Kim, S., Seo, A. Y., & Lee, T. G. (2021). Evaluation of optimal conditions for anionic surfactant removal in wastewater. Chemosphere, 263, 128174. https://doi.org/10.1016/j.chemosphere.2020.128174.
Pereira, V. J., Weinberg, H. S., Linden, K. G., & Singer, P. C. (2007). UV degradation kinetics and modeling of pharmaceutical compounds in laboratory grade and surface water via direct and indirect photolysis at 254 nm. Environmental Science and Technology, 41, 1682–1688. https://doi.org/10.1021/es061491b.
Pettersson, A., Adamsson, M., & Dave, G. (2000). Toxicity and detoxification of Swedish detergents and softener products. Chemosphere, 41, 1611–1620. https://doi.org/10.1016/S0045-6535(00)00035-7.
Pham, T. T., Mai, T. D., Pham, T. D., Hoang, M. T., Nguyen, M. K., & Pham, T. T. (2016). Industrial water mass balance as a tool for water management in industrial parks. Water Resources and Industry, 13, 14–21. https://doi.org/10.1016/j.wri.2016.04.001.
Rashid, T. U., Kabir, S. M. F., Biswas, M. C., & Bhuiyan, M. A. R. (2020). Sustainable wastewater treatment via dye–surfactant interaction: A critical review. Industrial and Engineering Chemistry Research, 59, 9719–9745. https://doi.org/10.1021/acs.iecr.0c00676.
Renault, F., Sancey, B., Charles, J., Morin-Crini, N., Badot, P.-M., Winterton, P., & Crini, G. (2009). Chitosan flocculation of cardboard-mill secondary biological wastewater. Chemical Engineering Journal, 155, 775–783. https://doi.org/10.1016/j.cej.2009.09.023.
Rosen, M. J. (1972). The relationship of structure to properties in surfactants. Hoboken, NJ, USA: Wiley Online Library.
Singh, B., & Ahuja, N. (2000). Pharmaceutical experimental design (Drugs and the Pharmaceutical Sciences, vol. 92), Edited by G.A. Lewis, D. Mathieu and R. Phan-Tan-Luu, 1st Edition, Marcel Dekker, New York, 1999. vi+498 pp., 23.5×15.5 cm., 1.9 lb., Hardcover, ISBN 0–8247–9860–0, Price. International Journal of Pharmaceutics, 195, 247–248. https://doi.org/10.1016/S0378-5173(99)00384-1.
Siyal, A. A., Shamsuddin, M. R., Low, A., & Rabat, N. E. (2020). A review on recent developments in the adsorption of surfactants from wastewater. Journal of Environmental Management, 254. https://doi.org/10.1016/j.jenvman.2019.109797.
Soloman, P. A., Ahmed Basha, C., Velan, M., Balasubramanian, N., & Marimuthu, P. (2009). Augmentation of biodegradability of pulp and paper industry wastewater by electrochemical pre-treatment and optimization by RSM. Separation and Purification Technology, 69, 109–117. https://doi.org/10.1016/j.seppur.2009.07.002.
Sridhar, R., Sivakumar, V., & Thirugnanasambandham, K. (2015). Response surface modeling and optimization of upflow anaerobic sludge blanket reactor process parameters for the treatment of bagasse based pulp and paper industry wastewater. Desalination and Water Treatment, 57, 1–12. https://doi.org/10.1080/19443994.2014.999712.
Saurabh D., Rutuja D., & Eswara P. (2023). Surfactants Market by Type?: Global Opportunity Analysis and Industry Forecast, 2020-2027. https://www.alliedmarketresearch.com/surfactant-market.
Wei, N., Zhang, Z., Liu, D., Wu, Y., Wang, J., & Wang, Q. (2015). Coagulation behavior of polyaluminum chloride: Effects of pH and coagulant dosage. Chinese Journal of Chemical Engineering, 23, 1041–1046. https://doi.org/10.1016/j.cjche.2015.02.003.
Wong, S. S., Teng, T. T., Ahmad, A. L., Zuhairi, A., & Najafpour, G. (2006). Treatment of pulp and paper mill wastewater by polyacrylamide (PAM) in polymer induced flocculation. Journal of Hazardous Materials, 135, 378–388. https://doi.org/10.1016/j.jhazmat.2005.11.076.
Yongabi, K. (2010). Biocoagulants for water and waste water purification: A review. International Review of Chemical Engineering, 2, 444–458.
Yuan, C. L., Xu, Z. Z., Fan, M. X., Liu, H. Y., Xie, Y. H., & Zhu, T. (2014). Study on characteristics and harm of surfactants. Journal of Chemical and Pharmaceutical Research, 6, 2233–2237.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harif, S., Aboulhassan, M.A., Bammou, L. et al. Improving the Removal of Anionic Surfactant in Cardboard Industry Wastewater by Coagulation/Flocculation: Process Optimization with Response Surface Methodology. Water Air Soil Pollut 234, 477 (2023). https://doi.org/10.1007/s11270-023-06435-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06435-0