Abstract
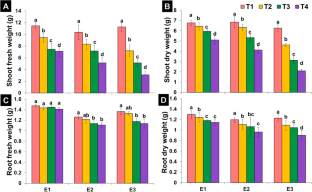
In the present study, three ecotypes of Xanthium strumarium L. were collected from different ecological regions, i.e., Uchalli (E1), Sargodha (E2), and Samundri (E3) in Punjab province, Pakistan. All ecotypes were assessed for their salt resistance and remediation capacity at different NaCl levels (T1 (control), T2 (50 mM), T3 (100 mM), and T4 (150 mM)). Xanthium responses to varied NaCl levels were investigated for growth, anatomical, and physio-biochemical attributes. A comparative account of different attributes revealed a performance difference between three ecotypes. Biomass of all ecotypes was reduced, but the minimum reduction in biomass was noticed in E1 as compared to other ecotypes. Comparison of control and NaCl-treated Xanthium plants indicated highest reduction (96.3%) for both chlorophyll a and chlorophyll b observed under T4 in E3 as compared to E1 and E2. Maximum increase in carotenoids was observed in E2 (82.2%) under T4. Shoot Na+ correspondingly increased in all ecotypes with NaCl levels and maximum Na+ in E3. Minimum absorption of Cl− ion was observed in E1. Osmoprotectants in three ecotypes were much higher under elevated NaCl levels than that of control plants. A significant increase in total soluble sugars was recorded in E1 (76.82%) and E2 (45.35%) as compared to E3. Additionally, significant anatomical changes in stem, leaf, and root of X. strumarium L. were observed in all three ecotypes grown under NaCl conditions. E1 ecotype showed large vascular bundle cell area (119.23%), (110.95%), (56.34%), epidermal thickness (152.21%), (187.40%), (117.28%) of leaf, root, and stem, respectively, as compared to E2 and E3 ecotypes. Similarly, E1 ecotypes showed the highest percentage increase in root sclerenchyma cell area (93.46%), stem epidermal cell area (246.66%), stem cortical cell area (114.23%), and stem sclerenchyma cell area (92.5%) as compared to E2 and E3 ecotypes. These findings endorse differential capabilities of different ecotypes of the same plant with reference to the changes in studied attributes. Overall, results indicate that X. strumarium L. withstood high NaCl stress (150 mM) and can be an eco-friendly source for the phytoremediation of saline soils. It could serve as a foundation for future research on the plant adaptability. We recommend use of this plant for restoring saline-degraded and marginal soils.








Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Abouelsaad, I., & Renault, S. (2018). Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. Journal of Plant Physiology, 226, 136–144.
Aghaleh, M., Niknam, V., Ebrahimzadeh, H., & Razavi, K. (2011). Effect of salt stress on physiological and antioxidative responses in two species of Salicornia (S persica and S europaea). Acta Physiologiae Plantarum, 33(4), 1261–1270.
Akcin, A., & Yalcin, E. (2016). Effect of salinity stress on chlorophyll, carotenoid content, and proline in Salicornia prostrata Pall and Suaeda prostrata Pall subsp prostrata (Amaranthaceae). Brazilian Journal of Botany, 39(1), 101–106.
Akhter, N., Aqeel, M., Shahnaz, M. M., Alnusairi, G. S., Alghanem, S. M., Kousar, A., Hashem, M., Kanwal, H., Alamri, S., & Ilyas, A. (2021). Physiological homeostasis for ecological success of Typha (Typha domingensis Pers) populations in saline soils. Physiology and Molecular Biology of Plants, 27(4), 687–701.
Akhter, N., Habiba, O., Hina, M., Shahnaz, M. M., Alzuaibr, F. M., Alamri, S., Hashem, M., Khalid, N., Aqeel, M., & Noman, A. (2022). Structural, Biochemical, and physiological adjustments for toxicity management, accumulation, and remediation of cadmium in wetland ecosystems by typha domingensis pers. Water, Air, & Soil Pollution, 233(5), 1–23.
Akram, M., Akhtar, S., Javed, I., Wahid, A., & Rasul, E. (2002). Anatomical attributes of different wheat (Triticum aestivum) accessions/varieties to NaCl salinity. International Journal of Agriculture and Biology, 4, 166–168.
Akram, S., Siddiqui, M., Hussain, B., Al Bari, M., Mostofa, M. G., Hossain, M. A., & Tran, L.-S.P. (2017). Exogenous glutathione modulates salinity tolerance of soybean (Glycine max (L) Merrill) at reproductive stage. Journal of Plant Growth Regulation, 36(4), 877–888.
Alam, M., Juraimi, A. S., Rafii, M. & Abdul Hamid, A. (2015). Effect of salinity on biomass yield and physiological and stem-root anatomical characteristics of purslane (Portulaca oleracea L) accessions. BioMed research international, 2015.
Alorfi, H. S., Alshehry, A. A., Ghandourah, M. A., Bawakid, N. O., Elfaky, M. A., Ali, A. M., & Alarif, W. M. (2020). Cytotoxic isoprenoids from Xanthium strumarium linn. Pharmacognosy Magazine, 16(70), 391.
Aqeel, M., Khalid, N., Tufail, A., Ahmad, R. Z., Akhter, M. S., Luqman, M., Javed, M. T., Irshad, M. K., Alamri, S., Hashem, M. & Noman, A. (2021). Elucidating the distinct interactive impact of cadmium and nickel on growth, photosynthesis, metal-homeostasis, and yield responses of mung bean (Vigna radiata L) varieties. Environmental Science and Pollution Research, 1–15.
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiology, 24(1), 1–15.
Awasthi, O., Pathak, R., & Pandey, S. (1999). Anatomical variation in leaf lamina of ber seedling and budded plants grown at different sodicity levels. Indian Journal of Horticulture, 56(1), 29–33.
Barhoumi, Z., Djebali, W., Chaïbi, W., Abdelly, C., & Smaoui, A. (2007). Salt impact on photosynthesis and leaf ultrastructure of Aeluropus littoralis. Journal of Plant Research, 120(4), 529–537.
Beinsan, C., Camen, D., Sumalan, R. & Babau, M. (2009). 'Study concerning salt stress effect on leaf area dynamics and chlorophyll content in four bean local landraces from Banat area', 44th Croatian & 4th International Symposium on Agriculture, Opatija, Bosnia and Herzegovina.
Bhattarai, S., Biswas, D., Fu, Y.-B., & Biligetu, B. (2020). Morphological, physiological, and genetic responses to salt stress in alfalfa: A review. Agronomy, 10(4), 577.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254.
Devi, S., Nandwal, A., Angrish, R., Arya, S., Kumar, N., & Sharma, S. (2016). Phytoremediation potential of some halophytic species for soil salinity. International Journal of Phytoremediation, 18(7), 693–696.
Ditta, A., Imtiaz, M., Mehmood, S., Rizwan, M. S., Mubeen, F., Aziz, O., Qian, Z., Ijaz, R., & Tu, S. (2018). Rock phosphate-enriched organic fertilizer with phosphate-solubilizing microorganisms improves nodulation, growth, and yield of legumes. Communications in Soil Science and Plant Analysis, 49(21), 2715–2725.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P., & t. & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356.
FAO. (2009). How to feed the world in 2050. Population and Development Review, 35, 837–839.
Farhangi-Abriz, S., & Torabian, S. (2017). Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicology and Environmental Safety, 137, 64–70.
Flowers, T. J., Galal, H. K., & Bromham, L. (2010). Evolution of halophytes: Multiple origins of salt tolerance in land plants. Functional Plant Biology, 37(7), 604–612.
Flowers, T. J. & Colmer, T. D. (2008). Salinity tolerance in halophytes. New Phytologist, 945–963.
Freire, M. B. G. S., Freire, F. J., Pessoa, L. G. M., Souza, E. R. d. & Gheyi, H. R. (2021). 'Salt affected soils in the Brazilian semiarid and phytoremediation as a reclamation alternative', Saline and Alkaline Soils in Latin America, Springer, pp. 119–139.
Gupta, B. & Huang, B. (2014). Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. International Journal of Genomics, 2014.
Haque, M. I., Siddiqui, S. A., Jha, B. & Rathore, M. S. (2021). Interactive effects of abiotic stress and elevated CO2 on physio-chemical and photosynthetic responses in Suaeda species. Journal of Plant Growth Regulation, 1–19.
He, Y., & Ma, M. (2018). Responses of seed germination of the invasive plant Xanthium italicum to environmental factors. Acta Ecology Sinica, 38, 1226–1234.
Hu, L., Chen, L., Liu, L., Lou, Y., Amombo, E., & Fu, J. (2015). Metabolic acclimation of source and sink tissues to salinity stress in bermudagrass (Cynodon dactylon). Physiologia Plantarum, 155(2), 166–179.
Jackson, M. L. (1962). Soil chemical analysis-Advanced Course, Madison, Wisconsin Dept. of Soil Science, Univ. of Wisconsin
Jiang, J.-L., Tian, Y., Li, L., Yu, M., Hou, R.-P., & Ren, X.-M. (2019). H2S alleviates salinity stress in cucumber by maintaining the Na+/K+ balance and regulating H2S metabolism and oxidative stress response. Frontiers in Plant Science, 10, 678.
Karakas, S., Dikilitas, M. & Tıpırdamaz, R. (2020). Phytoremediation of salt-affected soils using halophytes. Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture, 1–18.
Katschnig, D., Broekman, R., & Rozema, J. (2013). Salt tolerance in the halophyte Salicornia dolichostachya Moss: Growth, morphology and physiology. Environmental and Experimental Botany, 92, 32–42.
Kilic, S., Cavusoglu, K., & Kabar, K. (2007). Effects of 24-epibrassinolide on salinity stress induced inhibition of seed germination, seedling growth and leaf anatomy of barley. Süleyman Demirel Üniversitesi Fen Edebiyat Fakültesi Fen Dergisi, 2(1), 41–52.
Kirk, J., & Allen, R. (1965). Dependence of chloroplast pigments synthesis on protein effects on actilione. Biochem. Biph. Res. Cann, 27, 523–530.
Kumar, S., Beena, A., Awana, M., & Singh, A. (2017). Physiological, biochemical, epigenetic and molecular analyses of wheat (Triticum aestivum) genotypes with contrasting salt tolerance. Frontiers in Plant Science, 8, 1151.
Moore, S., & Stein, W. H. (1948). Photometric ninhydrin method for use in the chromatography of amino acids. Journal of Biological Chemistry, 176, 367–388.
Naheed, R., Zahid, M., Aqeel, M., Maqsood, M. F., Kanwal, H., Khalid, N., Hashem, M., Alamri, S. & Noman, A. (2022). Mediation of growth and metabolism of Pisum sativum in salt stress potentially be credited to thiamine. Journal of Soil Science and Plant Nutrition, 1–14.
Nasir, F. A., Batarseh, M., Abdel‐Ghani, A. H. & Jiries, A. (2010). Free amino acids content in some halophytes under salinity stress in arid environment Jordan. CLEAN–Soil Air Water 38 (7) 592–600.
Niknam, V., Sharifizadeh, B., Ebrahimzadeh, H., Zarre, S., & Izadpanah, M. (2004). Comparative study of proteins in seeds of some species of Trigonella from Iran. Iranian International Journal of Science, 5(1), 1–11.
Noreen, S., Faiz, S., Akhter, M. S., & Shah, K. H. (2019). Influence of foliar application of osmoprotectants to ameliorate salt stress in sunflower (Helianthus annuus L). Sarhad Journal of Agriculture, 35(4), 1316–1325.
Nurzhanova, A., Mukasheva, T., Berzhanova, R., Kalugin, S., Omirbekova, A., & Mikolasch, A. (2021). Optimization of microbial assisted phytoremediation of soils contaminated with pesticides. International Journal of Phytoremediation, 23(5), 482–491.
Ola, H. A. E., Reham, E. F., Eisa, S., & Habib, S. (2012). Morpho-anatomical changes in salt stressed kallar grass (Leptochloa fusca L Kunth). Research Journal of Agriculture and Biological Sciences, 8(2), 158–166.
Pan, G., Liu, W., Zhang, H., & Liu, P. (2018). Morphophysiological responses and tolerance mechanisms of Xanthium strumarium to manganese stress. Ecotoxicology and Environmental Safety, 165, 654–661.
Pan, G., Zhang, H., Liu, P., Xiao, Z., Li, X., & Liu, W. (2019). Effects of manganese stress on phenology and biomass allocation in Xanthium strumarium from metalliferous and non-metalliferous sites. Ecotoxicology and Environmental Safety, 172, 308–316.
Parida, A. K., Veerabathini, S. K., Kumari, A., & Agarwal, P. K. (2016). Physiological, anatomical and metabolic implications of salt tolerance in the halophyte Salvadora persica under hydroponic culture condition. Frontiers in Plant Science, 7, 351.
Parihar, P., Singh, S., Singh, R., Singh, V. P., & Prasad, S. M. (2015). Effect of salinity stress on plants and its tolerance strategies: A review. Environmental Science and Pollution Research, 22(6), 4056–4075.
Qadir, M., Schubert, S., Ghafoor, A., & Murtaza, G. (2001). Amelioration strategies for sodic soils: A review. Land Degradation & Development, 12(4), 357–386.
Rabhi, M., Hafsi, C., Lakhdar, A., Hajji, S., Barhoumi, Z., Hamrouni, M. H., Abdelly, C., & Smaoui, A. (2009). Evaluation of the capacity of three halophytes to desalinize their rhizosphere as grown on saline soils under nonleaching conditions. African Journal of Ecology, 47(4), 463–468.
Saule, A., Akmaral, N., Subhash, M., Aygul, A., Saule, K., Saule, A., Asil, N., Anar, Z., Saltanat, A., & Ravilya, A. (2013). The effect of salinity on growth and anatomical attributes of barley seedling (Hordeum vulgare L). African Journal of Biotechnology, 12(18), 2366–2377.
Shabala, S., Hariadi, Y., & Jacobsen, S.-E. (2013). Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. Journal of Plant Physiology, 170(10), 906–914.
Shabani, M. (2016). CO2 bio-sequestration by Chlorella vulgaris and Spirulina platensis in response to different levels of salinity and CO2. Proceedings of the International Academy of Ecology and Environmental Sciences, 6(2), 53.
Shabani, N., & Sayadi, M. (2012). Evaluation of heavy metals accumulation by two emergent macrophytes from the polluted soil: An experimental study. The Environmentalist, 32(1), 91–98.
Shahzad, R., Khan, A. L., Bilal, S., Waqas, M., Kang, S.-M., & Lee, I.-J. (2017). Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environmental and Experimental Botany, 136, 68–77.
Silva, B., Batista, B., & Lobato, A. (2021). Anatomical changes in stem and root of soybean plants submitted to salt stress. Plant Biology, 23(1), 57–65.
Singh, J., Singh, V., & Sharma, P. (2018). Elucidating the role of osmotic, ionic and major salt responsive transcript components towards salinity tolerance in contrasting chickpea (Cicer arietinum L) genotypes. Physiology and Molecular Biology of Plants, 24(3), 441–453.
Slama, I., Abdelly, C., Bouchereau, A., Flowers, T., & Savouré, A. (2015). Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Annals of Botany, 115(3), 433–447.
Steel, R. G., Torrie, J. H., & Dickey, D. A. (1997). Principles and procedures of statistics (pp. 4–7). McGraw-Hill.
Stirzaker, R., Cook, F., & Knight, J. (1999). Where to plant trees on cropping land for control of dryland salinity: Some approximate solutions. Agricultural Water Management, 39(2–3), 115–133.
Tavakkoli, E., Rengasamy, P., & McDonald, G. K. (2010). High concentrations of Na+ and Cl– ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. Journal of Experimental Botany, 61(15), 4449–4459.
Ventura, Y., Myrzabayeva, M., Alikulov, Z., Omarov, R., Khozin-Goldberg, I. & Sagi, M. (2014). Effects of salinity on flowering, morphology, biomass accumulation and leaf metabolites in an edible halophyte. AoB Plants, 6.
Vijayan, K., Chakraborti, S. P., Ercisli, S., & Ghosh, P. D. (2008). NaCl induced morpho-biochemical and anatomical changes in mulberry (Morus spp). Plant Growth Regulation, 56(1), 61–69.
Voltolini, C. H., Reis, A. & Santos, M. (2009). Leaf morphoanatomy of rheophyte Dyckia distachya Hassler (Bromeliaceae). Revista Brasileira de Biociências, 7 (4).
Wasim, M. & Naz, N. (2020). Anatomical adaptations of tolerance to salt stress in Cenchrus ciliaris L., a saline desert grass. JAPS: Journal of Animal & Plant Sciences, 30 (6).
Wolf, B. (1982). A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Communications in Soil Science and Plant Analysis, 13(12), 1035–1059.
Xue, C., Gao, Y., Qu, B., Tai, P., Guo, C., Chang, W. & Zhao, G. (2021). Hybridization with an invasive plant of Xanthium strumarium improves the tolerance of its native congener X. sibiricum to cadmium. Frontiers in Plant Science, 1543.
Yadav, S. P., Bharadwaj, R., Nayak, H., Mahto, R., Singh, R. K., & Prasad, S. K. (2019). Impact of salt stress on growth, productivity and physicochemical properties of plants: A Review. International Journal of Chemical Studies, 7(2), 1793–1798.
Zheng, H., & Li, J. (1999). Saline plants in Songnen Plain and restoration of alkaline-saline grass (pp. 179–206). China, Science Press of China.
Zojaji, F., Hassani, A., & Sayadi, M. (2015). A comparative study on heavy metal content of plants irrigated with tap and wastewater. International Journal of Environmental Science and Technology, 12(3), 865–870.
Acknowledgements
The authors are highly thankful to the Government College for Women University, Faisalabad, Pakistan, for providing experimental station and lab facilities.
Author information
Authors and Affiliations
Contributions
NA, AN: Conceived the idea and designed the experiment. NA, MA, MFI, SN, and AN: Wrote first draft. MA, MKI, and AN: Writing, review, editing. NA, MMS: Performed experiment, investigation. NA, MA: Statistical analysis. MA, RAM, OMA, TH, and AN: Critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akhter, N., Aqeel, M., Maqsood, M.F. et al. Comparative Assessment of Remediation Potential of Xanthium strumarium Ecotypes in NaCl-Affected Root Zone. Water Air Soil Pollut 233, 509 (2022). https://doi.org/10.1007/s11270-022-05990-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05990-2