Abstract
Current environmental trends show an increase in pollutant concentrations in water bodies. The alarming concern this poses has made it crucial to remove pollutants from water and eliminate them, owing to the host of issues they cause to the ecosystem. While sorption is a popular method of treating wastewater, there are several concerns regarding its accessibility, affordability, efficiency, and functioning toward a circular sustainable economy. The current study focuses on the feasibility of using sugarcane bagasse (SCB) to remove oil and diesel from water and proposes to do so without turning SCB into biochar, contrary to the usual method. Raw SCB was chemically treated using sodium hydroxide and acetic acid. With little pre-treatment, it was found to have improved sorption abilities with low input materials and environmental toxicity. Sorption experiments conducted on treated and untreated SCB showed the effects of different parameters on the oil or diesel removal efficiency. Furthermore, FTIR, BET, and SEM studies were conducted to characterize the intrinsic morphology and structure of the sorbent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In present times of increasing environmental pollution, an effective method to remove contaminants is sorption due to its versatility, competitive performance, lack of sludge generation, and easy application in treatment systems with high effluent flow rates (Mohamed et al., 2022). Biosorption has emerged as a promising technique to resolve the problem of several pollutants, oil pollutants being one of them. The use of sorbents for the rectification of oil pollution has attracted considerable attention in recent decades and has proven to be both economical and efficient. Various sorbents, such as wheat bran (Farajzadeh & Monji, 2004), rice husk (Ajmal et al., 2003), vegetable and fruit waste (Liu et al., 2021), sugarcane bagasse, soya beans (Raziq Rahimi Kooh et al., 2015), straw (Liu et al., 2020), peat (Allen et al., 2004), biomass (Bell & Tsezos, 1987), algae (Roy et al., 2008), coconut shells (Islam et al., 2016), tree bark (Ighalo & Adeniyi, 2020), almond shells (Maaloul et al., 2017), eggshells (Tsai et al., 2006), tea waste (Amarasinghe & Williams, 2007), sawdust (Bulut & Tez, 2007), and more (Mathew et al., 2016) have been developed over the years and have shown varying degrees of success, as shown in Table 1.
The use of these sorbents provides solutions to various other problems, such as heavy metal removal, industrial waste absorption, atmospheric pollutant removal, and removal of pesticides and fertilizers from the environment (Singh et al., 2020). As such, there is an immediate need to further take up this area of study to develop a better understanding of different sorbents. Currently, the focus is on the development of low-cost yet effective adsorbents from different biological sources, among which sugarcane bagasse (SCB) has been widely studied.
SCB is a popular adsorbent that is used in several industrial applications (Karnitz et al., 2007). Sugarcane (Saccharum officinarum) is widely cultivated as a tropical plant species in several countries including Brazil, China, India, Mexico, and South Africa which form a large proportion of the sugar industries in the world. India is the second-largest producer of sugarcane after Brazil, with an average productivity of 79.68 tonnes/ha per annum (Hiloidhari et al., 2018). Unlike non-tropical countries, India also does not face the problem of sugarcane being a seasonal crop, as it is grown throughout the year in various parts of the country, excluding a couple of months (Status Paper on Sugarcane, 2013). After the sugar has been extracted from crop sugarcane, the bagasse waste product is discarded or utilized for heat production. However, the incineration of bagasse for fuel enables the emission of carbon dioxide and, hence, global warming. SCB also faces problems of having a hygroscopic nature, high moisture (> 50%), and low density, giving it a low ash yield (Chen et al., 2021). Hence, to minimize these effects while utilizing bagasse for maximum usability, other applications must be developed. In this study, SCB was investigated as a sorbent source.
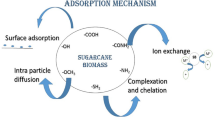
SCB has high mechanical strength and contains several hydroxyl and carboxyl groups, giving it the potential to increase sorption through ion exchange and complexation (Gong et al., 2022a). SCB has thus been used in various forms, such as aerogels (Kumar et al., 2021; Thai et al., 2020), concrete and cement (Quedou et al., 2021; Saad Agwa et al., 2022), and building materials (Araújo de Almeida & Colombo, 2021; Mehrzad et al., 2022). For sorption, sugarcane fly ash and biochar have been most popularly used due to their porosity (Wong et al., 2020) and high surface area (Freitas & Farinas, 2017), as seen in some studies (Alvarenga & Correa, 2021; Oliveira et al., 2019). However, it has been noted that the thermal conversion process needed to develop this biochar is usually high temperatures ranging from 300 to 1000 °C. Despite this, biochar yield is usually ≤ 50% in most studies (Iwuozor et al., 2022). A lot of the remaining residue is wasted, and thus, we have proposed using it in its natural form. Sugarcane also has a high retention power and low implantation costs, and using it in its solid natural form provides a sustainable solution to both the solid residue produced by the sugarcane industry, as well as a cheap alternative to other sorbents (Boni et al., 2016). Studies have found that SCB consists of approximately 42% cellulose, 25% hemicellulose, and 20% lignin (Ezeonuegbu et al., 2021). Among these, lignin confers a hydrophobic nature (along with thermal stability) onto the sorbent that enables good sorptive capacity toward oil (Zhang et al., 2020), while hemicellulose and cellulose form the hydrophilic structures of SCB. Lignin also acts as a barrier between the oil and hemicellulose components and is both accessible and functional owing to its mechanical resistance (Suteu et al., 2010). The sorption capacity of SCB depends chiefly on the fractions of the structures present in it, and the effect of chemical treatment has been investigated.
The treatment of SCB using sodium hydroxide (NaOH) is a popular method of removing lignin by cleaving ester and ether bonds in the lignin carbohydrate complex. It is also one of the most economical alkalis available for strong treatment (Chang et al., 2017). However, while NaOH treatment and delignification have been widely studied for saccharification processes, their effect on sorption has not been studied. Dilute acid removes hemicelluloses and cellulose by acid hydrolysis (Yu et al., 2013). Acetic acid, in particular, eliminates the problem of corrosion and degradation of products shown by other acids. These pre-treatment strategies provide different results based on their interaction with the functional groups of SCB and have hence been investigated in this study.
The vegetable oil chosen for the study was sunflower oil, as it is one of the most widely used vegetable oils in the world, particularly in India. As such, it forms a major oil constituent in most kitchen wastewater and has therefore been studied to understand its removal.
2 Materials and Methods
2.1 Materials
Raw sugarcane bagasse (RSCB) used in the study was procured from a local vendor in Manipal, India. Emulsion samples were prepared using regular water sources, store brought sunflower oil, and diesel were from a local petrol station. Gum Arabic (Gum acacia or Gum sudani) was used as an emulsifier and was obtained from Merck, Germany, while sodium hydroxide and acetic acid were procured from Taloja, India.
2.2 Preparation of Sorbent Raw Sugarcane Bagasse
The soluble sugar and other impurities present in the bagasse were washed off by boiling, and then the RSCB was steamed for four hours. The bagasse was then soaked in water overnight, strained, and then dried under the sun. The dry RSCB was then ground into powder of three different sizes (size 1: > 1.18 mm; size 2: 0.212–1.18 mm; size 3: < 0.212 mm) and kept away for further use.
2.3 Preparation of Treated Sugarcane Bagasse
RSCB was separately treated with sodium hydroxide (NaOH) and acetic acid (CH3COOH) of strength 0.1 N each to form sodium hydroxide-treated SCB (SSCB) and acetic-acid-treated SCB (ASCB). For the treatment, RSCB was separately soaked in NaOH or acetic acid overnight (Fig. 1), followed by washing with boiled water, cooling, and drying. Upon soaking, it was observed that sodium hydroxide caused a darker color in the SCB, whereas acetic acid caused no such visual change. The dry bagasse was then ground into powder of three different sizes (size 1: 1.18–2.00 mm; size 2: 0.212–1.18 mm; size 3: 0.212 mm–pan) and kept away for further use.
2.4 Preparation of Oil-in-Water and Diesel-in-Water Emulsions
To prepare emulsions, 0.04 g of Gum Arabic was used as an emulsifier in a 100 mL mixture of vegetable oil or diesel (as required) and water. An Incubator Shaker (Lead Instruments® Ltd., Bangalore, India) was used at different temperatures and agitation speeds to form emulsions under different conditions.
2.5 Sorption Using Sugarcane Bagasse
The sorption behavior of three types of bagasse (RSCB, SSCB, and ASCB) was studied by conducting the sorption of oil or diesel from the emulsion in an incubator shaker. Experiments were conducted considering various temperatures, agitation speeds, contact times, initial oil (or diesel) concentrations, and adsorbent dosages. Upon completion of sorption, the solution was passed through a filter paper to separate the adsorbent from the solution.
The removal efficiency of the sorption process was then calculated by measuring the absorbance before and after sorption using an Ultraviolet Visible spectrometer (UV-1800, Shimadzu, Japan) with wavelengths of 276 nm (for oil) and 295 nm (for diesel) using Eq. (1).
2.6 Characterization of Sugarcane Bagasse Using SEM, FTIR, and BET Analysis
The structure of the SCB was analyzed using Scanning Electron Microscopy (SEM, ZEISS), the functional groups were analyzed using Fourier transform infrared spectroscopy (FTIR, Shimadzu – 8400S), and the surface area was analyzed using a Brunauer–Emmett–Teller (BET) Surface Area Analyzer (Smart Instruments Co. Pvt. Ltd.).
A summary of the methodology is provided in the flowchart in Fig. 2.
3 Results and Discussion
3.1 Calibration of Emulsion
Solutions of the prepared emulsions in water were made in 0%, 20%, 40%, 60%, 80%, and 100% emulsion concentrations. The absorbance was then measured using an Ultraviolet Visible spectrometer, and a calibration chart was plotted for both oil and diesel emulsions. The calibration equations are shown in the calibration charts in Fig. 3.
3.2 Sorption Experiments of Oil and Diesel Using Sugarcane Bagasse
Sorption experiments were conducted on the emulsions with one varying parameter, keeping all other parameters constant. The incubator shaker used was set to a general value of 30 ℃ temperature, 180 RPM agitation speed, and 15 min contact time, and the emulsions were of 0.5% initial oil (or diesel) concentration with adsorbent of size 2 used for sorption. The effects of these parameters on the percentage of oil (or diesel) removed were observed.
The mechanism of oil/diesel sorption involves the following steps: diffusion of molecules on the surface of the sorbent, capillary action resulting in the entrapment of molecules in the sorbent structure, and agglomeration of the droplets in the rough and porous structures of the sorbent (Tansel & Pascual, 2011).
3.2.1 Effect of Sorbent Size
The size of the adsorbent is an important factor in the sorption process. As seen in Fig. 4a, the oil removal and, subsequently, the adsorptive properties of the RSCB were found to be better with the decrease in particle size. This was due to the adsorbent surface area increasing with its declining particle size, which proportionally increased the sorption rates (Al-Ghouti & Al-Absi, 2020; Nuria Fiol, 2013; Senthil Kumar, 2015).
However, upon chemical treatment of the SCB, as seen in Fig. 4b,c, the oil removal varies with size such that the larger particles show better or almost equal removal efficiency than the smaller ones. Other authors have also reported the same (Jin et al., 2022; Suresh Kumar et al., 2019) and have validated it through kinetic studies and structural analysis.
In the case of the diesel-in-water emulsion, RSCB showed the best removal efficiency with medium-sized particles, as shown in Fig. 5a. Upon treatment, Fig. 5b,c shows opposite trends, where NaOH confers larger particles with better removal efficiency, while acetic acid exhibits the opposite trend. The increased efficiency for smaller sizes of ASCB can be attributed to the larger surface area, which decreases as the adsorbent size increases. However, in the case of the SSCB, assuming that sorption occurs through the pores of the particles, the increase in diesel removal as the adsorbent size increases may be due to the increase in the intrinsic pore size of the adsorbent (Anggono et al., 2019; Goh et al., 2014).
3.2.2 Effect of Temperature
Sorption is a temperature-dependent process, and SCB of all types was found to exhibit the same trend for the same type of emulsion, as shown in Fig. 6. For oil, at low temperatures, sorption occurs through physisorption only, through weak Van der Waal’s forces. With an increase in temperature, this force decreased, causing a decrease in the removal of oil (Bello & Ahmad, 2011). After a certain point, the oil molecules reach the activation energy for chemisorption, due to which it takes over the sorption process. This is because of the chemical interactions between the oil molecules and the adsorbent surface. An increase in temperature also reduces the viscosity of the oil. An increase in the temperature may also increase the porosity and total pore volume, and the interior cell structure of the adsorbent material to stretch, making it easier for larger oil molecules to adsorb (Mishra & Mukherji, 2012). Furthermore, while this trend remains the same across all SCBs, ASCB shows the least minimum point and reaches it much later than the other two, making it more suitable for the removal of oil.
In the case of diesel removal, it was observed that as the temperature increased, sorption first increased to a maximum and then decreased. This can be due to the increasing temperature causing more sorption sites to open up, increasing the removal efficiency. However, once equilibrium was reached and all sorption sites were saturated, the rate of desorption exceeded the rate of sorption owing to the higher kinetic energy of the diesel molecules (Belhaj et al., 2020), leading to a decrease in diesel removal. This trend continues for all types of SCB; however, it is observed that SSCB reaches a higher peak than the others, making it better suited for diesel removal.
3.2.3 Effect of Agitation Speed
The graphical data presented in Fig. 7a show that in the case of RSCB, the pollutant removal efficiency increased as the agitation speed increased. This is due to the increased diffusion rate of the oil molecules into the adsorbent, which was caused by the reduced liquid-surface boundary layer at increased turbulence. However, this reached a maximum point, after which an overall decrease was observed upon increasing the agitation speed. Similar sorption observations have been reported previously (Jamil et al., 2011; Omri et al., 2016). As suggested by Kuśmierek and Świątkowski (2015), this could be because, at very high agitation speeds, particles of both the adsorbate and the adsorbent attain sufficient kinetic energy to collide with each other, causing detachment of the loosely bound adsorbate molecules.
A similar trend is observed in the case of diesel, as shown in Fig. 7b, where the initial decrease may be attributed to the lack of driving force until a certain point, after which the agitation speed enables an increase in sorption followed by a decrease, as seen in the case of oil.
3.2.4 Effect of Contact Time
The contact time in sorption can be used to determine the optimum time for the process to be carried out to achieve maximum sorption efficiency. As seen in Fig. 8a, the oil removal percentage initially increased with time due to de-emulsification and increased contact of oil molecules with the adsorbent surface which aided the penetration of oil into its pores. However, this was observed only until a certain point, after which oil removal was found to decrease. Once equilibrium is attained, the rate of desorption overcomes the rate at which sorption takes place and causes the oil molecules that had been previously adsorbed to exit the pores of the adsorbent (Olufemi & Otolorin, 2017). A similar trend is observed in Fig. 8b with the removal of diesel.
In the case of oil removal, it is observed that the SSCB has the lowest peak among the three types of SCB. Furthermore, in the case of ASCB, it is also seen that the peak is attained much faster due to acid hydrolysis (Canilha et al., 2011; Zhao et al., 2018).
3.2.5 Effect of Sorbent Dosage
The amount of adsorbent used for the sorption process played a key role in planning the entire process. The maximum removal at the minimum adsorbent dosage is a vital parameter that needs to be considered for the economic and effective removal of oil.
As shown in Fig. 9a, at small concentrations of the adsorbent, for the same initial oil concentration, the removal of oil was observed to increase with the increased availability of active sites (S. S. D. Elanchezhiyan & Meenakshi, 2016). ASCB attains this maximum peak at the lowest dose, owing to the hydrolysis of hemicelluloses enabling a super oleophilic nature compared to RSCB or SSCB (Neureiter et al., 2002). However, this decreased with an increase in adsorbent dosage. This may be due to the formation of adsorbent aggregates at higher doses, which reduces the number of available sites for the sorption process. A similar trend was observed in Fig. 9b for the removal of diesel. While the same reasons can be applied, it is also observed that there is a repetitive trend of increase and decrease in oil removal with the adsorbent dosage. This may be explained by kinetic studies performed to understand the aggregation of the adsorbent molecules, as reported by a few authors (Becker et al., 1990).
3.2.6 Effect of Initial Sorbate Concentration
From Fig. 10a, it is seen that the oil removal is initially observed to increase with an increase in the initial oil concentration for the same amount of adsorbent. This is due to the availability of unoccupied active sites which is much higher than the amount of oil introduced. However, at higher initial oil concentrations, the removal of oil decreases because of the lack of available sites for the oil to adsorb (Kerrou et al., 2021).
A similar trend was observed in the case of diesel, as shown in Fig. 10b. In both cases, the treated SCB shows more or nearly equal removal percentage to that of RSCB; however, it does not have a huge variation effect on the same.
3.2.7 Effect of pH
The emulsions were prepared using water that was slightly acidified using a dil. HCl or basified with dil. NaOH solution. The emulsion pH values under acidic, neutral, and basic conditions were 2.13, 6.34, and 10.46. This effect was then computed, as shown in Fig. 11.
As shown in Fig. 11a, around the neutral pH value, the removal of oil was found to be quite high, whereas a decrease in the pH increased the oil removal. This can be attributed to a layer of positive charge formed over the surface of the bagasse which helped attract the negatively charged ends of the oil molecules, resulting in the de-emulsification of the solution and thus facilitating the sorption process. However, even a large decrease in pH did not significantly increase the removal of oil and manual acidification of the emulsion was not necessary (S. S. Elanchezhiyan et al., 2018a, 2018b). Under alkaline conditions obtained by the addition of NaOH solution to the emulsion, the surface charge of the adsorbents became negative owing to the hydroxyl group present in the aqueous medium, which could be explained by the electrostatic repulsive force and lower degree of de-emulsification (S. S. D. Elanchezhiyan et al., 2018a, 2018b). In addition, NaOH can also react with the oil present in the emulsion, resulting in a saponification reaction, thus decreasing the amount of oil available for sorption (Boni et al., 2016).
In the case of diesel, as seen in Fig. 11b, increasing the pH first decreases the removal percentage and begins to increase after crossing the neutral pH. Diesel is a mildly acidic solution and hence, when brought into an acidic environment, shows repulsion away from the emulsion solution into the adsorbate surface. In the case of basic solutions, there is a negative surface charge on the adsorbent surface, which may repel some of the positively charged diesel molecules, causing reduced diesel removal as compared to that of an acidic medium.
3.3 Fourier Transform Infrared Spectroscopy
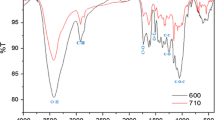
FTIR spectroscopy was employed to understand the functional group composition of SCB and was performed for RSCB, SSCB, and ASCB to check for any compositional changes after the chemical treatment of SCB. Previous studies have indicated that sorption is strongly related to the nature of the functional group properties of the system (Abdullah et al., 2010; Wang et al., 2012). In the case of oil/diesel removal, this was further confirmed to be the O–H, C = O, and C-O functional groups (Wahi et al., 2013), which was also confirmed in this study. Overall, it was observed that while the transmittance bands changed, the functional groups do not change after chemical treatment. The band at wavenumbers 4000–2500 cm−1 (as shown in the green box in Fig. 12) in both spectra indicates the presence of C-H and O–H functional groups, whereas the band at wavenumbers 2000–1500 cm−1 (highlighted in the yellow box in Fig. 12) indicates the presence of C = O and C = C functional groups. A higher transmittance at the same wavenumber indicates an increase in the number of functional groups. The presence of C = O can also be seen at a peak just above 1000 cm−1.
3.4 Scanning Electron Microscopy Imaging
The surface morphology of SCB was characterized using SEM. The images obtained from the RSCB are shown in Fig. 13.
The SEM images in Fig. 13a,b show a folded layered structure of the fibrous SCB, which enables an increase in the specific surface area, thereby increasing the area for sorption (Gong et al., 2022b). Furthermore, Fig. 13b depicts the pore distribution in the SCB, which acts as the site of sorption. The pores are of different sizes, and because of the irregular nature of SCB, they are distributed such that the areas of sorption are increased.
Upon comparing the different types of SCB before and after sorption, Fig. 14 was obtained from the SEM analysis. The SEM images shown in Fig. 14 can be used to compare the surface morphologies of the SCB before and after sorption. In general, we see that chemical treatment of SCB has resulted in the treated SCB having a more wrinkled and layered structure than RSCB. It was also observed that the pores were smaller in the treated SCB than in the RSCB.
From Fig. 14e, it is observed that the pores of the SSCB are irregularly shaped and farther apart than those of the RSCB in Fig. 14a. A similar visual is also seen in the case of ASCB, with the additional observation of smaller pores (Fig. 14e) than that of RSCB (Fig. 14a). Both visuals also showed a marked difference in texture post-treatment, depicting the changes in composition. The raised surfaces and decreased roughness in the case of SSCB have been noted by other researchers as well (Carvalho et al., 2016; Fu et al., 2022) and are indicative of better access to cellulose and hemicelluloses. In contrast, ASCB images show more roughness than the RSCB pre-sorption images. This is in line with other studies on acid pre-treatment of SCB (Sindhu et al., 2010) and is likely indicative of acid hydrolysis being able to remove any external fibers and hemicellulose while increasing the surface area. After sorption, a marked difference in sorption is seen in SSCB and ASCB, as shown in Fig. 14d,f, respectively, in comparison to RSCB. RSCB can retain the droplets owing to its larger pores, whereas, in both SSCB and ASCB, the adsorbate retention is patchy.
3.5 Brunauer–Emmett–Teller Surface Area Analysis
Upon conducting BET surface area analysis for the three types of SCB, the specific surface areas were obtained for size 2 RSCB, SSCB, and ASCB, as shown in Table 2.
The above results show an improvement in the surface area upon treatment with either NaOH or acetic acid. This is in accordance with an increase in the pore diameter as the reagents interact with the functional groups of the SCB. However, the obtained results are lower than those reported in the literature, despite agreeing with the values obtained in other studies (da Penha Bezerra et al., 2022; Priyanto, 2021). This may be attributed to the lack of activation of SCB by the treatment methods used, resulting in a lower surface area for gas sorption. Similar results for the specific surface area were obtained by Harripersadth et al. (2020) using SCB. The International Union of Pure and Applied Chemistry (IUPAC) convention was used to classify porous materials into three groups: micropore (pore diameter < 2 nm), mesopore (pore diameter: 2–50 nm), and macropore (pore diameter > 50 nm). The SCBs are mesoporous material.
4 Discussion and Future Implications
From the above experiments and results, the potential of SCB as a sorbent is highlighted. In the past decade, studies conducted using SCB have yielded varying results (Ali et al., 2011; Boni et al., 2016; Brandão et al., 2010). In contrast, despite not making use of sugarcane biochar (Tomazini da Conceição et al., 2022), our study has shown that even RSCB can provide a sorption efficiency averaging around 70–80%. This opens up possible investigations of SCB used as a sorbent in its raw form without activation as biochar, thus reducing the problem of equipment and capital reduction.
While the study has considered individual parameters for understanding the sorption abilities of SCB, it is always interactive effects that take place in real-life situations. Thus, the present study may be used as a precursor before modeling the interactive effects between the parameters to develop a system that can ascertain the operational parameters for optimum functioning both technically and economically.
Furthermore, while we did not attempt doping the sorbent to activate any functional groups, the characterization studies conducted may be used to understand the elements that may be used to enhance the sorbent capacity owing to these functional groups. Studies have shown that sorbent characteristics changes upon activation by certain compounds, which could potentially be investigated in this case as well (Bianchi et al., 2022; Kayvani Fard et al., 2016; Kerrou et al., 2021; Kurniawan et al., 2019).
5 Conclusion
In this study, sugarcane bagasse was found to be an effective, low-cost adsorbent without being converted into biochar. Following the treatment methods used, it was found that even with minimal chemical treatment, good sorption results were obtained across various individual parameters. The resulting removal process does not require sophisticated equipment or extreme process conditions, making the process accessible to anyone and everyone who may deal with sugarcane waste. The findings of this study can be expanded to better develop and optimize the sorption process for different pollutants at various scales. As only individual effects were studied in this paper, a key factor to be studied in the future is the interaction effects among the considered parameters. Furthermore, while the FTIR studies enabled an understanding of the number of functional groups changing upon treatment, the nature of the groups themselves is unknown. To increase the pollutant removal efficiency, better characterization of these functional groups may be conducted, followed by their introduction into the sorbent. Along the same lines, an improvement in the specific surface area obtained from BET would enable more sorption for lower amounts of adsorbent, which may be possible by activating bagasse. However, care needs to be taken to keep the activation process green and sustainable to minimize the negative environment it may cause.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Abdullah, M. A., Rahmah, A. U., & Man, Z. (2010). Physicochemical and sorption characteristics of Malaysian Ceiba pentandra (L.) Gaertn. as a natural oil sorbent. Journal of Hazardous Materials, 177(1–3), 683–691. https://doi.org/10.1016/J.JHAZMAT.2009.12.085
Ajmal, M., Rao, R. A. K., Anwar, S., Ahmad, J., & Ahmad, R. (2003). Adsorption studies on rice husk: Removal and recovery of Cd(II) from wastewater. Bioresource Technology, 86(2), 147–149. https://doi.org/10.1016/S0960-8524(02)00159-1
Alaa El-Din, G., Amer, A. A., Malsh, G., & Hussein, M. (2018). Study on the use of banana peels for oil spill removal. Alexandria Engineering Journal, 57(3), 2061–2068. https://doi.org/10.1016/J.AEJ.2017.05.020
Al-Ghouti, M. A., & Al-Absi, R. S. (2020). Mechanistic understanding of the adsorption and thermodynamic aspects of cationic methylene blue dye onto cellulosic olive stones biomass from wastewater. Scientific Reports, 10(1), 15928. https://doi.org/10.1038/s41598-020-72996-3
Ali, N., El-Harbawi, M., Jabal, A. A., & Yin, C. Y. (2011). Characteristics and oil sorption effectiveness of kapok fibre, sugarcane bagasse and rice husks: Oil removal suitability matrix. Environmental Technology, 33(4), 481–486. https://doi.org/10.1080/09593330.2011.579185
Allen, S. J., Mckay, G., & Porter, J. F. (2004). Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. Journal of Colloid and Interface Science, 280(2), 322–333. https://doi.org/10.1016/J.JCIS.2004.08.078
Alvarenga, A. D., & Correa, D. S. (2021). Composite nanofibers membranes produced by solution blow spinning modified with CO2-activated sugarcane bagasse fly ash for efficient removal of water pollutants. Journal of Cleaner Production, 285, 125376. https://doi.org/10.1016/J.JCLEPRO.2020.125376
Amarasinghe, B. M. W. P. K., & Williams, R. A. (2007). Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chemical Engineering Journal, 132(1–3), 299–309. https://doi.org/10.1016/J.CEJ.2007.01.016
Anggono, J., Purwaningsih, H., Sugondo, S., Henrico, S., Sewucipto, S., & Patel, J. (2019). Structural evaluation on sugarcane bagasse treated using sodium and calcium hydroxide. E3S Web of Conferences, 130, 01018. https://doi.org/10.1051/e3sconf/201913001018
Araújo de Almeida, M., & Colombo, R. (2021). Construction of green roofs via using the substrates made from humus and green coconut fiber or sugarcane bagasse. Sustainable Chemistry and Pharmacy, 22, 100477. https://doi.org/10.1016/J.SCP.2021.100477
Becker, O. M., Silverberg, M., & Ben-Shaul, A. (1990). Kinetically controlled aggregation in reactive adsorbate overlayers. Israel Journal of Chemistry, 30(1–2), 179–188. https://doi.org/10.1002/IJCH.199000017
Belhaj, A. F., Elraies, K. A., Mahmood, S. M., Zulkifli, N. N., Akbari, S., & Hussien, O. S. E. (2020). The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: A review. Journal of Petroleum Exploration and Production Technology, 10(1), 125–137. https://doi.org/10.1007/S13202-019-0685-Y
Bell, J. P., & Tsezos, M. (1987). Removal of hazardous organic pollutants by biomass adsorption. Water Pollution Control Federation, 59(4), 191–198. http://www.jstor.org/stable/25043223
Bello, O. S., & Ahmad, M. A. (2011). Adsorptive removal of a synthetic textile dye using cocoa pod husks. Toxicological and Environmental Chemistry, 93(7), 1298–1308. https://doi.org/10.1080/02772248.2011.590490
Bianchi, C. L., Djellabi, R., della Pina, C., & Falletta, E. (2022). Doped-polyaniline based sorbents for the simultaneous removal of heavy metals and dyes from water: Unravelling the role of synthesis method and doping agent. Chemosphere, 286, 131941. https://doi.org/10.1016/J.CHEMOSPHERE.2021.131941
Boni, H. T., de Oliveira, D., Ulson de Souza, A. A., & Ulson de Souza, S. M. A. G. (2016). Bioadsorption by sugarcane bagasse for the reduction in oil and grease content in aqueous effluent. International Journal of Environmental Science and Technology, 13(4), 1169–1176. https://doi.org/10.1007/S13762-016-0962-Y
Brandão, P. C., Souza, T. C., Ferreira, C. A., Hori, C. E., & Romanielo, L. L. (2010). Removal of petroleum hydrocarbons from aqueous solution using sugarcane bagasse as adsorbent. Journal of Hazardous Materials, 175(1–3), 1106–1112. https://doi.org/10.1016/J.JHAZMAT.2009.10.060
Bulut, Y., & Tez, Z. (2007). Removal of heavy metals from aqueous solution by sawdust adsorption. Journal of Environmental Sciences, 19(2), 160–166. https://doi.org/10.1016/S1001-0742(07)60026-6
Canilha, L., Santos, V. T. O., Rocha, G. J. M., Almeida E Silva, J. B., Giulietti, M., Silva, S. S., Felipe, M. G. A., Ferraz, A., Milagres, A. M. F., & Carvalho, W. (2011). A study on the pretreatment of a sugarcane bagasse sample with dilute sulfuric acid. Journal of Industrial Microbiology and Biotechnology, 38(9), 1467–1475. https://doi.org/10.1007/s10295-010-0931-2
da Penha Bezerra, W. F., Dognani, G., de Alencar, L. N., Parizi, M. P. S., Boina, R. F., Cabrera, F. C., & Job, A. E. (2022). Chemical treatment of sugarcane bagasse and its influence on glyphosate adsorption. Matéria (Rio de Janeiro), 27(1), e13142. https://doi.org/10.1590/s1517-707620220001.1342
de Carvalho, D. M., de Queiroz, J. H., & Colodette, J. L. (2016). Assessment of alkaline pretreatment for the production of bioethanol from eucalyptus, sugarcane bagasse and sugarcane straw. Industrial Crops and Products, 94, 932–941. https://doi.org/10.1016/J.INDCROP.2016.09.069
Chang, M., Li, D., Wang, W., Chen, D., Zhang, Y., Hu, H., & Ye, X. (2017). Comparison of sodium hydroxide and calcium hydroxide pretreatments on the enzymatic hydrolysis and lignin recovery of sugarcane bagasse. Bioresource Technology, 244, 1055–1058. https://doi.org/10.1016/J.BIORTECH.2017.08.101
Chen, X., Liang, J., Liao, P., Huang, W., He, J., & Chen, J. (2021). Effect of process parameters and raw material characteristics on the physical and mechanical quality of sugarcane bagasse pellets. Biomass and Bioenergy, 154, 106242. https://doi.org/10.1016/J.BIOMBIOE.2021.106242
Elanchezhiyan, S. S. D., & Meenakshi, S. (2016). Facile synthesis of metal incorporated chitin for the recovery of oil from oil-in-water emulsion using adsorptive method. Journal of Cleaner Production, 139, 1339–1350. https://doi.org/10.1016/j.jclepro.2016.08.119
Elanchezhiyan, S. S. D., Muthu Prabhu, S., & Meenakshi, S. (2018a). Treatment of emulsified oil using biopolymer assisted materials. Polymer Composites, 39, E261–E270. https://doi.org/10.1002/pc.24103
Elanchezhiyan, S. S., Prabhu, S. M., & Meenakshi, S. (2018b). Effective adsorption of oil droplets from oil-in-water emulsion using metal ions encapsulated biopolymers: Role of metal ions and their mechanism in oil removal. International Journal of Biological Macromolecules, 112, 294–305. https://doi.org/10.1016/j.ijbiomac.2018.01.118
Ezeonuegbu, B. A., Machido, D. A., Whong, C. M. Z., Japhet, W. S., Alexiou, A., Elazab, S. T., Qusty, N., Yaro, C. A., & Batiha, G. E. S. (2021). Agricultural waste of sugarcane bagasse as efficient adsorbent for lead and nickel removal from untreated wastewater: Biosorption, equilibrium isotherms, kinetics and desorption studies. Biotechnology Reports, 30, e00614. https://doi.org/10.1016/J.BTRE.2021.E00614
Farajzadeh, M. A., & Monji, A. B. (2004). Adsorption characteristics of wheat bran towards heavy metal cations. Separation and Purification Technology, 38(3), 197–207. https://doi.org/10.1016/J.SEPPUR.2003.11.005
Freitas, J. V., & Farinas, C. S. (2017). Sugarcane bagasse fly ash as a No-Cost adsorbent for removal of phenolic inhibitors and improvement of biomass saccharification. ACS Sustainable Chemistry and Engineering, 5(12), 11727–11736. https://doi.org/10.1021/ACSSUSCHEMENG.7B03214
Fu, Y., Gao, H., Yu, H., Yang, Q., Peng, H., Liu, P., Li, Y., Hu, Z., Zhang, R., Li, J., Qi, Z., Wang, L., Peng, L., & Wang, Y. (2022). Specific lignin and cellulose depolymerization of sugarcane bagasse for maximum bioethanol production under optimal chemical fertilizer pretreatment with hemicellulose retention and liquid recycling. Renewable Energy, 200, 1371–1381. https://doi.org/10.1016/J.RENENE.2022.10.049
Goh, E. G., Xu, X., & McCormick, P. G. (2014). Effect of particle size on the UV absorbance of zinc oxide nanoparticles. Scripta Materialia, 78–79, 49–52. https://doi.org/10.1016/J.SCRIPTAMAT.2014.01.033
Gong, X. L., Lu, H. Q., Li, K., & Li, W. (2022a). Effective adsorption of crystal violet dye on sugarcane bagasse–bentonite/sodium alginate composite aerogel: Characterisation, experiments, and advanced modelling. Separation and Purification Technology, 286, 120478. https://doi.org/10.1016/J.SEPPUR.2022.120478
Gong, X. L., Lu, H. Q., Li, K., & Li, W. (2022b). Effective adsorption of crystal violet dye on sugarcane bagasse–bentonite/sodium alginate composite aerogel: Characterisation, experiments, and advanced modelling. Separation and Purification Technology, 286, 120478. https://doi.org/10.1016/J.SEPPUR.2022.120478
Harripersadth, C., Musonge, P., Makarfi Isa, Y., Morales, M. G., & Sayago, A. (2020). The application of eggshells and sugarcane bagasse as potential biomaterials in the removal of heavy metals from aqueous solutions. South African Journal of Chemical Engineering, 34, 142–150. https://doi.org/10.1016/j.sajce.2020.08.002
Hiloidhari, M., Araújo, K., Kumari, S., Baruah, D. C., Ramachandra, T. V., Kataki, R., & Thakur, I. S. (2018). Bioelectricity from sugarcane bagasse co-generation in India-An assessment of resource potential, policies and market mobilization opportunities for the case of Uttar Pradesh. Journal of Cleaner Production, 182, 1012–1023. https://doi.org/10.1016/J.JCLEPRO.2018.02.087
Ibrahim, S., Wang, S., & Ang, H. M. (2010). Removal of emulsified oil from oily wastewater using agricultural waste barley straw. Biochemical Engineering Journal, 49(1), 78–83. https://doi.org/10.1016/J.BEJ.2009.11.013
Ifelebuegu, A. O., Anh Nguyen, T. V., Ukotije-Ikwut, P., & Momoh, Z. (2015). Liquid-phase sorption characteristics of human hair as a natural oil spill sorbent. Journal of Environmental Chemical Engineering, 3(2), 938–943. https://doi.org/10.1016/J.JECE.2015.02.015
Ighalo, J. O., & Adeniyi, A. G. (2020). Adsorption of pollutants by plant bark derived adsorbents: An empirical review. Journal of Water Process Engineering, 35, 101228. https://doi.org/10.1016/J.JWPE.2020.101228
Islam, M. S., Ang, B. C., Gharehkhani, S., & Afifi, A. B. M. (2016). Adsorption capability of activated carbon synthesized from coconut shell. Carbon Letters, 20(1), 1–9. https://doi.org/10.5714/CL.2016.20.001
Iwuozor, K. O., Chizitere Emenike, E., Ighalo, J. O., Omoarukhe, F. O., Omuku, P. E., & George Adeniyi, A. (2022). A Review on the thermochemical conversion of sugarcane bagasse into biochar. Cleaner Materials, 6, 100162. https://doi.org/10.1016/J.CLEMA.2022.100162
Jamil, N., Ahsan, N., Munawar, M. A., Anwar, J., & Shafique, U. (2011). Removal of toxic dichlorophenol from water by sorption with chemically activated carbon of almond shells-A green approach. Journal of the Chemical Society of Pakistan, 33(5), 640.
Jin, Z., Xiao, S., Dong, H., Xiao, J., Tian, R., Chen, J., Li, Y., & Li, L. (2022). Adsorption and catalytic degradation of organic contaminants by biochar: Overlooked role of biochar’s particle size. Journal of Hazardous Materials, 422, 126928. https://doi.org/10.1016/J.JHAZMAT.2021.126928
Jmaa, S. B., & Kallel, A. (2019). Assessment of performance of Posidona oceanica (L.) as biosorbent for crude oil-spill cleanup in seawater. BioMed Research International, 2019, 6029654. https://doi.org/10.1155/2019/6029654
Karnitz, O., Gurgel, L. V. A., de Melo, J. C. P., Botaro, V. R., Melo, T. M. S., de Freitas Gil, R. P., & Gil, L. F. (2007). Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse. Bioresource Technology, 98(6), 1291–1297. https://doi.org/10.1016/J.BIORTECH.2006.05.013
Kayvani Fard, A., Rhadfi, T., Mckay, G., Al-marri, M., Abdala, A., Hilal, N., & Hussien, M. A. (2016). Enhancing oil removal from water using ferric oxide nanoparticles doped carbon nanotubes adsorbents. Chemical Engineering Journal, 293, 90–101. https://doi.org/10.1016/J.CEJ.2016.02.040
Kerrou, M., Bouslamti, N., Raada, A., Elanssari, A., Mrani, D., & Slimani, M. S. (2021). The use of sugarcane bagasse to remove the organic dyes from wastewater. International Journal of Analytical Chemistry, 2021, 5570806. https://doi.org/10.1155/2021/5570806
Khalid, F. E., Ahmad, S. A., Zakaria, N. N., Shaharuddin, N. A., Sabri, S., Azmi, A. A., Khalil, K. A., Verasoundarapandian, G., Gomez-Fuentes, C., & Zulkharnain, A. (2021). Application of cogon grass (Imperata cylindrica) as biosorbent in diesel-filter system for oil spill removal. Agronomy, 11(11), 2273. https://doi.org/10.3390/AGRONOMY11112273
Kumar, G., Dora, D. T. K., Jadav, D., Naudiyal, A., Singh, A., & Roy, T. (2021). Utilization and regeneration of waste sugarcane bagasse as a novel robust aerogel as an effective thermal, acoustic insulator, and oil adsorbent. Journal of Cleaner Production, 298, 126744. https://doi.org/10.1016/J.JCLEPRO.2021.126744
Kurniawan, R. Y., Dwi, I., Kurniawan, O., Atmaja, L., & Widiastuti, N. (2019). Synthesis N-doped activated carbon from sugarcane bagasse for CO2 adsorption. IPTEK The Journal for Technology and Science, 30(3), 80–87. https://doi.org/10.12962/J20882033.V30I3.5469
Kuśmierek, K., & Świątkowski, A. (2015). The influence of different agitation techniques on the adsorption kinetics of 4-chlorophenol on granular activated carbon. Reaction Kinetics, Mechanisms and Catalysis, 116(1), 261–271. https://doi.org/10.1007/S11144-015-0889-1
Liu, Q., Li, Y., Chen, H., Lu, J., Yu, G., Möslang, M., & Zhou, Y. (2020). Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. Journal of Hazardous Materials, 382, 121040. https://doi.org/10.1016/J.JHAZMAT.2019.121040
Liu, Q., Chen, Z., Huang, L., Mujtaba Munir, M. A., Wu, Y., Wang, Q., Ma, L., Xu, S., Wen, Z., & Feng, Y. (2021). The effects of a combined amendment on growth, cadmium adsorption by five fruit vegetables, and soil fertility in contaminated greenhouse under rotation system. Chemosphere, 285, 131499. https://doi.org/10.1016/J.CHEMOSPHERE.2021.131499
Lv, N., Wang, X., Peng, S., Luo, L., & Zhou, R. (2018). Superhydrophobic/superoleophilic cotton-oil absorbent: Preparation and its application in oil/water separation. RSC Advances, 8(53), 30257–30264. https://doi.org/10.1039/c8ra05420g
Maaloul, N., Oulego, P., Rendueles, M., Ghorbal, A., & Díaz, M. (2017). Novel biosorbents from almond shells: Characterization and adsorption properties modeling for Cu(II) ions from aqueous solutions. Journal of Environmental Chemical Engineering, 5(3), 2944–2954. https://doi.org/10.1016/J.JECE.2017.05.037
Mathew, B. B., Jaishankar, M., George Biju, V., & Beeregowda, K. N. (2016). Role of bioadsorbents in reducing toxic metals. Journal of Toxicology, 2016, 4369604. https://doi.org/10.1155/2016/4369604
Mehrzad, S., Taban, E., Soltani, P., Samaei, S. E., & Khavanin, A. (2022). Sugarcane bagasse waste fibers as novel thermal insulation and sound-absorbing materials for application in sustainable buildings. Building and Environment, 211, 108753. https://doi.org/10.1016/J.BUILDENV.2022.108753
Mishra, P. K., & Mukherji, S. (2012). Biosorption of diesel and lubricating oil on algal biomass. 3 Biotech, 2(4), 301–310. https://doi.org/10.1007/s13205-012-0056-6
Mohamed, N. B., Ngadi, N., Wong, S., Yahya, N. Y., Hassan, O., Inuwa, I. M., Opotu, L. A., & Ali, N. (2022). Facile synthesis of polyethylenimine-modified sugarcane bagasse adsorbent for removal of anionic dye in aqueous solution. Scientific African, 16, e01135. https://doi.org/10.1016/J.SCIAF.2022.E01135
Neureiter, M., Danner, H., Thomasser, C., Saidi, B., & Braun, R. (2002). Dilute-acid hydrolysis of sugarcane bagasse at varying conditions. Applied Biochemistry and Biotechnology, 98(1), 49–58. https://doi.org/10.1385/ABAB:98-100:1-9:49
Nuria Fiol, T. B. (2013). Adsorption on activated carbon from olive stones: Kinetics and equilibrium of phenol removal from aqueous solution. Journal of Chemical Engineering & Process Technology, 04(06), 1000165. https://doi.org/10.4172/2157-7048.1000165
Oliveira, J. A., Cunha, F. A., & Ruotolo, L. A. M. (2019). Synthesis of zeolite from sugarcane bagasse fly ash and its application as a low-cost adsorbent to remove heavy metals. Journal of Cleaner Production, 229, 956–963. https://doi.org/10.1016/J.JCLEPRO.2019.05.069
Olufemi, B. A., & Otolorin, F. (2017). Comparative adsorption of crude oil using mango (Mangnifera indica) shell and mango shell activated carbon. Environmental Engineering Research, 22(4), 384–392. https://doi.org/10.4491/eer.2017.011
Omri, A., Wali, A., & Benzina, M. (2016). Adsorption of bentazon on activated carbon prepared from Lawsonia inermis wood: Equilibrium, kinetic and thermodynamic studies. Arabian Journal of Chemistry, 9, S1729–S1739. https://doi.org/10.1016/J.ARABJC.2012.04.047
Peng, D., Cheng, S., Li, H., & Guo, X. (2021). Effective multi-functional biosorbent derived from corn stalk pith for dyes and oils removal. Chemosphere, 272, 129963. https://doi.org/10.1016/J.CHEMOSPHERE.2021.129963
Priyanto, A. (2021). Adsorption and characterization of activated sugarcane bagasse using natrium hydroxide. Indonesian Journal of Chemical Research, 8(2), 202–209. https://doi.org/10.30598//ijcr.2021.7-ade
Quedou, P. G., Wirquin, E., & Bokhoree, C. (2021). Sustainable concrete: Potency of sugarcane bagasse ash as a cementitious material in the construction industry. Case Studies in Construction Materials, 14, e00545. https://doi.org/10.1016/J.CSCM.2021.E00545
Raziq Rahimi Kooh, M., Khairud Dahri, M., Lim, L. L. B., Hoon Lim, L., Muhammad Raziq Rahimi Kooh, B., Darussalam, B., Tungku Link, J., Gadong, P., & Seri Begawan, B. B. (2015). Batch adsorption studies on the removal of acid blue 25 from aqueous solution using Azolla pinnata and soya bean waste. Arabian Journal for Science and Engineering, 41(7), 2453–2464. https://doi.org/10.1007/S13369-015-1877-5
Roy, D., Greenlaw, P. N., & Shane, B. S. (2008). Adsorption of heavy metals by green algae and ground rice hulls. Journal of Environmental Science and Health. Part A: Environmental Science and Engineering and Toxicology, 28(1), 37–50. https://doi.org/10.1080/10934529309375861
Saad Agwa, I., Zeyad, A. M., Tayeh, B. A., Adesina, A., de Azevedo, A. R. G., Amin, M., & Hadzima-Nyarko, M. (2022). A comprehensive review on the use of sugarcane bagasse ash as a supplementary cementitious material to produce eco-friendly concretes. Materials Today: Proceedings, 65(2), 688-696. https://doi.org/10.1016/J.MATPR.2022.03.264
Sahu, J. N., Karri, R. R., & Jayakumar, N. S. (2021). Improvement in phenol adsorption capacity on eco-friendly biosorbent derived from waste Palm-oil shells using optimized parametric modelling of isotherms and kinetics by differential evolution. Industrial Crops and Products, 164, 113333. https://doi.org/10.1016/J.INDCROP.2021.113333
Senthil Kumar, K. (2015). Adsorption studies on treatment of cooking oil mill effluent using crab shell chitosan. Journal of Chemical and Pharmaceutical Research, 7(11), 19–29.
Sidiras, D., Batzias, F., Konstantinou, I., & Tsapatsis, M. (2014). Simulation of autohydrolysis effect on adsorptivity of wheat straw in the case of oil spill cleaning. Chemical Engineering Research and Design, 92(9), 1781–1791. https://doi.org/10.1016/J.CHERD.2013.12.013
Sindhu, R., Binod, P., Satyanagalakshmi, K., Janu, K. U., Sajna, K. V., Kurien, N., Sukumaran, R. K., & Pandey, A. (2010). Formic acid as a potential pretreatment agent for the conversion of sugarcane bagasse to bioethanol. Applied Biochemistry and Biotechnology, 162(8), 2313–2323. https://doi.org/10.1007/S12010-010-9004-2
Singh, S., Kumar, V., Datta, S., Dhanjal, D. S., Sharma, K., Samuel, J., & Singh, J. (2020). Current advancement and future prospect of biosorbents for bioremediation. Science of The Total Environment, 709, 135895. https://doi.org/10.1016/J.SCITOTENV.2019.135895
Status Paper on Sugarcane. (2013). Retrieved July 4, 2022, from https://farmer.gov.in/imagedefault/pestanddiseasescrops/sugarcane.pdf
Suresh Kumar, P., Korving, L., Keesman, K. J., van Loosdrecht, M. C. M., & Witkamp, G. J. (2019). Effect of pore size distribution and particle size of porous metal oxides on phosphate adsorption capacity and kinetics. Chemical Engineering Journal, 358, 160–169. https://doi.org/10.1016/J.CEJ.2018.09.202
Suteu, D., Malutan, T., & Bilba, D. (2010). Removal of reactive dye Brilliant Red HE-3B from aqueous solutions by industrial lignin: Equilibrium and kinetics modeling. Desalination, 255(1–3), 84–90. https://doi.org/10.1016/J.DESAL.2010.01.010
Tansel, B., & Pascual, B. (2011). Removal of emulsified fuel oils from brackish and pond water by dissolved air flotation with and without polyelectrolyte use: Pilot-scale investigation for estuarine and near shore applications. Chemosphere, 85(7), 1182–1186. https://doi.org/10.1016/J.CHEMOSPHERE.2011.07.006
Tayeb, A. M., Farouq, R., Mohamed, O. A., & Tony, M. A. (2019). Oil spill clean-up using combined sorbents: A comparative investigation and design aspects. International Journal of Environmental Analytical Chemistry, 100(3), 311–323. https://doi.org/10.1080/03067319.2019.1636976
Thai, Q. B., Nguyen, S. T., Ho, D. K., Tran, T. du, Huynh, D. M., Do, N. H. N., Luu, T. P., Le, P. K., Le, D. K., Phan-Thien, N., & Duong, H. M. (2020). Cellulose-based aerogels from sugarcane bagasse for oil spill-cleaning and heat insulation applications. Carbohydrate Polymers, 228, 115365. https://doi.org/10.1016/J.CARBPOL.2019.115365
Tomazini da Conceição, F., Braga Moruzzi, R., Augusto Prado Duarte, G., Galileu Speranza, L., Lucia Pereira Antunes, M., & Donnini Mancini, S. (2022). Biochar from sugarcane bagasse for reactive dye adsorption considering a circular economy approach. Journal of Textile Engineering & Fashion Technology, 8(4), 126–132. https://doi.org/10.15406/jteft.2022.08.00310
Tsai, W. T., Yang, J. M., Lai, C. W., Cheng, Y. H., Lin, C. C., & Yeh, C. W. (2006). Characterization and adsorption properties of eggshells and eggshell membrane. Bioresource Technology, 97(3), 488–493. https://doi.org/10.1016/J.BIORTECH.2005.02.050
Wahi, R., Chuah, L. A., Choong, T. S. Y., Ngaini, Z., & Nourouzi, M. M. (2013). Oil removal from aqueous state by natural fibrous sorbent: An overview. Separation and Purification Technology, 113, 51–63. https://doi.org/10.1016/J.SEPPUR.2013.04.015
Wang, J., Zheng, Y., & Wang, A. (2012). Effect of kapok fiber treated with various solvents on oil absorbency. Industrial Crops and Products, 40(1), 178–184. https://doi.org/10.1016/J.INDCROP.2012.03.002
Wong, Y. J., Arumugasamy, S. K., Chung, C. H., Selvarajoo, A., & Sethu, V. (2020). Comparative study of artificial neural network (ANN), adaptive neuro-fuzzy inference system (ANFIS) and multiple linear regression (MLR) for modeling of Cu (II) adsorption from aqueous solution using biochar derived from rambutan (Nephelium lappaceum) peel. Environmental Monitoring and Assessment, 192(7), 1–20. https://doi.org/10.1007/S10661-020-08268-4
Yu, Q., Zhuang, X., Lv, S., He, M., Zhang, Y., Yuan, Z., Qi, W., Wang, Q., Wang, W., & Tan, X. (2013). Liquid hot water pretreatment of sugarcane bagasse and its comparison with chemical pretreatment methods for the sugar recovery and structural changes. Bioresource Technology, 129, 592–598. https://doi.org/10.1016/J.BIORTECH.2012.11.099
Zhang, Y., Zhang, Y., Cao, Q., Wang, C., Yang, C., Li, Y., & Zhou, J. (2020). Novel porous oil-water separation material with super-hydrophobicity and super-oleophilicity prepared from beeswax, lignin, and cotton. Science of The Total Environment, 706, 135807. https://doi.org/10.1016/J.SCITOTENV.2019.135807
Zhao, X., Wen, J., Chen, H., & Liu, D. (2018). The fate of lignin during atmospheric acetic acid pretreatment of sugarcane bagasse and the impacts on cellulose enzymatic hydrolyzability for bioethanol production. Renewable Energy, 128, 200–209. https://doi.org/10.1016/j.renene.2018.05.071
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
G, A.Y., Machado, A.A. & Mulky, L. A Comparative Study of Treatment Methods of Raw Sugarcane Bagasse for Adsorption of Oil and Diesel. Water Air Soil Pollut 234, 213 (2023). https://doi.org/10.1007/s11270-023-06210-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06210-1