Abstract
The efficacy of a fine bubble (FB) diffuser in enhancing the performance of an attached biofilm hydrogenotrophic denitrification (HD) reactor was evaluated. HD reactors equipped with an FB diffuser (FB reactor) and an air stone (AS) diffuser that produced ordinary bubbles (AS reactor) were operated in parallel at different hydraulic retention times (HRTs) in a synthetic groundwater treatment experiment. A reduction in H2 consumption of approximately 77% was achieved using the FB diffuser to reach a gas-liquid mass transfer coefficient similar to that of the AS diffuser. The high gas dissolution efficiency of the FB diffuser resulted in an effective nitrogen removal rate (NRR) enhancement, requiring less H2 supply. The highest value of NRR at 53.0±9.8 g-N/m3/d was obtained in the FB reactor at a minimum HRT of 3 h, which was two-fold higher than the corresponding value from the AS reactor. The FB reactor also had the lowest requirement of H2 for denitrification reaching 0.1 m3-H2/g-N in this condition, which was ten-fold lower than that in the AS reactor. Furthermore, the suspended sludge concentration in the FB reactor was lower than that in the AS reactor, indicating that the application of the FB diffuser can minimize excess suspended sludge accumulation inside the HD reactor. Microbial community analysis showed the predominance of Thauera spp. reaching a relative abundance of 15.7–27.3% in the FB reactor, suggesting a contribution to the HD. This finding can provide insight into the application of the FB diffuser for optimizing nitrate-contaminated groundwater treatment technology by HD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Groundwater is a major drinking water resource worldwide. Recently, groundwater contamination by nitrate nitrogen (NO3–-N) has become prevalent in several countries, including Africa (Lapworth et al., 2017), India (Suthar, et al., 2009), Nepal (Shakya et al., 2019), and Japan (Nakagawa et al., 2017). Intake of NO3–-N leads to methemoglobinemia and produces nitrosamine and nitrite nitrogen (NO2–-N) with toxic and carcinogenic contaminants (World Health Organization, 2017). A groundwater treatment system capable of NO3–-N removal is necessary to provide safe drinking water, which is targeted in the United Nations Sustainable Development Goal 6: Ensure access to water sanitation for all (United Nations, 2016).
Hydrogenotrophic denitrification (HD) is a chemoautotrophic bacterial denitrification that uses H2 as an electron donor, as shown in Eq. (1) (Lee and Rittmann, 2002). NO3–-N is reduced to N2 gas by the metabolic pathways of bacteria that can perform HD. The application of HD for nitrate-contaminated groundwater treatment and providing safe drinking water have received attention because of their advantages over the utilization of other biological processes and physicochemical treatment technology. H2 utilized as an electron donor is not harmful to human health and is not an environmentally hazardous material. Additionally, no post-treatment is required to remove the electron donor and excess sludge for the following reasons (Rezania, et al., 2005; Vasiliadou et al., 2009): (1) H2 is removed from water naturally and does not produce any other derivative, and (2) the HD process has a low biomass yield. H2 can be produced by water electrolysis so that the electron donor can be easily provided to the HD reactor. A physicochemical treatment process using an ion exchange resin and reverse osmosis membrane filter is an alternative to the biological NO3–-N removal process (Peter-Varbanets et al., 2009). However, waste brine containing highly concentrated NO3–-N is produced during this treatment (Shrimali and Singh, 2001), which leads to further environmental pollution and requires additional treatment processes compared to the groundwater treatment by HD.
Substantial research has been conducted on the NO3–-N removal performance of the denitrification systems using HD (Chang et al., 1999; Smith et al., 2005). The biggest drawback of the HD process is the low solubility of H2 in the water phase. Thus, the HD process requires a large dose of H2 to enhance denitrification activity owing to its low solubility, which increases the operational costs. To enhance the gas-dissolving efficiency, previous studies developed HD systems utilizing, a hollow fiber membrane filter (Zhang et al., 2009), and a sealed and pressurized system (Epsztein et al., 2016). However, these systems require thorough maintenance, such as frequent cleaning of the surface of the hollow fiber to remove biofouling (Karanasios et al., 2010), and strict management of the gas pressure to avoid hydrogen expression.
The application of fine bubble (FB) diffusers, which can generate micrometer (1–100 μm) and nanometer (< 1 μm) size gas bubbles (International Organization for Standardization, 2017), has the potential to enhance gas diffusion efficiency and minimize the H2 dose. The FB has a longer retention time than an ordinary gas bubble, and thus, it can be retained in the water. Additionally, according to the Young–Laplace equation, the pressure of the gas bubble increases when the size of the bubble decreases (Li et al., 2009); thus, the gas dissolution efficiency can be enhanced. Therefore, research has been conducted to introduce FB into wastewater treatment. Chu et al. (2008) applied the FB for textile wastewater treatment and found a 20% increase in the chemical oxygen demand removal efficiency compared with the bubble contactor. According to extensive research, the application of the FB into the HD reactor may also lead to H2 dissolution enhancement, minimization of H2 volume, and enhancement of denitrification performance. Furthermore, several types of the FB diffusers have been proposed, such as ultrasonication (Kim et al., 2000), decompression (Ikeura et al., 2011), venturi-type (Vilaida et al., 2019), and nano-ceramic membrane systems (Ahmed et al., 2018). These diffusers were operated in underwater and did not require a pressure increase of the reactor, minimizing the risk of the reactor exploding.
Very few studies have been conducted on the introduction of an FB diffuser into the denitrification system by HD. To the best of our knowledge, only our previous study demonstrated the application of the FB diffuser into groundwater treatment by HD (Eamrat et al., 2020). In the study, the FB diffuser critically enhanced gas diffusing efficiency compared to the air stone (AS) diffuser and found that H2 utilization efficiency for denitrification could be enhanced by six times. But, the effects of the FB diffuser on the enhancement of the denitrification rate and the inner environment of the reactor are not well understood; this information is crucial for the optimization of the HD reactor–equipped FB diffuser. Thus, the present study evaluated the effects of an FB diffuser on the performance of HD reactor. First, the H2 dissolution efficiency of an FB diffuser and an AS diffuser—producing bubbles of ordinary size—was compared. Subsequently, the attached biofilm HD reactors equipped with FB and AS diffusers were operated in parallel to compare the denitrification performance, the amount of sludge inside the reactor, and the composition of the microbial community.
2 Methodology
2.1 Evaluation of the H2 Dissolution Efficiency of the Fine Bubble (FB) Diffuser
An FB diffuser, which can generate approximately 85 nm of gas bubbles by passing H2 through a ceramic dispersal tube diffuser (AZ-015-0276, Anzaikantetsu, Kanagawa, Japan), was utilized for the experiment. To understand the physicochemical characteristics of this diffuser, its gas-liquid mass transfer coefficient (KLa) was evaluated and compared with that of an AS diffuser (S103-H, Sudo, Aichi, Japan). The AS diffuser could generate a bubble size of approximately 2.20±0.25×103 μm in size (Eamrat et al., 2020). KLa was determined using Eq. (2) (American Society of Civil Engineers, 2007).
where C*, Ct, and Ct0, represent the dissolved hydrogen (DH) concentration at saturation concentration (1.6 mg/L), DH concentration at measuring time (t), and DH concentration at the initial time (t0), respectively.
KLa determination was conducted by H2 supply to 1 L of tap water in a graduated cylinder (φ3 cm×20 cm), following a previously described procedure (Eamrat et al., 2020). The graduated cylinder was mixed well at 500 rpm by a magnetic stirrer, and the water temperature was set to 25±2 °C. The initial dissolved oxygen (DO) concentration was set to < 0.5 mg/L by supplying Ar gas before starting the H2 supply. The H2 (99.99% purity) was supplied by a gas cylinder. Because the FB diffuser required gas pressure to effectively provide the bubbles, H2 was supplied at the manufacturer’s recommended optimal pressure of 0.15 MPa. For the AS diffuser, KLa measurements were conducted at 10, 50, 100, 150, 200, and 250 mL/min for H2 flow rates, to compare with the KLa of the FB diffuser. The real-time DH concentration was monitored using a portable DH meter (ENH-2000, TRUSTLEX, Osaka, Japan) until the DH concentration reached a stable value. All measurements were conducted in triplicate.
2.2 Laboratory-Scale HD Reactor Setup and Operating Conditions
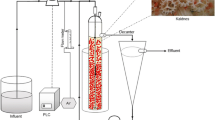
A schematic of the laboratory-scale HD reactor is shown in Fig. 1. The reactor was a polyacrylic-made cylindrical reactor with a working volume of 5 L (φ13cn×46 cm). Polyester non-woven fabric was used as the bacterial carrier in this study. A 500 cm3 carrier (20 cm×12.5 cm×1 cm; 2 pieces) was fixed to the HD reactor at a filling ratio of 10% (v/v). The carrier density was approximately 0.0167 g/cm3. To prevent H2 release, the water surface of the HD reactor was covered by polypropylene beads. Two reactors were prepared and equipped with an FB diffuser (hereafter referred to as the FB reactor) and an AS diffuser (hereafter referred to as the AS reactor). To set the equal gas diffusing efficiency in each reactor, the H2 was supplied at 0.15 MPa for the FB reactor and 100 mL/min for the AS reactor following the results of the KLa determination experiment. Water recirculation was conducted at approximately 25 L/min using a magnetic pump to thoroughly mix the water. The reactor was operated at room temperature. And, the water temperature of each reactor was 24±2 °C while in the experiment. The seed sludge was well-acclimated in the parent HD reactor, similar to a previous study (Rujakom et al., 2020). The initial seed sludge was added to both reactors at a mixed liquor-suspended sludge (MLSS) concentration of 72 mg/L. To immobilize the seed sludge on the bacterial carrier, the FB and AS reactors were left overnight with water recirculation and H2 supply and without synthetic groundwater supply before the start of the operation.
Synthetic groundwater was prepared from tap water by adding reagents, as summarized in Table 1. In this study, the NO3–-N concentration was set to 10 mg-N/L. Sodium bicarbonate was added as the sole carbon source for HD. The concentration was set to 205.9 mg/L, which is sufficient to induce HD, based on Eq. (1). The hydraulic retention time (HRT) gradually decreased from 12 to 6 h and 3 h. Each operational condition lasted for approximately 10–14 days, because the HD performance stabilized within 14 days in a previous study (Eamrat et al., 2020).
2.3 Water Quality Analysis and Denitrification Rate Calculation
The influent and effluent water from each reactor was collected and filtered using a 1-μm glass filter (GF/B, Cytiva, Tokyo, Japan). The NO2–-N and NO3–-N concentrations were analyzed using the N-(1-naphthyl)-ethylene-diamine method and ultraviolet spectrophotometric screening method (APHA/AWWA/WEF, 1998), respectively, using a spectrophotometer (UV-1800, SHIMADZU, Kyoto, Japan). The nitrogen loading rate (NLR), nitrogen removal rate (NRR), and nitrate removal efficiency (NRE) were calculated using Eqs. (3), (4), and (5), respectively.
where CDINin and CDINout represent the summation of NO3–-N and NO2–-N concentrations in the influent and effluent, respectively, and Q and V are the water flow rate and working volume, respectively. To elucidate the efficacy of H2 savings, the H2 supply rate and H2 requirement for 1 g of nitrogen removal were calculated using Eqs. (6) and (7).
where QH2 represents the H2 flow rate to the HD reactor.
The monitoring of pH, DO concentration, oxidation-reduction potential (ORP), and DH was performed at regular intervals during influent and effluent water sampling, using a pH meter (D-71, HORIBA, Kyoto, Japan), a multimeter for DO and ORP analysis (WQ-300, HORIBA), and a portable DH meter (ENH-2000, TRUSTLEX), respectively.
2.4 Measurement of Suspended Sludge Concentration and Attached Biomass Concentration on the Bacterial Carrier in the Reactor
Suspended sludge and attached biomass concentrations were determined for each reactor after the operation of each HRT. The bacterial carrier was removed from each reactor before the suspended sludge measurement. After the suspended sludge was mixed well, a portion of the suspension was used for the MLSS analysis following the standard protocol (APHA/AWWA/WEF, 1998). To measure the attached biomass, three 1-cm3 pieces (1 cm × 1 cm × 1 cm) were randomly taken. The carrier was then squeezed to suspend the attached sludge in distilled water according to previously reported methods (Nguyen et al., 2010; Zhang et al., 2016). The suspension was applied to MLSS analysis, which was used to calculate the attached biomass in 1 cm3 of the carrier. The total attached biomass (TAB) was estimated by multiplying the attached biomass with the total volume of the bacterial carrier. All measurements were conducted in triplicate.
Furthermore, the biofilm-forming efficiency was determined in the final operational period at an HRT of 3 h. Two pieces of a glass slide (2.6 cm × 7.6 cm × 0.1 cm) were sunk into each reactor. After the operation, the biofilm formed on one side was wiped, and the glass slide was kept in a desiccator to dry after gently rinsing with distilled water. Subsequently, the glass slide was dipped in 1.0% of the crystal violet staining solution (Merck KGaA, Darmstadt, Germany) for 30 s to stain the biofilm and again dried in a desiccator after removing excess dye by washing with distilled water.
2.5 Microbial Community Structure Analysis
The microbial community structure was analyzed during the reactor operation. The suspension from the suspended sludge analysis was partially used for DNA extraction after centrifugation at 6000 rpm for 10 min. Approximately 0.1 g of suspended sludge (SS) was used for total DNA extraction using the FastDNA SPIN Kit for Soil (MP Biomedicals, CA, USA). Total bacterial abundance was evaluated by quantitative PCR (qPCR) using primers515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) (Caporaso et al., 2011). The qPCR assay was conducted in a 25 μL of a mixture comprising 12.5 μL of SYBR® Premix EX TaqTM (II) (TaKaRa Bio, Shiga, Japan), 9.5 μL of deionized water, 0.5 μL of each primer (50 nM), and 2 μL of temperate. The PCR cycles were as follows: initial activation at 95 °C for 10 s; 35 cycles of denaturation at 94 °C for 15 s, annealing at 55 °C for 30 s, and extension at 72°C for 20 s, and a final extension step at 72 °C for 10 s. Genomic DNA from Escherichia coli (LA PCRTM genome DNA set, TaKaRa) was used as the standard for the qPCR assay. The measurements were conducted in duplicate.
The extracted DNA was also subjected to high-throughput DNA sequencing by using a next-generation sequencing analysis service (Food Analysis and Biotechnology Company; FASMAC, Atsugi, Japan). All analyses mentioned below were conducted using the analysis service. The V4 hypervariable region of the 16S rRNA gene was analyzed by Illumina MiSeq gene sequencer using the specific primers (5′-ACACTCTTTCCCTACACGACGCTCTTCCGATCT-GTGCCAGCMGCCGCGGTAA-3′; the sequence for 2nd PCR is underlined) and 806R (5′-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-GGACTACHVGGGTWTCTAAT-3′; the sequence for 2nd PCR is underlined). The read sequences were grouped into operational taxonomic units with a 3% of divergence of their homology and were compared to the SILVA database in the QIIME platform.
2.6 Statistical Analysis
The results obtained in this study are presented as mean ± standard deviation (SD). Statistical significance was determined using Student’s t-test after evaluating the variance ratio test in Microsoft Excel (Microsoft, Washington, USA). Statistical significance was set at p < 0.05.
3 Results and Discussion
3.1 Enhancement of H2 Dissolution Efficiency and Denitrification Performance by the FB Diffuser Application
The KLa variations in the FB and AS reactors are summarized in Fig. 2. The KLa of the FB diffuser at 0.15 MPa was reached at 70±12×10–3 S–1 (mean ± SD). Conversely, the KLa of the AS diffuser increased from 3±1×10–3 to 122±26×10–3 S–1 by increasing the H2 flow rate. At a 100 mL/min of gas flow rate, KLa reached 61±20×10–3 S–1, which was similar to the KLa of the FB diffuser (p > 0.05). The actual gas flow rate supplied by the FB diffuser was estimated to be 23 mL/min, calculated from the pressure loss over a certain period, suggesting that the FB diffuser could save approximately 77% of H2 to reach a similar KLa compared to the AS diffuser.
The performances of the FB and AS reactors are summarized in Table 2. At 12 h HRT, the FB and AS reactors showed similar denitrification performance. Denitrification performance was different at 6 h and 3 h HRT. The FB reactor maintained its denitrification performance with a minimum HRT of 3 h. Under these operational conditions, the NRR and NRE of the FB reactor reached 53.0±9.8 g-N/m3/d and 68.2±13.0%, respectively, which was approximately two-fold higher than that of the AS reactor (p < 0.05). The effluent NO3–-N concentration of the FB reactor was approximately 0.6±0.1 mg-N/L, whereases that of the AS reactor was 4.5±4.3 mg-N/L. The concentration of NO2–-N in the effluent reached 2.6±1.5 mg-N/L, which was similar to that of the AS reactor (2.2±1.7 mg-N/L). The higher pH of the FB reactor, compared with that of the AS reactor (Table S1), also supported the higher denitrification performance, because the HD induced a pH increase, as shown in Eq. (1). Furthermore, the FB reactor showed a linear increase in the NRR by reducing the HRT (Fig. S1). A strong inverse correlation was observed between HRT and NRR (r = 0.90, p < 0.05) in the FB reactor, whereas the AS reactor showed a tendency to suppress the NRR with a weak inverse correlation (r = 0.31, p > 0.05). The H2 requirement of the FB reactor at 3 h of HRT was approximately 0.1 m3-H2 for 1 g of nitrogen removal, which was approximately 10-fold lower than that for the corresponding value for the AS reactor (1.1 m3-H2/g-N; Table S2).
The application of the FB diffuser showed a significant H2-saving effect on nitrogen removal in the HD reactor, similar to our previous report (Eamrat et al., 2020). Although H2 was supplied to maintain a similar gas diffusing efficiency, the FB reactor showed higher denitrification performance than that of AS reactor at HRT 6 h and 3 h. The FB diffuser used in this study can produce numerous numbers of bubbles reaching 3.19×109 in 1 mL according to the company-provided information. Numerous numbers of H2 bubbles that did not dissolve into the liquid phase were available as a continuous supply of DH to bacteria in the HD reactor. The detection of higher DH concentrations in the FB reactor than in the AS reactor is indicative of this phenomenon (Table S1). Because the quantity of H2 remains sufficient for HD, the denitrification performance can be enhanced by an increase in NLR associated with the HRT decrease in the FB reactor. Thus far, the results suggest that the application of an FB diffuser may help minimize the reaction time and reduce the reactor size in groundwater treatment by HD. A similar effect has been reported in previous studies on the application of FB diffusers in wastewater treatment by physicochemical or biochemical processes. Fujita et al. (2021) demonstrated that an FB diffuser could enhance the physicochemical oxidation of benzaldehyde and could shorten the reaction time by approximately five-fold compared to an ordinary diffuser. Similarly, Yao et al. (2021) reported that micro-nano aeration enhanced the biological ammonium removal performance by approximately 3.2-fold compared to conventional aeration.
3.2 Minimization of the Excess Suspended Sludge Accumulation in the Reactor
The MLSS and TAB values for each reactor are shown in Fig. 3. The MLSS of the FB reactor was significantly lower than that of the AS reactor (p < 0.05; Fig. 3a). At 3 h of HRT, the MLSS of the FB reactor was 87.8±8.7 mg/L, which was approximately 1.4 times lower than that of the AS reactor under the same conditions (122.0±17.8 mg/L). Conversely, the TABs of the FB and AS reactors were similarly maintained at each HRT (p > 0.05; Fig. 3b). All TABs in each reactor were gradually increased by the HRT shift, showing sludge immobilization along with the reactor operation. At the end of operation at 3 h HRT, the TAB in the FB reactor reached 39.8±9.5 g/reactor, whereases that in the AS reactor was 46.8±12.1 g/reactor. After 6 h of HRT, the TAB of the AS reactor was almost equal to that in 12 h of HRT. The highest MLSS of the AS reactor was found in this set of operations; therefore, the accumulated sludge on the carrier might have detached and become suspended sludge in this reactor.
MLSS was always lower in the FB reactor compared with the AS reactor, which suggested the interruption of suspended sludge accumulation inside the reactor. FB provides a washing effect by coating the suspended solid with an electrical charge difference (Han et al., 2006; Temesgen et al., 2017). Thus, the previous study applied an FB diffuser to several washing approaches, such as the flotation process (Reis et al., 2019) and the removal of fouling on the membranes in wastewater treatment systems (Hashimoto et al., 2022). Our experimental results also indicate the washing effect of FB, resulting in the removal of suspended sludge from the reactor. Furthermore, the biofilm that formed on the glass slide collected from the FB reactor was dispersed (Fig. S2), indicating that the FB diffuser interrupted biofilm formation on the inner wall of the reactor. A similar result was found in a previous report that nanobubbles could act as a physical barrier to protein adsorption on a material surface (Wu et al., 2006, Wu et al., 2008). These two factors—washing out from the reactor and interruption of sludge attached to the inner wall of the reactor—could be potential factors leading to a lower MLSS in the FB reactor compared to the AS reactor. Based on a comparison of TAB values, it appears that these factors might not significantly affect the attached sludge on the bacterial carrier (Fig. 3b), since biofilm formation also occurred in the inner part. Therefore, enough amount of the sludge for HD could maintain on the bacterial carrier in the FB reactor while in operation, which might also support the NRR increase by reducing the HRT.
Thus far, suppression of the excess suspended sludge accumulation inside the reactor may help to minimize the sludge withdrawal frequency of the reactor, which is a significant advantage for FB diffuser application. Besides, the washed-out sludge may lead to an increase the sludge loading to the final filtration step. The stability of the effluent suspended sludge concentration and its effect on post-treatment during the long-term operation of the FB reactor should be further investigated. The elucidation of the optimum operational condition for an HD reactor with an FB diffuser and its performance stability deserves further investigation to develop a nitrate-contaminated groundwater treatment system.
3.3 Differences in Microbial Community Structure in the Reactor
The total bacterial abundance in the initial sludge was 2.0×109 copies/g-SS (Fig. S3). The total bacterial abundance in the suspended sludge of the FB and AS reactors was similar in the range of 1.9×109–5.0×109 copies/g-SS, suggesting that the number of the bacteria in the sludge was similarly maintained during operation. The microbial community structure of sludge from the HD reactor is summarized in Fig. 4. In the seed sludge, Azoarcus spp. were the dominant bacteria composing 19.6% of the total bacterial abundance. After the operation, the dominant taxa changed in each HD reactor. In the FB reactor, Thauera spp. was selectively enriched and existed as the dominant bacteria, representing 15.7–27.3% of the relative abundance under all operational conditions. The highest relative abundance of this genus was 27.3% after 3 h of HRT. Although an unidentified genus in the family Rikenellaceae comprised 36.4% of total bacterial abundance during operation at an HRT of 6 h, Thauera. spp. was the second-most dominant genus, comprising 15.7% of total bacterial abundance. The relative abundance of the unidentified genus (family Rikenellaceae) did not increase at 12 h and 3 h of HRTs and comprised 2.3% and 12.7%, respectively, of the total bacterial abundance. Besides this, Thauera spp. was not the dominant bacteria in the AS reactor. Although the highest relative abundance of this genus was 24.1% during operation at an HRT of 3 h, its abundance under other experimental conditions was only 12.7% and 13.7%, respectively. The dominant bacteria in the AS reactor, which were Erysipelothrix spp. (e.g., 14.7% and 25.8% at HRTs of 3 h and 6 h, respectively) and Methyloversatilis spp. (e.g., 22.5% for at HRT of 3 h), differed depending on the HRTs.
The experimental results showed the selective enrichment of the genus Thauera spp. in the FB reactor. Thauera spp. has been regularly detected in H2-supplying denitrification reactors (Chen et al., 2015; Eamrat et al., 2017). Mao et al. (2013) also reported the selective enrichment of Thauera spp. with the detection of HD by the cultivation of activated sludge and anaerobic digestion sludge in serum bottles filled with H2. The results of our previous study also implied that Thauera spp. might cooperate with denitrification, showing a strong correlation between the relative abundance of the NO2–-N reduction gene and the relative abundance of this genus (Rujakom, et al., 2020). The FB reactor showed rich DH (Table S1) and higher denitrification performance (Table 2) than that of the AS reactor, suggesting that Thauera spp. might have contributed to the HD. Insufficient H2 supply in the AS reactor at 3h of HRT might induce suppression of denitrification, despite the predominance of the Thauera spp. in the sludge. An unidentified genus in the Rikenellaceae family was occasionally found in each reactor. The sequence of this taxon (253 bp in length) was further analyzed using the EzBioCloud analytical service (https://www.ezbiocloud.net/) and showed 100% similarity to the sequence of the uncultured bacterium of the order Bacteroidales (clone 3E) found in the hydrogen-producing microbial electrolysis cell (Croese et al., 2011). Thus, this bacterium might prefer an environment with H2 and be enriched in the FB and AS reactors. Methyloversatilis spp. and Erysipelothrix spp. were the predominant bacteria in the AS reactor. Methyloversatilis spp. were frequently found in anoxic environments, such as those similar to up-flow anaerobic sludge blanket reactors (Han et al., 2018) and denitrification reactors fed with methanol (Baytshtok et al., 2009). Erysipelothrix spp. have also been detected in the biofilm of denitrification reactors (Mielcarek et al., 2017; Pishgar et al., 2019). Because the conditions of the AS reactor were anoxic and reductive (Table S1), Methyloversatilis spp. and Erysipelothrix spp. may also exist as members of the bacterial community. The predominance of these bacteria might be related to the maintenance of total bacterial abundance in the SS (Fig. S3), and the increase in MLSS and TAB (Fig.3) in the AS reactor, despite the lower denitrification performance than that of the FB reactor.
4 Conclusions
The present study demonstrates the efficacy of FB diffuser application in enhancing the performance of an attached biofilm HD reactor. The reduction in H2 consumed by approximately 77% can be achieved using FB diffuser to reach similar KLa compared to the AS diffuser producing ordinary bubble. The high gas dissolution efficiency of the FB diffuser can enhance the NRR of the HD reactor while reducing the amount of H2 required. The highest value of NRR at 53.0±9.8 g-N/m3/d was obtained in the FB reactor at a minimum HRT of 3 h, which was two-fold higher than the corresponding value from the AS reactor. The FB reactor also had the lowest requirement of H2 for denitrification reaching 0.1 m3-H2/g-N in this condition, which was ten-fold lower than that in the AS reactor. Additionally, the application of FB diffuser significantly suppressed the accumulation of excess suspended sludge inside the HD reactor. In terms of the microbial community structure, Thauera spp. was the predominant genus, representing 15.7–27.3% of the total bacterial abundance during reactor operation, highlighting its contribution to HD in the FB reactor. Optimization of the operational condition of the HD reactor with an FB diffuser and evaluation of its long-term performance deserve further investigation in order to develop an effective groundwater treatment technology for nitrate-contaminated groundwater.
References
Ahmed, A. K. A., Sun, C., Hua, L., Zhang, Z., Zhang, Y., Zhang, W., & Marhaba, T. (2018). Generation of nanobubbles by ceramic membrane filters: The dependence of bubble size and zeta potential on surface coating, pore size and injected gas pressure. Chemosphere, 203, 327–335. https://doi.org/10.1016/J.CHEMOSPHERE.2018.03.157
American Society of Civil Engineers. (2007). Measurement of oxygen transfer in clean water (pp. 2–6). American Society of Civil Engineers/EWRI.
APHA/AWWA/WEF. (1998). Standard methods for the examination of water and wastewater (20th ed.). American Public Health Association/American Water Works Association/Water Environmental Federation.
Baytshtok, V., Lu, H., Park, H., Kim, S., Yu, R., & Chandran, K. (2009). Impact of varying electron donors on the molecular microbial ecology and biokinetics of methylotrophic denitrifying bacteria. Biotechnology and Bioengineering, 102(6), 1527–1536. https://doi.org/10.1002/BIT.22213
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Lozupone, C. A., Turnbaugh, P. J., Fierer, N., & Knight, R. (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America, 108(Suppl 1), 4516–4522. https://doi.org/10.1073/pnas.1000080107
Chang, C. C., Tseng, S. K., & Huang, H. K. (1999). Hydrogenotrophic denitrification with immobilized Alcaligenes eutrophus for drinking water treatment. Bioresource Technology, 69(1), 53–58. https://doi.org/10.1016/S0960-8524(98)00168-0
Chen, D., Wang, H., Ji, B., Yang, K., Wei, L., & Jiang, Y. (2015). A high-throughput sequencing study of bacterial communities in an autohydrogenotrophic denitrifying bio-ceramsite reactor. Process Biochemistry, 50(11), 1904–1910. https://doi.org/10.1016/J.PROCBIO.2015.07.006
Chu, L. B., Xing, X. H., Yu, A. F., Sun, X. L., & Jurcik, B. (2008). Enhanced treatment of practical textile wastewater by microbubble ozonation. Process Safety and Environmental Protection, 86(5), 389–393. https://doi.org/10.1016/J.PSEP.2008.02.005
Croese, E., Pereira, M. A., Euverink, G. J. W., Stams, A. J. M., & Geelhoed, J. S. (2011). Analysis of the microbial community of the biocathode of a hydrogen-producing microbial electrolysis cell. Applied Microbiology and Biotechnology, 92(5), 1083–1093. https://doi.org/10.1007/S00253-011-3583-X
Eamrat, R., Tsutsumi, Y., Kamei, T., Khanichaidecha, W., Tanaka, Y., & Kazama, F. (2017). Optimization of hydrogenotrophic denitrification behavior using continuous and intermittent hydrogen gas supply. Journal of Water and Environment Technology, 15(2), 65–75. https://doi.org/10.2965/jwet.16-059
Eamrat, R., Tsutsumi, Y., Kamei, T., Khanichaidecha, W., Ito, T., & Kazama, F. (2020). Microbubble application to enhance hydrogenotrophic denitrification for groundwater treatment. Environment and Natural Resources Journal, 18(2), 156–165. https://doi.org/10.32526/ENNRJ.18.2.2020.15
Epsztein, R., Beliavski, M., Tarre, S., & Green, M. (2016). High-rate hydrogenotrophic denitrification in a pressurized reactor. Chemical Engineering Journal, 286, 578–584. https://doi.org/10.1016/J.CEJ.2015.11.004
Fujita, H., Fukuju, T., Matsuda, T., Hata, T., Nishiuchi, Y., & Sakamoto, M. (2021). Oxidation of benzaldehyde to benzoic acid using O2 fine bubbles. Journal of the Japan Petroleum Institute, 64(1), 10–16. https://doi.org/10.1627/JPI.64.10
Han, M. Y., Kim, M. K., & Shin, M. S. (2006). Generation of a positively charged bubble and its possible mechanism of formation. Journal of Water Supply: Research and Technology-Aqua, 55(7–8), 471–478. https://doi.org/10.2166/AQUA.2006.055
Han, Y., Guo, J., Zhang, Y., Lian, J., Guo, Y., Song, Y., Wang, S., & Yang, Q. (2018). Anaerobic granule sludge formation and perchlorate reduction in an upflow anaerobic sludge blanket (UASB) reactor. Bioresource Technology Reports, 4, 123–128. https://doi.org/10.1016/J.BITEB.2018.09.012
Hashimoto, K., Onzuka, A., Nishijima, W., Yamazaki, M., Aoki, M., & Sao, T. (2022). Effect of fine bubbles for washing of monolith type porous ceramic membranes treating oil-in-water emulsions. Chemosphere, 305, 135487. https://doi.org/10.1016/J.CHEMOSPHERE.2022.135487
Ikeura, H., Kobayashi, F., & Tamaki, M. (2011). Removal of residual pesticide, fenitrothion, in vegetables by using ozone microbubbles generated by different methods. Journal of Food Engineering, 103(3), 345–349. https://doi.org/10.1016/J.JFOODENG.2010.11.002
Karanasios, K. A., Vasiliadou, I. A., Pavlou, S., & Vayenas, D. V. (2010). Hydrogenotrophic denitrification of potable water: A review. Journal of Hazardous Materials, 180(1–3), 20–37. https://doi.org/10.1016/j.jhazmat.2010.04.090
Kim, J. Y., Song, M. G., & Kim, J. D. (2000). Zeta potential of nanobubbles generated by ultrasonication in aqueous alkyl polyglycoside solutions. Journal of Colloid and Interface Science, 223(2), 285–291. https://doi.org/10.1006/JCIS.1999.6663
Lapworth, D. J., Nkhuwa, D. C. W., Okotto-Okotto, J., Pedley, S., Stuart, M. E., Tijani, M. N., & Wright, J. (2017). Urban groundwater quality in sub-Saharan Africa: Current status and implications for water security and public health. Hydrogeology Journal, 25(4), 1093–1116. https://doi.org/10.1007/s10040-016-1516-6
Lee, K. C., & Rittmann, B. E. (2002). Applying a novel autohydrogenotrophic hollow-fiber membrane biofilm reactor for denitrification of drinking water. Water Research, 36(8), 2040–2052. https://doi.org/10.1016/S0043-1354(01)00425-0
Li, P., Takahashi, M., & Chiba, K. (2009). Enhanced free-radical generation by shrinking microbubbles using a copper catalyst. Chemosphere, 77(8), 1157–1160. https://doi.org/10.1016/J.CHEMOSPHERE.2009.07.062
Mao, Y., Xia, Y., & Zhang, T. (2013). Characterization of Thauera-dominated hydrogen-oxidizing autotrophic denitrifying microbial communities by using high-throughput sequencing. Bioresource Technology, 128, 703–710. https://doi.org/10.1016/j.biortech.2012.10.106
Mielcarek, A., Rodziewicz, J., Janczukowicz, W., Dabrowska, D., Ciesielski, S., Thornton, A., & Struk-Sokołowska, J. (2017). Citric acid application for denitrification process support in biofilm reactor. Chemosphere, 171, 512–519. https://doi.org/10.1016/J.CHEMOSPHERE.2016.12.099
Nakagawa, K., Amano, H., Takao, Y., Hosono, T., & Berndtsson, R. (2017). On the use of coprostanol to identify source of nitrate pollution in groundwater. Journal of Hydrology, 550, 663–668. https://doi.org/10.1016/J.JHYDROL.2017.05.038
Nguyen, T. T., Ngo, H. H., Guo, W., Johnston, A., & Listowski, A. (2010). Effects of sponge size and type on the performance of an up-flow sponge bioreactor in primary treated sewage effluent treatment. Bioresource Technology, 101(5), 1416–1420. https://doi.org/10.1016/J.BIORTECH.2009.07.081
Peter-Varbanets, M., Zurbrügg, C., Swartz, C., & Pronk, W. (2009). Decentralized systems for potable water and the potential of membrane technology. Water Research, 43(2), 245–265. https://doi.org/10.1016/J.WATRES.2008.10.030
Pishgar, R., Dominic, J. A., Sheng, Z., & Tay, J. H. (2019). Denitrification performance and microbial versatility in response to different selection pressures. Bioresource Technology, 281, 72–83. https://doi.org/10.1016/J.BIORTECH.2019.02.061
Reis, A. S., Reis Filho, A. M., Demuner, L. R., & Barrozo, M. A. S. (2019). Effect of bubble size on the performance flotation of fine particles of a low-grade Brazilian apatite ore. Powder Technology, 356, 884–891. https://doi.org/10.1016/J.POWTEC.2019.09.029
Rezania, B., Cicek, N., & Oleszkiewicz, J. A. (2005). Kinetics of hydrogen-dependent denitrification under varying pH and temperature conditions. Biotechnology and Bioengineering, 92(7), 900–906. https://doi.org/10.1002/bit.20664
Rujakom, S., Shinoda, K., Singhopon, T., Nakano, M., Kamei, T., & Kazama, F. (2020). Effect of bicarbonate on the performance of hydrogen-based denitrification at different hydraulic retention times. Japanese Journal of Water Treatment Biology, 56(3), 33–45. https://doi.org/10.2521/jswtb.56.33
Shakya, B., Nakamura, T., Kamei, T., Shrestha, D. S., & Nishida, K. (2019). Seasonal groundwater quality status and nitrogen contamination in the shallow aquifer system of the Kathmandu Valley. Nepal. Water, 11(10), 2184. https://doi.org/10.3390/w11102184
Shrimali, M., & Singh, K. P. (2001). New methods of nitrate removal from water. Environmental Pollution, 112(3), 351–359. https://doi.org/10.1016/S0269-7491(00)00147-0
Smith, R. L., Buckwalter, S. P., Repert, D. A., & Miller, D. N. (2005). Small-scale, hydrogen-oxidizing-denitrifying bioreactor for treatment of nitrate-contaminated drinking water. Water Research, 39(10), 2014–2023. https://doi.org/10.1016/J.WATRES.2005.03.024
Suthar, S., Bishnoi, P., Singh, S., Mutiyar, P. K., Nema, A. K., & Patil, N. S. (2009). Nitrate contamination in groundwater of some rural areas of Rajasthan India. Journal of Hazardous Materials, 171(1–3), 189–199. https://doi.org/10.1016/j.jhazmat.2009.05.111
Temesgen, T., Bui, T. T., Han, M., Kim il, T. I., & Park, H. (2017). Micro and nanobubble technologies as a new horizon for water-treatment techniques: A review. Advances in Colloid and Interface Science, 246, 40–51. https://doi.org/10.1016/J.CIS.2017.06.011
Vasiliadou, I. A., Karanasios, K. A., Pavlou, S., & Vayenas, D. V. (2009). Experimental and modelling study of drinking water hydrogenotrophic denitrification in packed-bed reactors. Journal of Hazardous Materials, 165(1–3), 812–824. https://doi.org/10.1016/j.jhazmat.2008.10.067
World Health Organization (WHO). (2017). Guidelines for drinking-water quality (4th ed.). Publication of World Health Organization.
Wu, Z., Zhang, X., Zhang, X., Li, G., Sun, J., Zhang, Y., Li, M., & Hu, J. (2006). Nanobubbles influence on BSA adsorption on mica surface. Surface and Interface Analysis, 38(6), 990–995. https://doi.org/10.1002/SIA.2326
Wu, Z. H., Chen, H. B., Dong, Y. M., Mao, H. L., Sun, J. L., Chen, S. F., Craig, V. S. J., & Hu, J. (2008). Cleaning using nanobubbles: Defouling by electrochemical generation of bubbles. Journal of Colloid and Interface Science, 328(1), 10–14. https://doi.org/10.1016/J.JCIS.2008.08.064
Yao, G. J., Ren, J. Q., Zhou, F., Liu Di, Y. D., & Li, W. (2021). Micro-Nano aeration is a promising alternative for achieving high-rate partial nitrification. Science of the Total Environment, 795, 148899. https://doi.org/10.1016/J.SCITOTENV.2021.148899
Zhang, Y., Zhong, F., Xia, S., Wang, X., & Li, J. (2009). Autohydrogenotrophic denitrification of drinking water using a polyvinyl chloride hollow fiber membrane biofilm reactor. Journal of Hazardous Materials, 170(1), 203–209. https://doi.org/10.1016/j.jhazmat.2009.04.114
Zhang, X., Chen, X., Zhang, C., Wen, H., Guo, W., & Ngo, H. H. (2016). Effect of filling fraction on the performance of sponge-based moving bed biofilm reactor. Bioresource Technology, 219, 762–767. https://doi.org/10.1016/J.BIORTECH.2016.08.031
International Organization for Standardization (2017). Fine bubble technology—General principles for usage and measurement of fine bubbles - Part 1: Terminology [WWW Document]. ISO 20480-12017, Retrieved from https://www.iso.org/standard/68187.html (accessed 10.14. 2022)
United Nations (2016). Sustainable development goals, Retrieved from http://www.un.org/sustainabledevelopment/sustainable-development-goals/ Retrieved 5 December 2022
Vilaida, X., Kythavone, S., & Iijima, T. (2019). Effect of throat size on performance of microbubble generator and waste water treatment. IOP Conference Series: Materials Science and Engineering, 639(1). https://doi.org/10.1088/1757-899X/639/1/012031
Acknowledgements
The authors would like to thank Editage (www.editage.com) for the English language editing.
Data Availability Statement
All relevant data are included in the document or its Supplementary Information.
Funding
This research was funded by the Japanese Science and Technology Agency for partial financial support (Solution-Driven Co-creative R&D Program for SDGs [SOLVE for SDGs], grant number JPMJRX21I7).
Author information
Authors and Affiliations
Contributions
Writing—original draft, TK; writing—review and editing, HN, HK, TS, SR, RE, TN, KN; conceptualization, TK, HN, HK, TS; investigation, TK, HN; methodology; TK, HK, TN, KN; visualization, TK, HN. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamei, T., Narushima, H., Kodera, H. et al. Efficacy of a Fine Bubble Diffuser in Enhancing Attached Biofilm Hydrogenotrophic Denitrification Reactor Performance. Water Air Soil Pollut 234, 163 (2023). https://doi.org/10.1007/s11270-023-06175-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06175-1