Abstract
The pulp and paper industry have historically been one of the world’s largest consumers of freshwater resources and producers of wastewater discharges. More than 250 chemicals have been identified in effluents including chlorine-based organic and other toxic compounds. Some of these are resistant to biological degradation such as color-related compounds. The goal of this study was to analyze the real wastewater from the paper mill and to suggest a photocatalytic treatment method in order to reduce the organic pollutants load. The efficiency of the commonly used treatment photocatalytic processes (photo-TiO2 and photo-Fenton reagent) on the treatment of real paper mill effluent was investigated. Additionally, the performance of TiO2 photocatalytic reactions with and without the addition of hydrogen peroxide was also examined. For the experiment, a 500 mL pyrex UV reactor and a 125-W high-pressure mercury lamp, surrounded by pyrex filter blocking wavelengths below 290 nm were used. The approach has been applied for the treatment of effluents produced from a papermaking industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The pulp and paper industry are one of the most important in the world, but it discharges a variety of gaseous, liquid, and solid wastes into the environment. About 70% of the pollution originates from the pulp mill and the rest in the paper mill. It is estimated that each ton of paper produced 7 to 30 t of water that have to be discharged (Han et al., 2021; Pokhrel & Viraraghavan, 2004). Pollution of water bodies is of major concern because large volumes of wastewater are generated (Kurniawan et al., 2022; Puri & Verma, 2022; Tambosi et al., 2006).
More than 250 chemicals have been identified in effluents that are produced at different stages of papermaking, whereas some of these pollutants are naturally occurring wood extractives and other like xenobiotic compounds that are formed during the process of pulping and paper making. Organic matter in wastewaters from the papermaking process is usually biodegradable, but the initial values of chemical oxygen demand (COD) and 5-day biochemical oxygen demand (BOD5) are relatively high (Tambosi et al., 2006).
Pulp and paper mill effluents are treated with various methods, the most common being primary clarification, and in some cases, it is succeeded by secondary treatment, generally of a biological nature, although not always being effective enough. The wastewater treatment typically includes (i) neutralization, screening, sedimentation, and floatation to remove suspended solids and (ii) biological treatment to reduce the organic content in wastewater and destroy toxic organics (Han et al., 2021; Pokhrel & Viraraghavan, 2004). Among the various treatment processes, only a few are generally adopted by pulp and paper industry especially for tertiary treatment. Some of them include ozonation, Fenton’s reagent, and adsorption, while membrane technology is more efficient but are more expensive.
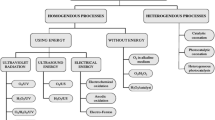
Advanced oxidation process (AOP) is complex effluent treatment involving the conversion of organic pollutants to short species and even to their complete mineralization through the generation of highly reactive free radical oxidants. Some AOP techniques comprise H2O2, O3 (ozonation), Fe(II)/Fe(III) with H2O2 (Fenton reaction), UV irradiation (direct photolysis), UV/H2O2, UV/catalyst/H2O2, UV/{Fe(II)/Fe(III) + H2O2} (Photo-Fenton), UV/O3 (photo-ozonation), and others. Photocatalysis is an important alternative because it can eliminate refractory residues (also known as photodegradation and photo-oxidation methods) (Jin & Davarpanah, 2020; Mitsika et al., 2021; Rodrigues et al., 2008; Sikarwar & Jain, 2015; Simões et al., 2021).
Among many AOPs, solar photo-Fenton process (combination of H2O2, Fe (II), and solar irradiation) has been proved to be rather effective in degradation and mineralization of single organic toxicants and the mixtures of various organic wastes, including those from pulp and paper mills. Although controversial issues exist regarding the dominant oxidizing species in the process, evidences indicated that both hydroxyl radical (•OH) and high valence Fe species (Fe3+ (O2H)2+, Feaq4+, Fe4+ = O, and Fe5+ = O) can contribute to the strong oxidizing powers of photo-Fenton process. The reactions forming oxidizing species are potentially responsible for direct attack on organic carbon.
Titanium dioxide is a catalyst widely employed in photocatalysis either in suspension or immobilized. It has low cost, is non-toxic, photostable in a wide range of pH, recoverable after wastewater treatment, and adequate for industrial scale. The solution/effluent pH is an important parameter in heterogeneous photocatalysis. It modulates the catalyst charge and consequently affects both pollutant adsorption and particle aggregation (Rodrigues et al., 2008). The photocatalytic reaction occurs when the semiconductor is activated by light. When the energy of a photon is equal to or greater than the band gap of the semiconductor, the photon is absorbed by the material and excites an electron into the conduction band (Dette et al., 2014). For TiO2, the band gap energy is 3.2 eV, which corresponds to absorption at wavelengths shorter than ca. 390 nm (Machado et al., 2003). One electron is promoted from valence band (producing positive holes, hVB +) to conduction band (eCB −). For successful photocatalysis, COD values must be lower than 800 mg L−1 as high suspended material content leads to light scattering effects. At the same time, organic matter tends to recover the catalyst surface (adsorption), which could diminish the number of photons that reaches photo-reactive sites on TiO2 (Daneshvar et al., 2003; Faisal et al., 2007). One alternative for wastewater treatment is to apply a physical–chemical procedure such as coagulation to eliminate most of the organic materials first, followed by photocatalysis as the second treatment (Rodrigues et al., 2008; Sheng et al., 2022).
The treatment efficiency of wastewater obtained from papermaking industry is typically investigated by controlling COD, TOC, and BOD5 (Balabanič et al., 2012; Latorre et al., 2007). To the best of our knowledge, only one published research provides comparative data for the treatment of the wastewater of papermaking industry using both TiO2 and photon-Fenton approaches (Ginni et al., 2017). Therefore, the purpose of the present research was to analyze the real wastewater from the paper mill and to suggest a photocatalytic treatment method in order to reduce the organic pollutants load. The efficiency of the commonly used treatment photocatalytic processes (photo-TiO2 and photo-Fenton reagent) on the treatment of real paper mill effluent was investigated. Additionally, the performance of TiO2 photocatalytic reactions with and without the addition of H2O2 was also examined. The approach has been applied for the treatment of effluents of a paper mill.
2 Materials and Methods
2.1 Characteristics of the Mill and the Waste Produced
The paper mill produces three kinds of paper: toilet paper, kitchen paper, and tissue. The kinds of pulp used are long fibber, short fibber, or chemic-thermo-mechanical pulp. The flow rate of the waste depends on the product process and the techniques used for each paper type. During the product process, there is no solid waste. Only water steam and gases such as CO2 are involved. The 70% of the wastewater is recycled.
The coagulation-flocculation method is used as in-plant wastewater treatment. The coagulant cationic polymer PERFORM—PA 9220 and the flocculant anionic polymer (POLYSAN RDF 9257) are used in this process. After coagulation-flocculation, the wastewater is passed through the sand filter and after discharged to the municipal sewage system.
The maximum concentration of the pollutants in the wastewater according to the regulation should much the following parameters (QCVN 40:2011/BTNMT n.d.): (i) pH, 5.5–9.5; (ii) color, 1:20; (iii) BOD, less than 70 mg L−1; (iv) COD, less than 250 mg L−1; and (v) total suspended solid (TSS), less than 120 mg L−1.
2.2 Chemicals
All chemicals used in this study were analytical grade and purchased from Sigma-Aldrich (Darmstadt, Germany) unless otherwise stated.
2.3 Analytical Methods
The following analytical methods were used for the analyses of the wastewater samples, being the standard methods for water and wastewater analysis.
Turbidity–Nephelometric Method
The turbidity measurements were performed using a portable turbidity-meter Turb 355 IR (WTW) having a measurement range of 0.01–1100 NTU. The method is equivalent to DIN EN ISO 7027. Turbidity-free water and stock and diluted turbidity standards were used for the instrument calibration.
Total Suspended mater
A volume of 100 mL of sample containing no more than 200 mg of total non-filtrable residue was filtrated using a pre-dried glass fiber filter under vacuum (Millipore WP 6,122,050). The filter was carefully removed and transferred to aluminum or stainless steel planchet as a support. Then, it was dried for at least for 1 h at 105 °C, cooled in desiccator, and finally weighted (Rice & Baird, 2017).
Spectrophotometric Method
The color determination of the filtrated samples was carried out using a Hitachi U-2000 spectrophotometer. Potassium chloroplatinate/cobaltous chloride standards were used for the instrument calibration.
BOD Measurements
For the determination of BOD, the dissolved oxygen (DO) was measured using an Oxi 330 (WTW) oximeter equipped with an OxiCal-SL (WTW) electrode. Before measurements the electrode was calibrated using the standards with specified DO concentration (determined by iodometric titration).
COD Measurements
Fifty milliliter sample was placed in the 500-mL refluxing flask followed by the addition of 1 g HgSO4, 5.0 mL of concentrated H2SO4, and boiling chips. The solution was cooled externally during the mixing to avoid possible loss of volatile materials of the sample. Then, 25 mL K2Cr2O7 solution (0.25 mol L−1) were added and mixed. The flask was attached to the condenser, and the remaining reagent was be added through the open end of the condenser. The mixture was refluxed for 2 h. After cooling, diluted samples were titrated with standard ferrous ammonium sulfate (Fe(NH4)2(SO4)2 (0.01 mol L−1), using ferroin as indicator.
TOC Measurements
TOC measurements were carried out by a Shimadzu V-CSH TOC analyzer.
Chlorine Residual
The Cl2 residual was determined according to EPA 330.5, US Standard Methods 4500-Cl2 G, and ISO 7393.
Chloride Determination
The sample was titrated directly if its pH value was between 7 and 10; otherwise, an adjustment should be made with H2SO4 or NaOH solution. Titration was accomplished with standard AgNO3 titrant using K2CrO4 solution as indicator.
Sulfide Determination
Sulfide and sulfite determinations were carried out according to EPA 376.2, US Standard Methods 4500-S2- D and ISO 10530.
Sulfite Determination
Sample (acidified with H2SO4) was titrated with iodide-iodate titrant using starch as indicator.
Ammonia Determination
Ammonia quantitation was performed using the Spectroquant Ammonium Test 1.14752.0001 which is based on EPA 350.1, APHA 4500-NH3 D, and ISO 7150/1 method.
Nitrite Determination
Nitrite quantitation was performed using the Spectroquant Nitrite Test 1.14776.0001 which is based on EPA 354.1, US Standard Methods 4500- NO2- b, and EN 26 777 method.
Phosphate Determination
Phosphate analysis was carried out after its complexation with molybdate followed by its spectrophotometric determination.
Phenols Determination
Phenols determination was performed using the Spectroquant Phenol Cell Test 1.14551.0001.
Chlorophenols Measurements
A volume of 100 mL sample is placed into 250-mL beaker, and 2.0 mL of NH4Cl solution was added, and the pH was adjusted with concentrated ammonia to 10. Then, 2 mL of each aminoantipyrine and potassium ferricyanide solutions were added and mixed. After 15 min, the absorbance of the solution was measured at 510 nm.
2.4 Photocatalysis Experimental Procedures
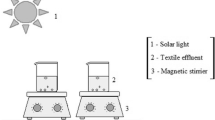
Photocatalysis experiments were conducted using real wastewater from the paper mill that was treated at room temperature. No filtering was applied before the experiment. A pyrex UV reactor and a 125 W high-pressure mercury lamp surrounded by pyrex filter blocking wavelengths below 290 nm, to simulate sunlight reaching earth surface, were used. The reactor was wrapped with Al foil in order to prevent loss of the light from the lamp and to keep the reaction mixture in the dark before the light application. A 500 mL sample was placed in the reactor and stirred during the whole experiment time by magnet stirrer.
2.4.1 Heterogeneous Photocatalysis Using TiO2
In this set of experiments, no pH adjustment was performed, and the reagents TiO2 (Degussa P25) and H2O2 (30% w/v, PanReac) were used without any purification. A portion of 500 mL sample was first mixed with catalyst during 30 min being kept in the dark, and then the light was applied. Only a small adsorption of organics on catalyst was observed during the 30-min equilibration period varying from 0 to 11% with different concentrations of TiO2. The duration of each experiment was 6 h. Peroxide was added just before applying the light. The parameters to control the process efficiency were TOC, COD, phenols concentration, and toxicity. The samples were taken each 30 min and filtered in the syringe through 0.45-μm Millipore filters.
2.4.2 Homogeneous Photocatalysis Using the Photo-Fenton Reaction
Before experiments, the sample pH was acidified to 2.5–3 using diluted acid having the same anions in order to avoid potential interferences. In our case, Fe(II) oxalate, Fe(III) nitrate, Fe(III) sulfate, and H2O2 (30% w/v) were used. The addition of H2O2 reagent was carried out just before the application of the light. Initially, a series of experiments was conducted to study the influence of the type of the anion and to find out at which stage—pre- or post-treatment—photo-Fenton process is more efficient. The duration of each experiment was 4 h. Samples were taken each 15 min to control the pH, the Fe, and H2O2 concentration. To control the efficiency of the process TOC, COD, phenols concentration, and toxicity measurements were used.
2.5 Toxicity Test Description
The luminescent bacteria Vibrio fischeri was used for the examination of toxic properties of the samples collected during the photodegradation processes. The inhibition of the marine bacteria V. fischeri was measured by the Microtox test system (Microtox 500 Analyzer, SDI) within short exposure times of 5 and 15 min. The experiments for the determination of the toxicity of the samples obtained from various stages of the photodegradation process were conducted using the 82% Microtox screening test protocol. The pH value of the samples was adjusted to 7 ± 1, prior to the toxicity tests, by the addition of HCl or NaOH solution (0.1 mol L−1 each).
3 Results and Discussion
3.1 Preliminary Experiments
Initially, the wastewater was analyzed according to standard methods in order to find out its composition and the efficiency of the in-plant treatment. For this purpose, samples were filtered through 45-µm filters (Millipore), and the analyses listed in Table 1 are performed. It should be noted that turbidity was analyzed without filtering. Samples were collected twice per month before and after coagulation-flocculation process and used as the in-plant waste treatment.
As it can be seen from the obtained results, the waste flow is not stable and varies greatly and especially parameters such as turbidity, TSS, BOD and COD, and color. The concentrations of NO3− and PO43− are also quite variable. The content of inorganic components (Cl2, Cl−, NO3−, NO2−, NH4+, SO32−, S2−, and PO43−) is quite low, and their effect are considered to be negligible. BOD and COD are not efficiently removed by in-plant treatment facility, and both values are relatively high in all samples tested, thus being the parameters of concern. The color of the waste it represents is another issue during the process. Comparing the results of Table 1 with the typical ones of the pulp and paper industry, we noticed that pH and BOD values of the tested wastewaters were higher than average. Higher pH values probably attributed due to slightly reductive conditions after in-plant treatment.
3.2 Heterogeneous Photocatalysis Using TiO2
The investigation of the effect of the catalyst concentration is of great importance in the case of heterogeneous photocatalysis using semiconductors since, an optimum concentration is reached, above which, no improvement but even deterioration is observed. This boundary denotes to the optimal of light absorption, since in higher catalyst concentrations, the particles of the catalyst increase the suspension’s opacity and light scattering. Furthermore, particle-to-particle interactions take place, such as agglomeration and collision, between ground state and activated TiO2 molecules causing reduced site density for surface holes and electrons and increased deactivation rate (Bizani et al., 2006, 2014a; Caliman et al., 2007).
The purpose of the initial series of experiments was to find out the optimal concentration of TiO2 photocatalyst. Five experiments were made with the same conditions and concentrations of TiO2 namely 0.5, 0.8, 1, 1.5, and 2 g L−1. The highest %TOC removal achieved was 27.6% in 6 h using TiO2 concentration of 1 g L−1 (Fig. 1). According to Fig. 2, the highest initial photodegradation rate was recorded at TiO2 concentration of 1 g L−1 and then decreased thereafter following the typical pathway for the TiO2 photocatalytic process. Excess TiO2 reagent can cause strong turbidity effect, which makes the passage of light through the heterogeneous solution difficult due to light scattering (Rodrigues et al., 2008). It should be noted that the initial rate was obtained from the time period of starting from 30 min before application of light when catalyst was added until 45 min after the light application in order to reduce the possibility of any interference from byproducts formation.
The effect of H2O2 concentration was also investigated in the range of 0.036–0.45 g L−1, while the TiO2 concentration was remained constant (1 g L−1) (Fig. 3). The maximum TOC removal (ca 30%) was achieved at 0.075 g L−1 H2O2, which is relatively negligible compared to without the addition of H2O2. Identical efficiency was also recorded at H2O2 concentration of 0.15 g/L, but the initial rate of the reaction was significantly lower. Additionally, higher H2O2 concentration can increase the toxicity of the final effluent and also the price of the treatment process.
The final experiments were conducted to assess in which stage of treatment the TiO2 photocatalysis can be applied. The wastewater before and after in-plant treatment was tested with TiO2 concentration of 1 g L−1 in the absence of H2O2. Before treatment no TOC removal was achieved in 6 h, while the COD removal was ca 12.9%. In the case of using water after in-plant treatment, a TOC, COD, and phenols removal of 28.83%, 33.33%, and 76.84% were obtained in 6 h, respectively (Fig. 4). Based on these findings, we concluded that TiO2 photocatalytic reaction has little impact on the purification of this kind of wastewater. Although high phenols load removal was achieved, it should be taken into consideration that the initial phenol concentration was quite low and the total TOC removal was also limited even in 6 h. TiO2 would probably be more useful in completely cleaning slight contamination, rather than the treatment of large quantities of heavy contaminated wastewater (Nakata & Fujishima, 2012). Another practical issue is the fact that TiO2 powder remains suspended in the water after the treatment process which has to be removed by filtration.
3.3 Homogeneous Photocatalysis Using the Photo-Fenton Reaction
Photo-Fenton oxidation technology is reported to be capable of reducing almost all parts of organics which consist of both soluble initial and microbial inert fraction of COD for biologically pretreated pulp and paper industry wastewater (Jiang et al., 2022; Shokri & Fard, 2022).
In this set of experiments, we initially investigated the effect of anion species of iron salt on the efficiency of the photo-Fenton process. Three salts namely iron sulfate, iron nitrate, and Mohr’s salt were tested (Fig. 5) using iron and H2O2 concentration of 10 and 40 mg L−1, respectively. The experiments indicated that iron sulfate had the lowest total efficiency and initial rate of the reaction. On the other hand, Mohr’s salt and iron nitrate showed the same total TOC removal efficiency, while the initial rate was slightly higher when iron nitrate was used. Based on these results, Mohr’s salt was selected as optimum. Typically, iron (II) provided higher photodegradation rates based on the following reaction:
which is faster than
The reaction rate constants have been assigned values of 40–80 M−1 s−1 and 3.1 × 10−3–9.0 × 10−3 M−1 s−1, respectively (Pignatello et al., 2007). However, because of the photocatalytic cycle, after a short time, the amount of hydroxyl radicals in the solution is stabilized in both cases, and the rate tends to be equalized. Similar observations are reported by other researchers (Orozco et al., 2008).
The effect of iron concentration on the performance of the process was examined in the range of 2.5–10 mg L−1. According to the experiments, a high total TOC removal efficiency (ca 90%) and relatively high initial rates were recorded in 4 h for all iron concentration studied. From practical point of view, we selected the lowest concentration of iron (2.5 mg L−1) as optimum as a compromise between the initial removal and the consumption of the reagent.
The effect of H2O2 concentration on the TOC removal performance was tested between 20 and 80 mg L−1. At lower H2O2 concentration, a high initial reaction rate was obtained although total TOC removal was almost identical. Slightly higher TOC removal was recorded using a H2O2 concentration of 20 mg L−1. However, elevated concentrations of H2O2 cause adverse effects by the scavenging of the photo-generating holes and hydroxyl radicals to the formation of peroxo-compounds (Bizani et al., 2014b). Thus, the value of 20 mg L−1 was selected and adopted for further experiments.
The final experiments were conducted to compare the efficiency of photo-Fenton treatment of wastes before and after the in-plant treatment. The efficiency of the process with waste before treatment was only 41.39% of TOC removal and was achieved in 4 h. The removal of COD and phenols was 4.08 and 77.89%, respectively. On the other hand, the after in-plant treatment resulted in 75.5%, 58.85%, and 89.13% for TOC, COD, and phenols removal, respectively, for the same period of time. As derived, photo-Fenton process proved to be an efficient technique that can be practically used as a wastewater treatment method for paper industry as a post-treatment approach.
3.4 Comparison of TiO2-Based Photocatalytic and Photo-Fenton Treatments
Comparing the efficiency of TiO2 and Fenton treatment, we concluded that photo-Fenton process is more efficient both in terms of initial and total TOC and COD removal rate (Fig. 6). The removal of phenols was adequate in both approaches but the initial concentration of phenols in tested wastewater was low. Analogous results were concluded by Amat et al., (2005).
3.5 Toxicity Test
The initial toxicity of waste is very low, being slightly higher in samples before in-plant treatment. Toxicity during the heterogeneous photocatalytic experiments with TiO2 was quite low during the whole experiment time, showing small increase in the middle of the process and being reduced by the end of experiment (6 h) to very low level (Fig. 7). No significant difference between the toxicity values after 5 and 15 min was observed. Thus, if TiO2 photocatalytic treatment is to be employed, the toxicity is not the parameter, which can cause a problem.
In the experiments with photo-Fenton reagent during 4 h, a stable linear increase of toxicity was observed both in the experiment with waste before and after in-plant treatment (data not shown). The difference between toxicity effect values after 5- and 15-min exposure periods was not significant.
The observed results probably attributed due to formation of the short-chained chlorinated byproducts, since the wastewater tested has residual chlorine. As the waste with increased toxicity hardly can be discharged to the environment, the longer experiment with Photo-Fenton reagent was made, and in 5 h already, the decrease of the toxicity effect was achieved. It should be noted that this test cannot be interpreted as a direct effect on living organisms, but should be understood like an indicator of the change in the toxicity during the time of experiment.
4 Conclusions
We concluded that TiO2 photocatalytic process is not efficient in removing organic pollution load from paper mill wastewater neither as pretreatment nor as post-treatment technique. No significant TOC removal was achieved even during 6-h experiment. Addition of H2O2 did not enhance the process to any significant extent.
Photo-Fenton process proved to be an efficient treatment method with high removal of TOC, COD, and phenols being achieved. The initial rate of reaction is high, and a satisfactory TOC removal level can be achieved quite fast, although longer treatment time is required to remove toxicity of the waste. Comparing to TiO2 photocatalytic process, the photo-Fenton is more efficient.
Photocatalysis should be applied as a post-treatment technique, because its efficiency after primary treatment is much higher than before primary treatment. This process can be scaled up to a pilot plant.
Data Availability
All the data associated with this paper are presented in the form of tables and figures.
References
Amat, A. M., Arques, A., López, F., & Miranda, M. A. (2005). Solar photo-catalysis to remove paper mill wastewater pollutants. Solar Energy, 79(4), 393–401. https://doi.org/10.1016/J.SOLENER.2005.02.021
Balabanič, D., Hermosilla, D., Merayo, N., Klemeničič, A. K., & Blanco, Á. (2012). Comparison of different wastewater treatments for removal of selected endocrine-disruptors from paper mill wastewaters. Journal Of Environmental Science And Health Part A, Toxic/Hazardous Substances & Environmental Engineering, 47(10), 1350–1363.
Bizani, E., Fytianos, K., Poulios, I., & Tsiridis, V. (2006). Photocatalytic decolorization and degradation of dye solutions and wastewaters in the presence of titanium dioxide. Journal of Hazardous Materials, 136(1), 85–94. https://doi.org/10.1016/J.JHAZMAT.2005.11.017
Bizani, E., Lambropoulou, D., Fytianos, K., & Poulios, I. (2014a). Photocatalytic degradation of molinate in aqueous solutions. Environmental Science and Pollution Research International, 21(21), 12294–12304. https://doi.org/10.1007/S11356-014-3086-2
Bizani, E., Lambropoulou, D., Fytianos, K., & Poulios, I. (2014b). Photocatalytic degradation of molinate in aqueous solutions. Environmental Science and Pollution Research, 21(21), 12294–12304. https://doi.org/10.1007/S11356-014-3086-2/FIGURES/7
Caliman, A. F., Cojocaru, C., Antoniadis, A., & Poulios, I. (2007). Optimized photocatalytic degradation of Alcian Blue 8 GX in the presence of TiO2 suspensions. Journal of Hazardous Materials, 144(1–2), 265–273. https://doi.org/10.1016/J.JHAZMAT.2006.10.019
Daneshvar, N., Salari, D., & Khataee, A. R. (2003). Photocatalytic degradation of azo dye acid red 14 in water: Investigation of the effect of operational parameters. Journal of Photochemistry and Photobiology a: Chemistry, 157(1), 111–116. https://doi.org/10.1016/S1010-6030(03)00015-7
Dette, C., Pérez-Osorio, M. A., Kley, C. S., Punke, P., Patrick, C. E., Jacobson, P., et al. (2014). TiO2 anatase with a bandgap in the visible region. Nano Letters, 14(11), 6533–6538. https://doi.org/10.1021/NL503131S/SUPPL_FILE/NL503131S_SI_001.PDF
Faisal, M., Abu Tariq, M., & Muneer, M. (2007). Photocatalysed degradation of two selected dyes in UV-irradiated aqueous suspensions of titania. Dyes and Pigments, 72(2), 233–239. https://doi.org/10.1016/J.DYEPIG.2005.08.020
Ginni, M., Adish Kumar, S., Rajesh Banu, J., & Tae Yeom, I. (2017). Synergistic photodegradation of pulp and paper mill wastewater by combined advanced oxidation process. Desalination and Water Treatment, 68, 160–169. https://doi.org/10.5004/DWT.2017.20337
Han, N., Zhang, J., Hoang, M., Gray, S., & Xie, Z. (2021). A review of process and wastewater reuse in the recycled paper industry. Environmental Technology & Innovation, 24, 101860. https://doi.org/10.1016/j.eti.2021.101860
Jiang, Y., Ran, J., Mao, K., Yang, X., Zhong, L., Yang, C., et al. (2022). Recent progress in Fenton/Fenton-like reactions for the removal of antibiotics in aqueous environments. Ecotoxicology and Environmental Safety, 236. https://doi.org/10.1016/J.ECOENV.2022.113464
Jin, Y., & Davarpanah, A. (2020). Using photo-Fenton and floatation techniques for the sustainable management of flow-back produced water reuse in shale reservoirs exploration. Water, Air, and Soil Pollution, 231(8). https://doi.org/10.1007/S11270-020-04812-7
Kurniawan, T. A., Othman, M. H. D., Adam, M. R., Goh, H. H., Mohyudin, A., Avtar, R., & Kusworo, T. D. (2022). Treatment of whitewater from pulp and paper industry using membrane filtrations. Chemical Papers, 76(8), 5001–5010. https://doi.org/10.1007/s11696-022-02226-9
Latorre, A., Malmqvist, A., Lacorte, S., Welander, T., & Barceló, D. (2007). Evaluation of the treatment efficiencies of paper mill whitewaters in terms of organic composition and toxicity. Environmental Pollution, 147(3), 648–655. https://doi.org/10.1016/J.ENVPOL.2006.09.015
Machado, A. E. H., de Miranda, J. A., de Freitas, R. F., Duarte, E. T. F. M., Ferreira, L. F., Albuquerque, Y. D. T., et al. (2003). Destruction of the organic matter present in effluent from a cellulose and paper industry using photocatalysis. Journal of Photochemistry and Photobiology a: Chemistry, 155(1–3), 231–241. https://doi.org/10.1016/S1010-6030(02)00393-3
Mitsika, E. E., Christophoridis, C., Kouinoglou, N., Lazaridis, N., Zacharis, C. K., & Fytianos, K. (2021). Optimized photo-Fenton degradation of psychoactive pharmaceuticals alprazolam and diazepam using a chemometric approach—Structure and toxicity of transformation products. Journal of Hazardous Materials, 403. https://doi.org/10.1016/J.JHAZMAT.2020.123819
Nakata, K., & Fujishima, A. (2012). TiO2 photocatalysis: Design and applications. Journal of Photochemistry and Photobiology c: Photochemistry Reviews, 13(3), 169–189. https://doi.org/10.1016/J.JPHOTOCHEMREV.2012.06.001
Orozco, S. L., Bandala, E. R., Arancibia-Bulnes, C. A., Serrano, B., Suárez-Parra, R., & Hernández-Pérez, I. (2008). Effect of iron salt on the color removal of water containing the azo-dye reactive blue 69 using photo-assisted Fe(II)/H2O2 and Fe(III)/H2O2 systems. Journal of Photochemistry and Photobiology a: Chemistry, 198(2–3), 144–149. https://doi.org/10.1016/J.JPHOTOCHEM.2008.03.001
Pignatello, J. J., Oliveros, E., & MacKay, A. (2007). Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry, 36(1), 1–84. https://doi.org/10.1080/10643380500326564
QCVN 40:2011/BTNMT. (n.d.). National Technical Regulation on Industrial Wastewater. https://circabc.europa.eu/sd/a/97d7bd92-e92e-4b42-a2b3-6a1d8d00d81d/National%20Technical%20Regulation%20%20on%20Industrial%20Wastewater.pdf. Accessed 3 November 2022
Pokhrel, D., & Viraraghavan, T. (2004). Treatment of pulp and paper mill wastewater—A review. Science of the Total Environment, 333(1–3), 37–58. https://doi.org/10.1016/J.SCITOTENV.2004.05.017
Puri, S., & Verma, A. (2022). Color removal from secondary treated pulp & paper industry effluent using waste driven Fe–TiO2 composite. Chemosphere, 303, 135143. https://doi.org/10.1016/j.chemosphere.2022.135143
Rice, E. W., & Baird, R. B. (2017). Methods for the examination of water and wastewater. American Public Health Association, American Water Works, 23. Accessed 25 August 2022
Rodrigues, A. C., Boroski, M., Shimada, N. S., Garcia, J. C., Nozaki, J., & Hioka, N. (2008). Treatment of paper pulp and paper mill wastewater by coagulation–flocculation followed by heterogeneous photocatalysis. Journal of Photochemistry and Photobiology a: Chemistry, 194(1), 1–10. https://doi.org/10.1016/J.JPHOTOCHEM.2007.07.007
Sheng W, Zhang Y, Song K, Xu J, Wu J, Liu B, Zhao X (2022) Mesoporous boron-doped TiO2/mussel shell for photocatalytic degradation of dye X-3B. Water, Air, and Soil Pollution, 233(4). https://doi.org/10.1007/S11270-022-05602-Z
Shokri, A., & Fard, M. S. (2022). A critical review in Fenton-like approach for the removal of polunslutants in the aqueous environment. Environmental Challenges, 7. https://doi.org/10.1016/J.ENVC.2022.100534
Sikarwar, S., & Jain, R. (2015). Nano photo catalytic degradation of the pharmaceutical agent balsalazide under UV slurry photo reactor. Water, Air, and Soil Pollution, 226(8). https://doi.org/10.1007/S11270-015-2531-2
Simões, A. J. A., Macêdo-Júnior, R. O., Santos, B. L. P., Silva, D. P., & Ruzene, D. S. (2021). A Bibliometric study on the application of advanced oxidation processes for produced water treatment. Water, Air, and Soil Pollution, 232(7). https://doi.org/10.1007/S11270-021-05247-4
Tambosi, J. L., di Domenico, M., Schirmer, W. N., José, H. J., & Moreira, R. F. P. M. (2006). Treatment of paper and pulp wastewater and removal of odorous compounds by a Fenton-like process at the pilot scale. Journal of Chemical Technology & Biotechnology, 81(8), 1426–1432. https://doi.org/10.1002/JCTB.1583
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bulskaya, I., Fytianos, K. Oxidative Treatment of Paper Mill Effluent by TiO2 Photocatalysis and Photo-Fenton Reagent. Water Air Soil Pollut 233, 522 (2022). https://doi.org/10.1007/s11270-022-06003-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-06003-y