Abstract
Microplastics (MP) are contaminants able to cause adverse effects on organisms. MPs are capable to interact with other environmental contaminants, including pesticides, altering their toxicity. The objective of the study was to research the sublethal effects (enzymatic activity) of pesticides alone and in combination with MPs. Cholinesterase enzymes are used as biomarkers to determine and evaluate the effects produced in organisms after exposure to pollutants. This study showed the acetylcholinesterase (AChE) enzymatic activity in the tissue of Solea senegalensis exposed to two environmental pollutants, the insecticide chlorpyrifos (CPF) and antibacterial triclosan (TCS) with and without microplastics (MPs). Solea senegalensis was chosen because it is a species in high demand because of its high economic value in southern Europe, as well as the use of this species in ecotoxicology and its increasing use as sentinel species, which justify using it to assess biological effects of pollutants. Toxicity tests were performed in organisms exposed to concentrations of between 5 and 80 μg/L CPF and 0.1 and 0.4 mg/L TCS for 96 h. In addition, each test incorporated MPs that were added at different concentrations in order to evaluate their role as a possible enhancer of the effects caused by the previous pollutants. In the case of CPF, the head and muscle tissue cholinesterase activity was inhibited from a concentration of 5 μg/L both without and with MPs, and the AChE enzymatic activity for the treatment with MPs was approximately half the activity for the treatment without MPs in the tissues studied. Besides, TCS inhibited the cholinesterase activity at a concentration of 0.3 mg/L in the muscle of S. senegalensis. In contrast, no significant differences were observed in the TCS + MP treatment compared to the controls. These results showed the importance of studies in assessing the anticholinesterase effects of pesticides combined with microplastics due to the abundance of these contaminants in the marine environment and the role of cholinesterase activity (biomarker) in the neurotransmission of key physiological processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microplastics (MPs) are tiny particles less than 5 mm in diameter that are present in air, soil, seas, oceans, plants, and animals. Plastics originate from plastic products, textiles, industry, agriculture, and waste in general and belong to the primary source of pollution. The secondary source of MPs arises from the physical and mechanical degradation of larger plastics over time (Garrido et al., 2019; Hermabessiere et al., 2017; Xia et al., 2020).
Cosmetic products present a serious threat to the environment because they are being discharged into water bodies and partially blocked by sewage treatment plants. Environmental associations have been fighting to ban MPs in PCCPs (pharmaceuticals and personal care products), and many countries have taken action and prohibited MPs or are in the process of doing so. Some cosmetics companies have even voluntarily renounced the use of microbeads (solid primary microplastics < 5 mm in diameter that are added to cosmetic products for cleansing and/or exfoliation of the skin). However, after the use of the microbeads, companies discharge them into the drain and they end up in wastewater treatment plants (WWTPs), from which they can escape into the seas and oceans. Once disposed, there is no efficient method of recovery and the environmental conditions do not allow for full biodegradation. Due to the possible effect of microbeads on the environment and human health and taking into account that alternatives have always been available at a similar cost, researchers have suggested banning microbeads in cosmetic products; nevertheless, the damage is done and they are still in the environment.
Microbeads, a type of MPs, are persistent and accumulate in the water and sediment column (Cole et al., 2011) where their small size facilitates the interaction with a large number of organisms (Bergmann et al., 2015) so that when their concentration increases, the bioavailability increases. MPs may induce toxic effects (Ferreira et al., 2016; Oliveira et al., 2013), as they may contain hazardous chemicals that were added during the manufacturing process to give them certain properties (chemical additives against heat, UV rays, corrosion, preservatives, etc.). For instance, MPs have been shown to influence the location, biotransformation, and/or toxicity of polybrominated diphenyl esters (PBDEs), a compound widely used as a flame retardant in plastics and foams (Meeker et al., 2009), in fish (Oliveira et al., 2013; Rochman et al., 2015) and other organisms (Avio et al., 2015; Chua et al., 2014; Paul-Pont et al., 2016).
In turn, MPs have the ability to interact with and retain environmental contaminants, such as heavy metals (mercury, chromium, cobalt, nickel, copper, zinc, cadmium, and lead) (Barboza et al., 2018; Holmes et al., 2012; Luís et al., 2015), polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) (Mato et al., 2001; Rochman et al., 2015), pharmaceuticals and personal care products (Fonte et al., 2016; Wardrop et al., 2016), and hydrophobic organic pollutants (Guo et al., 2012) such as the insecticide chlorpyrifos (CPF) and the antimicrobial triclosan (TCS). Therefore, many contaminants are introduced into organisms when they consume MPs, which can lead to an increase in the accumulation of these substances in the food chain (Batel et al., 2016; Setälä et al., 2014; Teuten et al., 2009). MPs can be ingested by different organisms (Besseling et al., 2013; De Sá et al., 2015; Fossi et al., 2012; Frias et al., 2014; Goldstein & Goodwin, 2013; Güven et al., 2017; Romeo et al., 2015; Rummel et al., 2016), including certain species that are part of the human diet (Battaglia et al., 2016; Neves et al., 2015; Rochman et al., 2015; Silva-Cavalcanti et al., 2017). Intake of MPs has been investigated in a large number of benthic organisms under controlled laboratory conditions (Cole et al., 2011; Wright et al., 2013). These studies demonstrate that earthworms (Arenicola marina), amphipods (Orchestia gammarellus), sea cucumbers (Holothuria sp.), sea urchins (Tripneustes gratilla), and mussels (Mytilus sp.), fish (Girella laevifrons), and flatfish (Soles sp.) are capable of feeding on MPs (Ahrendt et al., 2020; Avio et al., 2015; Graham & Thompson, 2009; Pellini et al., 2018; Thompson et al., 2004; Kaposi et al., 2014; Wegner et al., 2012). However, many aspects of MPs are still unknown, so some of the current challenges regarding this pollutant are to increase knowledge about their origin, fate, and behaviour in water, including the effects of its fragmentation, bioaccumulation, and impact on ecosystems and human health (Barboza & Giménez, 2015) and besides there is also little information on MP toxicity to fish in general and flatfish and sole in particular.
Pesticides are chemical products used in agriculture to minimize crop pests and assure agricultural production. The use of pesticides has increased in recent decades due to intensive agriculture. These compounds are not selective and can cause a variety of toxic effects on non-target species, such as insects, birds, fish, mammals, and humans (Aktar et al., 2009; Côlović et al., 2011; Costa et al., 2008; Ramesh & Muniswamy, 2009). Their use carries a very significant human and environmental cost. Pesticides present in the soil, air, surface water, groundwater, and wastewater pose risks to the environment, living organisms, and human health (Masia et al. 2015). Due to their mechanism of action, exposure to pesticides such as CPF appears to increase the predisposition to neurodegenerative diseases such as Alzheimer’s and Parkinson’s (Gupta et al., 2017). The use of the pesticide CPF has been used for more than 30 years as a quick, easy, and economical solution to control weeds, insects, and pests in general. However, in June 2020, the European Union banned the use of plant protection products that included CPF (European Commission, 2020). Nevertheless, the use of CPF outside the European Union has remained significant, which means that CPF continues to enter aquatic environments and, thus, spreads globally via ocean currents (Bonifacio et al., 2017). This pesticide has been one of the most used insecticides for pest control in Spain, especially in citrus farms and vineyards. CPF is liposoluble, with a high octanol:water coefficient and a high vapour tension at ordinary temperatures (Ferrer, 2003), so it is able to easily enter the environment or living beings as a result of the activities that take place in these areas. CPF is transferred from air or surface runoff into natural waters, where it accumulates in different organisms, especially fish, making them vulnerable to diverse effects. Deb & Das, (2013) reviewed the works on various effects of CPF in fish and they observed to this pesticide can adversely affect the non-target in fish. They reviewed cholinesterase inhibition, oxidative stress, disruption of the endocrine system, and behavioural, neuro-, and developmental toxicity were some of the probable manifestations of CPF toxicity in fish.
In the case of humans, ingestion, inhalation of aerosols, or contact with the skin and/or eyes are processes that can take place both in the occupational and non-occupational environment (Gupta et al., 2017). Most of the available studies focus on exposure to organophosphates through either accidental or voluntary ingestion by suicide. In these cases, the compound is rapidly absorbed, distributed through the bloodstream, and metabolized in the liver to finally be eliminated through the kidneys as metabolites or as the original compound (Castrejón-Godínez et al., 2014; Gupta et al., 2017). On the other hand, while absorption by inhalation is considered a rapid process, the entry of CPF through the skin is slower compared to other organophosphates (Gupta et al., 2017); however, this process is facilitated by skin lesions and/or warm temperatures (Ferrer, 2003).
Human activities that generate disturbances in the biota do not necessarily have to be on a large scale like agriculture; they can also be simpler and more everyday actions that, as a whole, involve large impacts on the environment. This is the case of such common activities as health care or personal hygiene through the frequent use of PPCPs, many of which contain triclosan (TCS). This compound suffers from multiple controversies about its toxic effects, and although it is not banned in Spain, good industry practices in the last few years are to discard it. In fact, in 2016, the FDA, the US drug agency, banned its use in soaps. As mentioned above, many PPCPs often contain MPs (Lusher et al., 2017). These MPs are usually of primary origin; that is they are industrially manufactured at a smaller size than previously established and then added to personal care and hygiene products. They are used for a wide variety of purposes, for example as an absorption phase for active ingredients, viscosity variation, and exfoliation. Some products contain both added plastic and ingredients such as the plastic in which they are packaged (Leslie, 2015). In these cases, the amount of added plastic represents 10% of the weight of the product and several thousand microbeads per gram of the product (Lassen et al., 2015). Both TCS and MPs are released with the natural use of the products containing them, and by rinsing them, they are introduced into the sewage network and later reach the wastewater treatment plants (which retain and eliminate them more or less efficiently) that discharge into rivers and seas. Some studies confirm that PPCPs are one of the main sources of primary MPs (Boucher & Friot, 2017). In the specific case of TCS and as pointed out by Singer et al., (2002), these systems offer a high general elimination rate (79% in biological degradation and 15% in sorption on the mud), with only 6% remaining to be released into the environment. However, despite the small percentage, the discharge of effluents from sewage treatment plants and sludge deposits on land are the main route of entry of TCS into the natural environment (Ying et al., 2007). Agüera et al., (2003) quantified the concentrations of the compound present in the wastewater from a treatment plant located in Almería (Spain) and revealed that this plant discharged concentrations of 400 ng/L and 800 ng/L in April and May, respectively, and up to 22,100 ng/L and 19,600 ng/L in June and July, respectively, into the Mediterranean Sea. These environmental values, together with the chemical properties of TCS, suggest that this pollutant can be highly persistent and easily bioaccumulated (Dhillon et al., 2015 in Maulvault et al., 2019), and many authors consider it to be toxic in aquatic organisms (Orvos et al., 2002; Perron et al., 2012).
Like the debate on the use of CPF, the biological safety of TCS has been strongly questioned during the last 10 years because several in vitro and in vivo studies have shown that TCS can disrupt cellular and metabolic functions and possibly induce cancer. To date, the relationship between TCS use and cancer is still controversial; however, it cannot be ignored that TCS has negative effects under certain laboratory conditions (García et al., 2016). Despite the contradictions, the majority of research concludes that TCS is an endocrine disruptor (Hedrick-Hopper et al., 2015; Matozzo et al., 2012; Wang & Tian, 2015), reduces growth (Gao et al., 2015), and causes impairment of the immune system, metabolism, and detoxification mechanisms (Mi et al., 2018; Regnault et al., 2016). Some studies point to a relationship between the presence of TCS and neurotoxic effects such as enzymatic inhibition of esterases responsible for the transmission of the nerve impulse, especially acetylcholinesterase (AChE) (Kim et al., 2018; Maulvault et al., 2019; Solé et al., 2012, 2015). On the contrary, other research denies the relationship between the antibacterial and the adverse effects of its consumption. Allmyr et al., (2009) observed an increase in TCS in plasma after exposure to toothpaste containing TCS, but this did not correspond to significant changes in thyroid hormones. The study by Cullinan et al., (2012) concluded that after 4 years of administration of toothpaste containing TCS, no effects on thyroid hormones were detected. Bhargava & Leonard, (1996) made a compilation of independent research concerning the toxicity of TCS, which clearly showed negative results for mutagenicity, reproduction, and embryonic development. Therefore, many authors concluded that the normal use of PPCPs containing TCS does not cause significant alterations (Allmyr et al., 2009), which supports the decision of the European Parliament and the Council to set the level at 0.3% as safe (Cullinan et al., 2012) in toothpastes, bars of soap, liquid soaps, shower gels, deodorants, facial powders, corrective creams, and nail products when the intended use is to clean the fingernails and toes before applying artificial nail systems and at a maximum concentration of 0.2% in the case of mouthwashes (Regulation (EC) No 1223/2009).
Senegalese sole is a species of great interest in aquaculture due to rapid growth and larval development. In the last few years, this species has been selected for toxicity tests of certain compounds that are present in the southern Mediterranean area, among them, monitoring studies of contaminants, in which tissues from the head and the muscle are chosen for the neurotoxic determination of the compound using cholinesterase enzymes (ChE) as a biomarker (Solé et al., 2012) after exposure to contaminants (Alves et al., 2015; Gomes et al., 2014; Oliveira et al., 2009; Solé et al., 2008, 2012; Solé & Sanchez-Hernandez, 2015; Albendín et al., 2021).
In view of the background, the general aim of the work was to study the possible adverse effects of MPs from commercial cream and their interaction with triclosan (biocide) and chlopryrifos (insecticide) on juveniles of Solea senegalensis, that is to determine the role of MPs from cosmetics as a possible enhancer of the effect produced by CPF and TCS.
2 Material and Methods
2.1 Chemicals
Acetylthiocholine iodide (AcSCh) (Sigma-Aldrich), 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) (Merck), acetone (Sharlau), 5-chloro-2-(2,4-dichlorophenoxy)phenol (triclosan (CAS 3380–34-5, Sigma-Aldrich), O,O-dietil O-3,5,6-tricloro-2-piridil fosforotionato (chlorpyrifos), (CAS 2921–88-2, Sigma-Aldrich), di-sodium hydrogen phosphate anhydrous (CAS 7558–79-4, Merck), sodium dihydrogen phosphate monohydrate (CAS 10049–21-5, Merck), and supermarket facial moisturiser creams were used. Bovine serum albumin (BSA) and the Bio-Rad Protein Assay were supplied by Bio-Rad (Madrid, Spain).
2.2 Biological Material
Solea senegalensis (n = 184) had an average body mass of 3.39 ± 0.56 g and were supplied by Marine Culture Laboratory (registration number ES110280000312) at the Marine and Environmental Sciences Faculty (University of Cádiz). The experimental protocols were in compliance with the current legislation concerning animal experimentation. This legislation includes Directive 2010/63/EU and Royal Decree 53/2013, both of which relate to the protection and correct treatment of animals used in animal experimentation.
2.3 Extraction and Characterisation of Microplastics
Commercial microbeads were extracted from a facial scrub according to the method published in Fendall and Sewell (2009). The facial scrub was a viscous liquid purchased at a supermarket close to Cádiz SPAIN.
MPs were extracted using woven wire sieves of varying mesh sizes. The diameter of the MPs ranged from 100 to 500 µm.
Attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR) was employed to record the spectra of the polymer. ATR-FTIR spectra were measured on a PerkinElmer Spectrum 100™ FTIR. Samples were placed onto the ATR accessory, and a constant force was applied using an integrated torque press. Spectra were subjected to a library search routine of the SpectrumTM software using a library set that included the Spectrum of the polymer.
Then, ATR-FTIR spectra were scanned from 4000 to 650 cm−1, averaging 16 scans per spectra at a resolution of 4 cm−1. The spectra were obtained with respect to the background and under the same measurement conditions. Afterwards, the internal reflection element (IRE) was carefully cleaned with soft paper and a blank spectrum was recorded in order to verify the total elimination of the measured sample to avoid cross-contamination between samples.
2.4 Bioassay
The 96-h toxicity tests were conducted with continuous aeration and water renewal every 24 h at a temperature of 20–21 °C and a photoperiod of 12 h light/12 h dark exposure. The pH values in the tests varied between 7.49 and 7.63. The dissolved oxygen was 75.4–98.3%, and the conductivity was 48.20–49.37 mS/cm. There were no significant variations in the parameters of the aquariums. Only oxygen levels decreased because no external aeration system was used, but it was always above 75% saturation. As the minimum dissolved oxygen established by the OECD (2004) for the correct performance of toxicity tests was 60%, it was determined that these variations did not affect the organisms and, therefore, also the test results. S. senegalensis was treated in accordance with the ethical guidelines of the European Union Council (Council Directive 86/609/EEC).
The fish were acclimatised to laboratory conditions prior to experimentation: the physico-chemical parameters were in line with those of the toxicity test and the fish were fed ad libitum until the day before the test.
S. senegalensis was exposed to nominal concentrations of TCS (0.1–0.4 mg/L) and three concentrations of this compound mixed with MPs (triclosan: 0.1, 0.2, and 0.3 mg/L; microplastic: 0.150 mg/L). In addition, S. senegalensis was exposed to five nominal concentrations of CPF (5, 10, 20, 40, and 80 μg/L) and three concentrations of this compound mixed with microplastics (chlorpyrifos: 5, 10, and 20 μg/L; microplastics: 0.150 mg/L), plus an untreated control and a solvent control (acetone < 0.01%). Eight fish for each concentration and control were used in each test series (four replicates for each treatment with two organisms). In these bioassays, no mortality of the organisms was observed for both compounds and their mixtures.
The MP concentration of 0.15 mg/L was chosen based on previous studies conducted with MPs of the same size (Ferreira et al., 2016; Fonte et al., 2016) because it is ecologically relevant according to the literature (Luís et al., 2015). CPF and TCS concentrations were prepared from a standard stock solution diluted in acetone. MP suspensions were prepared in Milli-Q water, placed in an ultrasonic bath for 20 min to achieve a homogeneous suspension, and kept in the dark at 2–4 °C to prevent microbial growth.
No food was supplied during exposure. Mortality was recorded every 24 h. After exposure, the fish were anesthetised with MS-222, sacrificed by decapitation, and the head and muscle tissues were dissected and stored at − 80 °C until processing.
2.5 Sample Preparation and Enzymatic Assays
The samples of S. senegalensis were kept ice-cold during the analyses for the determination of the cholinesterase activity. Muscle (0.9 ± 0.13 g) and head (0.5 ± 0.162 g) samples were cold homogenised in 0.1 M phosphate buffer (pH 7.4) at a rate of 1 mL phosphate buffer per 20 mg of S. senegalensis tissue in an Ultra-Turrax at high speed. Subsequently, the homogenate was centrifuged at 10,000 rpm for 30 min at 4 °C. The homogenate was filtered, and the supernatant was stored at − 80 °C until the analysis.
The ChE activity of S. senegalensis was obtained by applying the method of Ellman et al., (1961), modified for microplates as described by Albendín et al., (2021). A 96-well microplate was used in which 45 μL of homogenised sample, 5 μL of iso-OMPA inhibitor, and 250 μL of a reaction mixture containing Ellman’s reagent (DTNB) were added. The reaction mixture was prepared by adding 15 mL of 0.1 M phosphate buffer at pH 7.4, 0.5 mL of 10 mM dithiobisnitrobenzoic reagent (5,5′-dithiobis-(2-nitrobenzoic acid) or DTNB), and 100 μL of acetylthiocholine iodide (ASCh) dissolved in ultra-pure Milli-Q water. The final well concentrations were as follows: substrate (ASCh) 1.07 mM and DTNB 0.27 mM. The sample and the iso-OMPA inhibitor were incubated for 15 min, the reaction mixture was added, and immediately afterwards, the enzyme reaction rate was determined using a microplate reader (Bio-Rad, Mark Plus model) at 415 nm for 3 min every 30 s. Each sample and control was measured in triplicate. The percentages of the enzymatic activities including the concentration of the acetone control were calculated, considering the seawater control enzymatic activity as 100%.
The determination of protein concentration was carried out by the method of Bradford, (1976) adapted to microplates using bovine serum albumin (BSA) as the standard. In each well, 10 μL of sample or protein standard (0.625, 0.125, 0.25, and 0.5 mg/mL BSA) and 200 μL of Bradford’s reagent were added at the rate of 1:4. After the addition of the reagent, the mixture was left to react for 10 min at room temperature and then measured at 595 nm.
2.6 Statistical Analysis
Cholinesterase activity data was statistically analysed using the IBM SPSS Statistics for Windows Version 23 program. The assumptions of normality and homogeneity of variance of the data were tested using Shapiro–Wilk’s test and Levene’s test, respectively. When the assumption of normality was not satisfied, the Kruskal–Wallis test was used to determine whether there was any significant difference, and the Tukey or Mann–Whitney U test was used to determine the significant difference between groups.
When the assumption of normality was followed, the analysis of variance (one-way ANOVA) was employed to assess the differences in the inhibition of AChE activity among the different chemical compounds followed by Dunnet’s post hoc comparison test.
3 Results and Discussion
A large number of MPs are emitted annually into the seas and oceans, causing harm to aquatic organisms because they are consumed by fish (Dai et al., 2018; Wang et al., 2019). Therefore, MPs and other pollutants adsorbed by MPs accumulate in the exposed fish. Furthermore, organic pollutants present in MPs can affect the innate immunity of fish (Greven et al., 2016) and can even travel through the circulatory system of fish and damage organs such as the liver, causing adverse effects in aquatic organisms (Karami et al., 2017; Oliveira et al., 2013; Teuten et al., 2009). Likewise, there is evidence that chemical compounds can be adsorbed on microplastics (Ivar do Sul & Costa, 2014) and distributed throughout the body, being able to reach organs such as the liver, brain, and muscles (Karami et al., 2017; Oliveira et al., 2013; Teuten et al., 2009). Once there, they can inhibit enzymes such as cholinesterases, involved in nerve impulse transmission.
Our study examined this hypothesis by evaluating the effects of different treatments (CPF, TCS, CPF-MP, and TCS-MP) on cholinesterase enzyme in S. senegalensis. The MPs used were derived from the commercial creams identified as polyethilen. Although the Spanish authorities, following European regulations, planned to ban cosmetics and detergents containing intentionally added MPs by 2021, these MPs could remain in the environment and produce adverse effects on ecosystems.
In this study, MPs in the facial cleanser showed a wide size range between 1 and 5 mm. The composition of commercial microbeads was basically polyethylene (Fig. 1), and these MPs were added to the sole. The results obtained with the CPF and CPF + MP and TCS and TCS + MP bioassays in the muscle and head of S. senegalensis are shown in Figs. 2 and 3. In these assays, no mortality of the specimens was observed with both compounds and their mixtures with MPs. No physical or behavioural change was registered in the organisms exposed to this compound and its mixture with MPs.
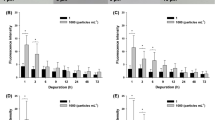
% AChE activity of S. senegalensis in the muscle after 96 h of exposure to different contaminant concentrations. a CPF, b CPF + MPs, c TCS, d TCS + MPs. Error bars represent standard deviations. *Significantly different between controls (SWC) and different treatments. SWC seawater control, ACC acetone control, CPF chlorpyrifos, MPs microplastics, TCS triclosan
% AChE activity of S. senegalesis in the head after 96 h of exposure to different contaminant concentrations. a CPF, b CPF + MPs (0.15 mg/L), c TCS, d TCS + MPs (0.15 mg/L). Error bars represent standard deviations. *Significantly different between controls (SWC) and different treatments. SWC seawater control, ACC acetone control, CPF chlorpyrifos, MPs microplastics, TCS triclosan
The AChE activity decreased as the insecticide concentration increased in the muscle and head of S. senegalensis. For the concentration of CPF (80 μg/L) without MPs, the cholinesterase activity was only 20% in the muscle of S. senegalensis. In addition, the AChE activity was also inhibited in the tests with CPF associated with MPs. Thus, MPs could exert a synergistic effect with the pesticide; for the same concentration, the AChE activity was reduced from 58.66 to 32.27% in concentrations of 10 μg/L CPF and 10 μg/L CPF + MP, respectively, and from 27.61 to 15.2% in concentrations of 20 μg/L CPF to 20 μg/L CPF + MP, respectively. Therefore, the activity obtained in the CPF + MP treatment was approximately half that of the single treatment.
Chlorpyrifos inhibited the AChE activity alone and combined with MPs in the muscle and head of S. senegalensis. These values coincided with the range of concentrations detected by other authors for individuals of the same species. According to Sánchez-Nogué & Varó Solé, (2013), CPF inhibited the muscle AChE activity in juvenile S. senegalensis and S. solea when concentrations were high (> 100 μM). Following the same concentration, Wheelock et al., (2005) suggested that high doses of the insecticide (approximately 100 μg/L) resulted in 100% mortality of juvenile Oncorhynchus tshawytscha salmon due to a decrease in the cholinesterase activity of 85% (brain) and 92% (muscle) compared to controls from a concentration of 7.3 μg/L CPF. For the species Oryzias latipes, exposure to sublethal concentrations of CPF caused a decrease in the AChE activity in the brain (Qiu et al., 2017). There was also evidence of a significant reduction in the AChE activity in the brain, liver, and muscle in Hypophthalmichthys molitrix carp exposed to another insecticide, deltamethrin (Ullah et al., 2021).
The AChE activity in the head of S. senegalensis, compared to the control, was altered in fish exposed to CPF and CPF + MPs (Fig. 3). The results showed that there was inhibition activity in the head of S. senegalensis in the presence of CPF. However, no significant differences between the same concentrations of chlorpyrifos and its mixture with MPs were observed.
In the muscle samples, the AChE activity decreased at TCS concentrations of 0.3 and 0.4 mg/L and showed values of 26.7% and 20.1%, respectively. Significant differences were observed with respect to the activity of the controls. The enzyme activity for TCS + MPs showed a tendency to decrease as the concentration of TCS increased in the muscle of S. senegalensis. However, the activity obtained at the maximum concentration of triclosan (0.3 mg/L TCS + 0.15 mg/L MPs) used in our test was higher than the activity values of the controls. Therefore, in view of these results and under these study conditions, the combination of TCS and MPs could not cause a significant variation in the AChE activity. The results obtained for TCS in this study can be compared with other authors, such as Matozzo et al., (2012), who showed that the AChE activity present in the gills of the clam Ruditapes philippinarum decreased when individuals were exposed to TCS concentrations between 300 and 900 ng/L. Falfushynska et al., (2017) examined the possible effects of typical compounds added to PPCPs on Pelophylax ridibundus, with TCS showing the greatest inhibition of the AChE enzyme activity. In both studies, the concentrations of TCS were 106 times lower than the concentrations used in our project. However, exposure to TCS increased the cholinesterase activity in Danio rerio embryos when they were exposed to 50 and 100 μg/L TCS, according to Falisse et al., (2017), and from 250 μg/L TCS, according to Oliveira et al., (2009). The latter authors researched on adults of the same species without obtaining significant changes in the enzyme activity.
The results obtained for the combined TCS + MPs assay showed no significant variations in the cholinesterase activity in the muscle or in the head of S. senegalensis. In comparison, Karami et al., (2017) did not detect significant changes in the cholinesterase activity of D. rerio larvae after exposure to low-density polyethylene fragments, which could indicate that this compound did not interact with organisms under short exposure conditions. Despite these results, the ability of MPs to depress acetylcholine activity has been described in juvenile goby fish Pomatoschistus microps (Oliveira et al., 2013) and in Mediterranean mussels, Mytilus galloprovincialis (Avio et al., 2015).
Nowadays, many studies show the effect of CPF and TCS on different organisms; however, there is limited information about the toxicity of both compounds individually or combined with MPs (ECCC, 2020; Albendín et al., 2021; Guilhermino et al., 2021). For many years, plastics seemed to be biochemically inert and not considered hazardous to the ecosystem health (Roy et al., 2011; Teuten et al., 2009), but there is a growing consensus among the scientific community and governments that a better understanding of the ecotoxicological effects of MPs, especially when combined with other pollutants, is needed (ECCC, Health Canada, 2020).
In our work, MPs combined with CPF exerted a synergistic effect with the pesticide. These results are in agreement with those obtained by other authors. Thus, revealed that CPF alone or combined with MPs induced a decrease in plasma AChE activity in common carp Cyprinus carpio. Similar results were observed in Prochilodus lineatus exposed to λ-cyhalothrin (insecticide) (Vieira & dos Reis Martínez, 2018) and in Oreochromis niloticus exposed to MPs and roxithromycin (Ding et al., 2018; Zhang et al., 2019), suggesting that these contaminated particles may inhibit acetylcholinesterase activity (Avio et al., 2017).
Barboza et al., (2018) reported that the increase in inhibition caused by the presence of MPs could indicate the neurotoxic nature of this pollutant. Furthermore, several authors suggested that MPs alone can decrease the cholinesterase activity (Avio et al., 2015, Oliveira et al., 2013), which would explain why cholinesterase activity of S. solea of the MP treatment alone is lower than the values obtained for the seawater and acetone control of the same assay. Oliveira et al., (2013) showed that exposure to MPs (polyethylene) alone in a 96-h test, with concentrations of 18.4 μg/L and 184 μg/L, reduced the acetylcholinesterase activity by 22% in samples of Pomatoschistus microps. Luís et al., (2015) found similar results to the previous study, as exposure to MPs (polyethylene) reduced the cholinesterase activity by approximately 20% in a test carried out with juveniles of the same species also exposed for 96 h.
The significant changes in the cholinestarase activity in S. senegalensis after exposure to CPF alone and in combination with MPs provide evidence that enzyme inhibition can be used as an effective monitoring tool to detect CPF and CPF-MP toxicity in fish. Although this study used aquatic organisms under laboratory conditions, it may not adequately reflect the toxicity of the CPF-MP combination in the natural environment. Therefore, the toxic effects of pollutants combined with MPs should be further evaluated to better illustrate the mechanism of toxicity, particularly at the environmental dose of CPF-MP in fish. In addition, longer exposure would help to better understand the risks associated with individual CPFs and TCS or combined with MPs.
Low concentrations of MPs and other pollutants have been reported even in the most remote areas of the earth (Cabrera et al., 2022; Chu et al., 2019; Waller et al., 2017). The novelty of this study shows that even at ecologically relevant concentrations of MPs and low concentrations of CPF individually and in combination with MPs, they can generate adverse effects on fish. In realistic environmental scenarios, MPs of different sizes and shapes may be retained in the water column for some time, adsorbing various classes of pollutants before they are taken up by organisms. Therefore, further studies are required to assess the dose-dependent biological impacts of MP alone and when combined with other pollutants, as MP acts as a vector that could enhance the effect of other pollutants.
4 Conclusion
The results of the inhibition of the AChE activity by CPF and TCS exposure in S. senegalensis indicated significant toxicity of both compounds. The association of the insecticide with the MPs resulted in an increase in toxicity in the case of CPF; however, the combination MPs + TCS did not show any variation in the toxicity of the antibacterial. Therefore, the presence of MPs seems to affect the toxicity of the antibacterial of the muscle, AChE inhibition was observed upon exposure to 0.3 mg/L of TCS alone but was not observed upon exposure to TCS (0.3 mg/L) + MPs.
Data Availability
The authors declare that the relevant data supporting the findings of this study are available within the article.
References
Agüera, A., Fernández-Alba, A. R., Piedra, L., Mézcua, M., & Gómez, M. J. (2003). Evaluation of triclosan and biphenylol in marine sediments and urban wastewaters by pressurized liquid extraction and solid phase extraction followed by gas. Analytica Chimica, 180(2), 193–205. https://doi.org/10.1016/S0003-2670(03)00040-0
Ahrendt, C., Perez-Venegas, D. J., Urbina, M., Gonzalez, C., Echeveste, P., Aldana, M., Pulgar, J., Galbán-Malagón, C. (2020). Microplastic ingestion cause intestinal lesions in the intertidal fish Girella laevifrons. Marine Pollution Bulletin, 151, 110795. https://doi.org/10.1016/j.marpolbul.2019.110795
Aktar, M. W., Sengupta, D., & Chowdhury, A. (2009). Impact of pesticides use in agriculture: Their benefits and hazards. Interdisciplinary Toxicology, 2, 1–12. https://doi.org/10.2478/v10102-009-0001-7
Albendín, M. G., Mánuel-Vez, M. P., & Arellano, J. M. (2021). In vivo cholinesterase sensitivity of gilthead seabream (Sparus aurata) exposed to organophosphate compounds: Influence of biological factors. Ecological Indicators, 121, 107176. https://doi.org/10.1016/j.ecolind.2020.107176
Allmyr, M., Panagiotidis, G., Sparve, E., Ulf, D., & Sandborgh-Englund, G. (2009). Human exposure to triclosan via toothpaste does not changeCYP3A4 activity or plasma concentrations of thyroid hormones. Nordic Pharmacological Society. Basic & Clinical Pharmacology & Toxicology, 105, 339–344. https://doi.org/10.1111/j.1742-7843.2009.00455.x
Alves, L. M., Lemos, M. F. L., Correia, J. P. S., da Costa, N. A. R., & Novais, S. C. (2015). The potential of cholinesterases as tools for biomonitoring studies with sharks: Biochemical characterization in brain and muscle tissues of Prionace glauca. Journal of Experimental Marine Biology and Ecology, 465, 49–55. https://doi.org/10.1016/j.jembe.2015.01.006
Avio, C. G., Gorbi, S., Milan, M., Benedetti, M., Fattorini, D., d’Errico, G., Pauletto, M., Bargelloni, L., & Regoli, F. (2015). Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environmental Pollution, 198, 211–222. https://doi.org/10.1016/j.envpol.2014.12.021
Avio, C. G., Gorbi, S., & Regoli, F. (2017). Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Marine Environment Research, 128, 2–11. https://doi.org/10.1016/j.marenvres.2016.05.012
Barboza, L. G. A., & Giménez, B. C. G. (2015). Microplastics in the marine environment: Current trends and future perspectives. Marine Pollution Bulletin, 97, 5–12. https://doi.org/10.1016/j.marpolbul.2015.06.008
Barboza, L. G. A., Russo Vieira, L., & Guilhermino, L. (2018). Single and combined effects of microplastics and mercury on juveniles of the European sea bass (Dicentrarchus labrax): Changes in behavioral responses and reduction of swimming velocity and resistance time. Environmental Pollution, 236, 1014–1019. https://doi.org/10.1016/j.envpol.2017.12.082
Batel, A., Linti, F., Scherer, M., Erdinger, L., & Braunbeck, T. (2016). Transfer of benzo[a] pyrene from microplastics to Artemia nauplii and further to zebra fish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environmental Toxicology and Chemistry, 35, 1656–1666. https://doi.org/10.1002/etc.3361
Battaglia, P., Pedà, C., Musolino, S., Esposito, V., Andaloro, F., & Romeo, T. (2016). Diet and first documented data on plastic ingestion of Trachinotus ovatus L. 1758 (Pisces: Carangidae) from the Strait of Messina (central Mediterranean Sea). The Italian Journal of Zoology, 83, 121–129. https://doi.org/10.1080/11250003.2015.1114157
Bergmann, M., Gutow, L., Klage, M. (2015). Marine anthropogenic litter. Springer International Publishing. https://doi.org/10.1007/978-3-319-16510-3
Besseling, E., Wegner, A., Foekema, E. M., van den Heuvel-Greve, M. J., & Koelmans, A. A. (2013). Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L). Environmental Science & Technology, 47, 593–600. https://doi.org/10.1021/es302763x
Bhargava, H. N., & Leonard, P. A. (1996). Triclosan, application and safety. American Journal of Infection Control, 24, 209–18. https://doi.org/10.12691/ajmr-2-6-3
Bonifacio, A. F., Ballesteros, M. L., Bonansea, R. I., Filippi, I., Amé, M. V., & Hued, A. C. (2017). Environmental relevant concentrations of a chlorpyrifos commercial formulation affect two neotropical fish species, Cheirodon interruptus and Cnesterodon decemmaculatus. Chemosphere, 188, 486–493.
Boucher, J., & Friot, D. (2017). Primary microplastics in the oceans: A global evaluation of sources. Gland, Switzerland: IUCN. https://doi.org/10.2305/IUCN.CH.2017.01
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 254, 248–254.
Cabrera, M., Gabriel, M. M., Valencia, B. G., Maisincho, L., Rodríguez-Barroso, R., Albendín, G., Sakali, A., Lucas-Solis, O., Conicelli, & Capparelli, V. (2022). Microplastics in a tropical Andean Glacier: A transportation process across the Amazon basin? Science of the Total Environment, 20, 150334. https://doi.org/10.1016/j.scitotenv.2021.150334
Castrejón-Godínez, M. L., Ortiz, L., Sánchez-Salinas, E. (2014). Plaguicidas: Generalidades, Usos e Impactos sobre el Ambiente y la Salud. In: Los Plaguicidas en México. Aspectos Generales, Toxicológicos y Ambientales. Ed. 1a, Cpa 1. Universidad Autónoma del Estado de Morelos-Consejo de Ciencia y Tecnología del Estado de Morelos.
Chu, X., Li, T., Li, A., Yan, A., & Shen, C. (2019). Transport of microplastic particles in saturated porous media. Water, 11(12), 2472. https://doi.org/10.3390/w11122474
Chua, E. M., Shimeta, J., Nugegoda, D., Morrison, P. D., & Clarke, B. O. (2014). Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes compressa. Environmental Science and Technology, 48, 8127–8134. https://doi.org/10.1021/es405717z
Cole, M., Lindeque, P., Halsband, C., & Galloway, T. S. (2011). Microplastics as contaminants in the marine environment: A review. Marine Pollution Bulletin, 62, 2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
Čolović, M. B., Krstić, Z. K., Ušćumlić, G. S., & Vasić, V. M. (2011). Single and simultaneous exposure of acetylcholinesterase to diazinon, chlorpyrifos and their photodegradationproducts. Pesticide Biochemistry and Physiology, 100(1), 16–22. https://doi.org/10.1016/j.pestbp.2011.01.010
Costa, L. G., Giordano, G., Guizzetti, M., & Vitalone, A. (2008). Neurotoxicity of pesticides: A brief review. Frontiers in Bioscience (Landmark Ed), 13(4), 1240–1249. https://doi.org/10.2741/2758
Cullinan, M. P., Palmer, J. E., Carle, A., West, M. J., & Seymour, G. (2012). Long term use of triclosan toothpaste and thyroid function. Science of the Total Environment, 416, 75–79. https://doi.org/10.1016/j.scitotenv.2011.11.063
Dai, Z., Zhang, H., Zhou, Q., Tian, Y., Chen, T., & Tu, C. (2018). Occurrence of microplastics in the water column and sediment in an inland sea affected by intensive anthropogenic activities. Environmental Pollution, 242B, 1557–1565. https://doi.org/10.1016/j.envpol.2018.07.131
De Sá, L. C., Luís, L. G., & Guilhermino, L. (2015). Efects of microplastics on juveniles of the common goby (Pomatoschistus microps): Confusion with prey, reduction of the predatory performance and e ciency, and possible influence of developmental conditions. Environmental Pollution, 196, 359–362. https://doi.org/10.1016/j.envpol.2014.10.026
Deb, N., & Das, S. (2013). Chlorpyrifos toxicity in fish: A review. Current World Environment, 8(1), 1–7. https://doi.org/10.12944/CWE.8.1.17
Dhillon, G. S., Kaur, S., Pulicharla, R., Brar, S. K., Cledon, M., Verma, M., et al. (2015). Triclosan: Current status, occurrence, environmental risks and bioaccumulation potential. International Journal of Environmental Research and Public Health, 12, 5657–5684. https://doi.org/10.3390/ijerph120505657
Ding, J., Zhang, S., Razanajatovo, R. M., Zou, H., & Zhu, W. (2018). Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environmental Pollution, 238, 1–9. https://doi.org/10.1016/j.envpol.2018.03.001
Ellman, G. L., Courtney, K. D., Andres, V., & Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7(2), 88–95.
European Chemicals Agency. (2020). Microplastics. http://data.europa.eu/eli/reg_impl/2020/18/oj
European Commission. (2020). Commission implementing regulation (EU) 2020/18 of 10 January 2020 concerning the non-renewal of the approval of the active substance chlorpyrifos, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. Brussels, Belgium.
Falfushynska, H. I., Gnatyshyna, L. L., Oksana, H., Horyn, O., & Stoliara, O. B. (2017). Vulnerability of marsh frog Pelophylax ridibundus to the typical wastewater effluents ibuprofen, triclosan and estrone, detected by multi-biomarker approach. Comparative Biochemistry and Physiology Part C Toxicology & Pharmacology, 202, 26–38. https://doi.org/10.1016/j.cbpc.2017.07.004
Falisse, E., Voisin, A. S., & Silvestre, F. (2017). Impacts of triclosan exposure on zebrafish early-life stage: Toxicity and acclimation mechanisms. Aquatic Toxicology, 189, 97–107. https://doi.org/10.1016/j.aquatox.2017.06.003
Fendall, L. S., & Sewell, M. A. (2009). Contributing to marine pollution by washing your face: Microplastics in facial cleaners. Marine Pollution Bulletin, 58(8), 1225–1228.
Ferreira, P., Fonte, E., Soares, M. E., Carvalho, F., & Guilhermino, L. (2016). Effects of multi-stressors on juveniles of the marine fish Pomatoschistus microps: Gold nanoparticles, microplastics and temperature. Aquatic Toxicology, 170, 89–103. https://doi.org/10.1016/j.aquatox.2015.11.011
Ferrer, A. (2003). Intoxicación por plaguicidas. Anales del Sistema Sanitario de Navarra, 26, 155–171.
Fonte, E., Ferreira, P., & Guilhermino, L. (2016). Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): Post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquatic Toxicology, 180, 173–185. https://doi.org/10.1016/j.aquatox.2016.09.015
Fossi, M. C., Panti, C., Guerranti, C., Coppola, D., Giannetti, M., et al. (2012). Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean n whale (Balaenoptera physalus). Marine Pollution Bulletin, 64, 2374–2379. https://doi.org/10.1016/j.marpolbul.2012.08.013
Frias, J., Otero, V., & Sobral, P. (2014). Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Marine Environment Research, 95, 89–95. https://doi.org/10.1016/j.marenvres.2014.01.001
Gao, L., Yuan, T., Cheng, P., Bai, Q., Zhou, C., Ao, J., et al. (2015). Effects of triclosan and triclocarban on the growth inhibition, cell viability, genotoxicity and multixenobiotic resistance responses of Tetrahymena thermophila. Chemosphere, 139, 434–440. https://doi.org/10.1016/j.chemosphere.2015.07.059
García, G., Sánchez, A., Galindo, E., Cerda, B. (2016). Triclosan in toothpaste, is there any real risk for the health?. Odovtos International Journal of Dental Sciences 18. https://doi.org/10.15517/ijds.v0i0.24102
Garrido, S., Linares, M., Campillo, A. J., & Albentosa, M. (2019). Effect of microplastics on the toxicity of chlorpyrifos to the microalgae Isochrysis galbana, clone t-ISO. Ecotoxicology and Environmental Safety, 173, 103–109. https://doi.org/10.1016/j.ecoenv.2019.02.020
Goldstein, M. C., Goodwin, D. S. (2013). Gooseneck barnacles (Lepas spp) ingest microplastic debris in the North Pacific subtropical gyre. PeerJ1e:184. https://doi.org/10.7717/peerj.184
Gomes, D. L., Lemos, M. F. L., Soares, A. M. V. M., Barata, C., & Faria, M. (2014). The use of cholinesterase as potential biomarker: In vitro characterization in the polychaete Capitella teleta. Marine Pollution Bulletin, 85, 179–185. https://doi.org/10.1016/j.marpolbul.2014.05.053
Graham, E. R., & Thompson, J. T. (2009). Deposit- and suspension-feeding sea cucumber (Echinodermata) ingest plastic fragments. Journal of Experimental Marine Biology and Ecology, 36, 22–29. https://doi.org/10.1016/j.jembe.2008.09.007
Greven, A. N., Merk, T., Karagoz, F., Mohr, K., Klapper, M., Jovanovic, B., & Palíc, D. (2016). Polycarbonate and polystyrene nanoplastic particles act as stressors to the innate immune system of fathead minnow (Pimephales promelas). Environmental Toxicology and Chemistry, 35(12), 3093–3100. https://doi.org/10.1002/etc.3501
Guilhermino, L., Martins, A., Lopes, C., Raimundo, J., Vieira, L. R., Barboza, L. G. A., Costa, J., Antunes, C., Caetano, M., & Vale, C. (2021). Microplastics in fishes from an estuary (Minho River) ending into the NE Atlantic Ocean. Marine Pollution Bulletin, 173, A113008. https://doi.org/10.1016/j.marpolbul.2021.113008
Guo, X. Y., Wang, X. L., Zhou, X. Z., Kong, X. Z., Tao, S., & Xing, B. S. (2012). Sorption of four hydrophobic organic compounds by three chemically distinct polymers: Role of chemical and physical composition. Environmental Science and Technology, 46, 7252–7259. https://doi.org/10.1021/es301386z
Gupta, R. C. M., Mukherjee, I. R., Doss, R. B., Malik, J. K., Milatovic, D. (2017). Chapter 35 - Organophosphates and Carbamates. In: Gupta RC (Ed) Reproductive and Developmental Toxicology, Second Ed Academic Press/Elservier, Amsterdam 609–631. https://doi.org/10.1016/B978-0-12-804239-7.00035-4
Güven, O., Gökdağ, K., Jovanović, B., & Kıdeyş, A. E. (2017). Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea and its occurrence in the gastrointestinal tract of fish. Environmental Pollution, 223, 286–294. https://doi.org/10.1016/j.envpol.2017.01.025
Hedrick-Hopper, T. L., Koster, L. P., & Diamond, S. L. (2015). Accumulation of triclosan from diet and its neuroendocrine effects in Atlantic croaker (Micropogonias undulatus) under two temperature regimes. Marine Environment Research, 112, A52-60. https://doi.org/10.1016/j.marenvres.2015.09.006
Hermabessiere, L., Dehaut, A., Pont, I., Lacroix, C., Jezequel, R., Soudant, P., & Duflo, G. (2017). Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere, 182, 781–793. https://doi.org/10.1016/j.chemosphere.2017.05.096
Holmes, L. A., Turner, A., & Thompson, R. C. (2012). Adsorption of trace metals to plastic resin pellets in the marine environment. Environmental Pollution, 160, 42–48. https://doi.org/10.1016/j.envpol.2011.08.052
Ivar do Sul, J. A., & Costa, M. F. (2014). The present and future of microplastic pollution in the marine environment (review). Environmental Pollution, 185, 352–364. https://doi.org/10.1016/j.envpol.2013.10.036
Kaposi, K. L., Mos, B., Kelaher, B. P., & Dworjanyn, S. A. (2014). Ingestion of microplastic has limited impact on a marine larva. Environmental Science and Technology, 48, 1638–1645. https://doi.org/10.1021/es404295e
Karami, A., Golieskardi, A., Choo, C. K., Larat, L., Galloway, T. S., & Salamatinia, B. (2017). The presence of microplastics in commercial salts from different countries. Scientific Reports, 7, 46173. https://doi.org/10.1038/srep46173
Kim, J., Oh, H., Ryu, B., Kim, U., Min Lee, J., et al. (2018). Triclosan affects axón formation in the neural development stages of zebrafish embryos (Danio rerio). Environmental Pollution, 236, 304–301. https://doi.org/10.1016/j.envpol.2017.12.110
Lassen, C., Foss Hansen, S., Magnusson, K., Noren, F., Bloch Hartmann, N. I., Rehne Jensen, P., Gisel Nielsen, T., Brinch, A. (2015). Microplastics: Occurrence, effects and sources of releases to the environment in Denmark. The Danish Environmental Protection Agency.
Leslie, HA. (2015). Plastic in cosmetics: Are we polluting the environment through our personal care? Plastic ingredients that contribute to marine microplastic litter. 2015. 33 p.
Luís, L. G., Ferreira, P., Fontea, E., Oliveira, M., & Guilhermino, L. (2015). Does the presence of microplastics influence the acute toxicity of chromium (VI) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquatic Toxicology, 164, 163–174. https://doi.org/10.1016/j.aquatox.2015.04.018
Lusher, A., Hollman, P. Mendoza-Hill, J. (2017). Microplastics in fisheries and aquaculture: Status of knowledge on their occurrence and implications for aquatic organisms and food safety. FAO: Fisheries and Aquaculture Technical Paper 615
Mato, Y., Isobe, T., Takada, H., Kanehiro, H., Ohtake, C., & Kaminuma, T. (2001). Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environmental Science and Technology, 35, 318–324. https://doi.org/10.1021/es0010498
Matozzo, V., Formenti, A., Donadello, G., & Marin, M. G. (2012). A multi-biomarker approach to assess effects of triclosan in the clam Ruditapes philippinarum. Marine Environmental Research, 74, 40–46. https://doi.org/10.1016/j.marenvres.2011.12.002
Masiá, A., Campo, J., Navarro-Ortega, A., Barceló, D., & Picó, Y. (2015). Pesticide monitoring in the basin of Llobregat River (Catalonia, Spain) and comparison with historical data. Science of The Total Environment, 503–504, 58–68. https://doi.org/10.1016/j.scitotenv.2014.06.095
Maulvault, A. L., Camacho, C., Vera, B., Alves, R., Anacleto, P., Cunha, S. S., Fernandes, J. O., Pouão-Ferreira, P., Paula, J. R., Rosa, R., Diniz, M., & Marques, A. (2019). Bioaccumulation and ecotoxicological responses of juvenile white seabream (Diplodus sargus) exposed to triclosan, warming and acidification. Environmental Pollution, 245, 427–442. https://doi.org/10.1016/j.envpol.2018.11.020
Meeker, J. D., Sathyanarayana, S., & Swan, S. H. (2009). Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philosophical Transactions of the Royal Society of London. Series B, 364, 2097–2113. https://doi.org/10.1098/rstb.2008.0268
Mi, C., Teng, Y., Wang, X., Yu, H., Huang, Z., Zong, W., & Zou, L. (2018). Molecular interaction of triclosan with superoxide dismutase (SOD) reveals a potentially toxic mechanism of the antimicrobial agent. Ecotoxicology and Environmental Safety, 15, 78–83. https://doi.org/10.1016/j.ecoenv.2018.01.055
Neves, D., Sobral, P., Ferreira, J. L., & Pereira, T. (2015). Ingestion of microplastics by commercial sh of the Portuguese coast. Marine Pollution Bulletin, 101, 119–126. https://doi.org/10.1016/j.marpolbul.2015.11.008
Oliveira, R., Domingues, I., Koppe Grisolia, C., et al. (2009). Effects of triclosan on zebrafish early-life stages and adults. Environmental Science and Pollution Research, 16, 679–688. https://doi.org/10.1007/s11356-009-0119-3
Oliveira, M., Ribeiro, A., Hylland, K., & Guilhermino, L. (2013). Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecological Indicators, 34, 641–647. https://doi.org/10.1016/j.ecolind.2013.06.019
Orvos, D. R., Versteeg, D. J., Inauen, J., Capdevielle, M., Rothenstein, A., & Cunningham, V. (2002). Aquatic toxicity of triclosan. Environmental Toxicology and Chemistry, 21, 1338–1349. https://doi.org/10.1002/etc.5620210703
Paul-Pont, I., Lacroix, C., González Fernández, C., Hégaret, H., Lambert, C., Le Goïc, N., Frère, L., Cassone, A.-L., Sussarellu, S., Fabioux, C., Guyomarch, J., Albentosa, M., Huvet, A., & Soudant, P. (2016). Exposure of marine mussels Mytilus spp, to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environmental Pollution, 216, 724–737. https://doi.org/10.1016/j.envpol.2016.06.039
Pellini, G., Gomiero, A., Fortibuoni, T., Ferrà, C., Grati, F., Tassetti, A. N, Polidori, P., Fabi, G., Scarcella, G. (2018). Characterization of microplastic litter in the gastrointestinal tract of Solea solea from the Adriatic Sea. Environment Pollution 943–952. https://doi.org/10.1016/j.envpol.2017.12.038
Perron, M. M., Ho, K. T., Cantwell, M. G., Burgess, R. M., & Pelleterier, M. C. (2012). Effects of triclosan on marine benthic and epibenthic organisms. Environmental Toxicology and Chemistry, 31, 1861–1866. https://doi.org/10.1002/etc.5620210701
Qiu, X., Nomichi, S., Che, K., Honda, M., Kang, I. K. J., Shimasaki, Y., & Oshima, Y. (2017). Short-term and persistent impacts of behaviors related to locotion, ansiety, and startle responses of Japaese medaka (Oryzias latipes) induced by acute, sublethal exposure to chlorpyrifos. Aquatic Toxicology, 192, 148–154. https://doi.org/10.1016/j.aquatox.2017.09.012
Ramesh, H., & Muniswamy, D. (2009). Behavioural responses of the freshwater fish, Cyprinus carpio (Linnaeus) following sublethal exposure to clorpirifós. Turkish Journal of Fisheries and Aquatic Sciences, 9, 233–238. https://doi.org/10.4194/trjfas.2009.0218
Regnault, C., Willison, J., Veyrenc, S., Airieau, A., Meresse, P., et al. (2016). Metabolic and immune impairments induced by the endocrine disruptors benzo[a]pyrene and triclosan in Xenopus tropicalis. Chemosphere, 155, 519–527. https://doi.org/10.1016/j.chemosphere.2016.04.047
Rochman, C. M., Hoh, E., Kurobe, T., & Teh, S. J. (2013). Ingested plastic transfers hazardous chemicals to sh and induces hepatic stress. Scientific Reports, 3, 3263.
Rochman, C. M., Tahir, A., Williams, S. L., Baxa, D. V., Lam, R., Miller, J. T., Teh, F. C., Werorilangi, S., & Teh, S. J. (2015). Anthropogenic debris in seafood: Plastic debris and bers from textiles in sh and bivalves sold for human consumption. Scientific Reports, 5, 14340. https://doi.org/10.1038/srep14340
Romeo, T., Pietro, B., Pedà, C., Consoli, P., Andaloro, F., & Fossi, M. C. (2015). First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Marine Pollution Bulletin, 95, 358–361. https://doi.org/10.1016/j.marpolbul.2015.04.048
Roy, P. K., Hakkarainen, M., Varma, I. K., & Albertsson, A.-C. (2011). Degradable polyethylene: Fantasy or reality. Environmental Science and Technology, 45, 4217–4227. https://doi.org/10.1021/es104042f
Rummel, C. D., Löder, M. G., Fricke, N. F., Lang, T., Griebeler, E. M., Janke, M., & Gerdts, G. (2016). Plastic ingestion by pelagic and demersal sh from the North Sea and Baltic Sea. Marine Pollution Bulletin, 102, 134–141. https://doi.org/10.1016/j.marpolbul.2015.11.043
Sánchez-Nogué, B., & Varó Solé, M. (2013). Comparative analysis of selected biomarkers and pesticide sensitivity in juveniles of Solea solea and Solea senegalensis. Enviromental Science and Pollution Research, 20, 3480–3488. https://doi.org/10.1007/s11356-012-1355-5
Setälä, O., Fleming-Lehtinen, V., & Lehtiniemi, M. (2014). Ingestion and transfer of microplastics in the planktonic food web. Environmental Pollution, 185, 77–83. https://doi.org/10.1016/j.envpol.2013.10.013
Silva-Cavalcanti, J. S., Silva, J. D. B., França, E. J., Araújo, M. C. B., & Gusmão, F. (2017). Microplastics ingestion by a commmon tropical freshwater shing resource. Environmental Pollution, 221, 218–226. https://doi.org/10.1016/j.envpol.2016.11.068
Singer, H., Müller, S. C., & Tixier, P. L. (2002). Triclosan: Occurrence and fate of a widely used biocide in the aquatic environment: Field measurements in wastewater treatment plants, surface waters and lake sediments. Environmental Science and Technology, 36, 4998–5004. https://doi.org/10.1021/es025750i
Solé, M., & Sanchez-Hernandez, J. C. (2015). An in vitro screening with emerging contaminants reveals inhibition of carboxylesterase activity in aquatic organisms. Aquatic Toxicology, 69, 215–222. https://doi.org/10.1016/j.aquatox.2015.11.001
Solé, M., Lobera, G., Aljinovic, B., Ríos, J., García de la Parra, L. M., Maynou, F., & Cartes, J. E. (2008). Cholinesterases activities and lipid peroxidation levels in muscle from shelf and slope dwelling fish from the NW Mediterranean: Its potential use in pollution monitoring. Science of the Total Environment, 402, 306–317. https://doi.org/10.1016/j.scitotenv.2008.04.041
Solé, M., Vega, S., & Varó, I. (2012). Characterization of type “B” esterases and hepatic CYP450 isoenzimes in Senegalese sole for their further application in monitoring studies. Ecotoxicology and Environmental Safety, 78, 72–79. https://doi.org/10.1016/j.ecoenv.2011.11.013
Teuten, E. L., Saquing, J. M., Knappe, D. R., Barlaz, M. A., Jonsson, S., Björn, A., Rowland, S. J., Thompson, R. C., Galloway, T. S., Yamashita, R., Ochi, D., et al. (2009). Transport and release of chemicals from plastics to the environment and to wildlife. Philosophical Transactions of the Royal Society B, 364, 2027–2045. https://doi.org/10.1098/rstb.2008.0284
Thompson, R. C., Olsen, Y., Mitchell, R. P., Davis, A., Rowland, J. S., John, A. W. G., McGonigle, D., & Russell, A. E. (2004). Lost at sea: Where is all the plastic? Science, 304, 838. https://doi.org/10.1126/science.1094559
Ullah, R., Tsui, M. T. K., Chen, H., Chow, A., Williams, C., & Ligaba-Osena, A. (2021). Microplastics interaction with terrestrial plants and their impacts on agriculture. Journal of Environmental Quality, 50(5), 1024–1041. https://doi.org/10.1002/jeq2.20264
Vieira, C. E. D., & dos Reis Martínez, C. B. (2018). The pyrethroid λ-cyhalothrin induces biochemical, genotoxic, and physiological alterations in the teleost Prochilodus lineatus. Chemosphere, 210, 958–967. https://doi.org/10.1016/j.chemosphere.2018.07.115
Waller, C. L., Griffiths, H. J., Waluda, C. M., & Thorpe, S. E. (2017). Microplastics in the Antarctic marine system: An emerging area of research. Science of the Total Environment, 598(15), 220–227. https://doi.org/10.1016/j.scitotenv.2017.03.283
Wang, Y., Zhang, D., Zhang, M., Mu, J., Ding, G., Mao, Z., Cao, Y., Jin, F., Cong, Y., Wang, L., Zhang, W., & Wang, J. (2019). Effects of ingested polystyrene microplastics on brine shrimp, Artemia parthenogenetica. Environmental Pollution, 244, 715–722. https://doi.org/10.1016/j.envpol.2018.10.024
Wang, C. F., Tian, Y. (2015). Reproductive endocrine-disrupting effects of triclosan: Population exposure, present evidence and potential mechanisms. Environmental Pollution, 206, 195–201. https://doi.org/10.1016/j.envpol.2015.07.001
Wardrop, P., Shimeta, J., Nugegoda, D., Morrison, P. D., Miranda, A., Tang, M., & Clarke, B. O. (2016). Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish. Environmental Science and Technology, 50, 4037–4044. https://doi.org/10.1021/acs.est.5b06280
Wegner, A., Besseling, E., Foekema, E. M., Kamermans, P., & Koelmans, A. A. (2012). Effects of nanopolystyrene on the feeding behaviour of the blue mussels (Mytilus edulis L.). Environmental Toxicology and Chemistry, 31, 2490–3297. https://doi.org/10.1002/etc.1984
Wheelock, C. E., Shan, G., & Ottea, J. (2005). Overwiew of carboxylterases and their role in the metabolism of insecticides. Journal of Pesticide Science, 30(2), 75–83. https://doi.org/10.1584/jpestics.30.75
Wright, S., Thompson, R. C., & Galloway, T. S. (2013). The physical impacts of microplastics on marine organisms: A review. Environmental Pollution, 178, 483–492. https://doi.org/10.1016/j.envpol.2013.02.031
Xia, X., Sun, M., Zhou, M., Chang, Z., & Li, L. (2020). Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. Larvae. Science of the Total Environment, 716, 136479. https://doi.org/10.1016/j.scitotenv.2019.136479
Ying, G. G., Yu, X. Y., & Kookana, R. S. (2007). Biological degradation of triclocarban and triclosan in a soil under aerobic and anaerobic conditions and comparison with environmental fate modelling. Environmental Pollution, 150, 300–305. https://doi.org/10.1016/j.envpol.2007.02.013
Zhang, P., Yan, Z., Lu, G., & Ji, Y. (2019). Single and combined effects of microplastics and roxithromycin on Daphnia magna. Environmental Science and Pollution Research, 26, 17010–17020. https://doi.org/10.1007/s11356-019-05031-2
Acknowledgements
The authors are grateful to the Laboratory of Marine Culture at the University of Cádiz for providing the experimental fish used in this study and to Maribel Arufe and Inmaculada Cabrera-Pozo for their invaluable assistance.
Funding
Funding for open access publishing: Universidad de Cádiz/CBUA. This project has received funding from the Ministerio de Ciencia, Investigación y Universidades (REF.: RTI2018-096771-B-100).
Author information
Authors and Affiliations
Contributions
Arellano, J. M.: conceptualization; formal analysis; investigation; data curation; writing—original draft preparation; writing—review and editing; supervision; project administration. Coello, M. D.: conceptualization; writing—review and editing; supervision; funding acquisition. Quiroga, J. M.: conceptualization, funding acquisition. Albendín, M. G.: methodology, formal analysis, investigation. Alarcón I.: methodology; writing—original draft preparation. Rodríguez-Barroso, R.: formal analysis, data curation. Aranda, V.: methodology.
Corresponding author
Ethics declarations
Animal Research
Ethical considerations for animal rights in this paper were considered and the study was approved by the Ethics Committee of the University of Cádiz (OEBA-CEEA. Art. 34, 38. RD 53/2013).
Ethics Approval and Consent to Participate
This study was approved by the Research and Ethics Committee of the University of Cádiz (OEBA-CEEA. Art. 34, 38. RD 53/2013).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Sole is exposed to different pesticide treatments.
• Selected triclosan concentrations do not indicate cholinesterase activity inhibition in muscle tissue.
• Solea senegalensis shows sensitivity to chlorpyrifos and microplastics combined with chlorpyrifos from concentrations of 5 μg/L.
• Cholinesterase activity decreased significantly in the case of chlorpyrifos both without microplastics and the combined treatment with microplastics.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albendín, M.G., Alarcón, I., Coello, M.D. et al. The Effects of Exposing Solea senegalensis to Microbeads with and Without Pesticides. Water Air Soil Pollut 234, 132 (2023). https://doi.org/10.1007/s11270-023-06096-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06096-z