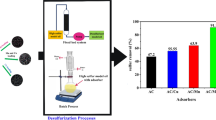
Abstract
In this work, new magnetic bentonite-based adsorbents combined with different carbon sources, namely, reduced graphene oxide and multiwall carbon nanotubes, were synthesized via co-precipitation method. The synthesized adsorbents were characterized using XRD, TGA, SEM, EDX, TEM, and BET analysis techniques. The adsorbents were then used to remove oil from aqueous solutions of water-in-oil emulsion by performing batch adsorption experiments. The experimental data were fitted to three isotherm models including Langmuir, Freundlich, and Sips models using non-linear regression and were compared using Akaike Information Criterion statistical model. The data analysis showed that Sips model best fits the experimental data for the adsorption of oil onto both adsorbents. The maximum adsorption capacity of oil from sips model were 81.65 mg/g and 77.12 mg/g for Fe3O4/Bent/rGO and Fe3O4//Bent/MWCNTs, respectively. The obtained kinetics data were fitted to pseudo-first-order, pseudo-second-order, and intraparticle diffusion models. Pseudo-second order kinetic model best fitted the kinetic data of both adsorbents. Overall, both adsorbents showed high removal efficiency reaching equilibrium in less than 50 min indicating that both adsorbents can be successfully utilized in industrial adsorption process.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Deterioration of water quality due to the continuous discharge of contaminated water induced by the world’s population growth, modernization, and rapid industrialization has become a global issue of concern (Mahmud et al., 2021; Zamora-Ledezma et al., 2021). More specifically, the frequent and continuous discharge of produced water that results from oil and gas industry became one of the serious problems worldwide causing considerable impact on ecological equilibrium, environment, and economy (Chao et al., 2020; Ejeta et al., 2022; Mofokeng et al., 2020). Produced water primarily consists of dissolved oil, hydrocarbon gases, organic acids, phenols, metals, and various chemical additives. The dissolved oil contaminants contains recalcitrant organic compounds such as benzene, toluene, xylene, waxes, and surfactants (Olajire, 2020). The disposal of produced water without proper treatment can interfere with the environmental sustainability; thus, produced water remediation is a crucial task (Liu et al., 2021; Zhang et al., 2020). Several treatment technologies have been used for oil contaminants removal. For instance, membranes and advanced oxidation are used for oily water treatment; however, their use is limited due to high capital and maintenance cost. Chemical flocculation attains high removal efficiency and requires small floor space, but it consumes huge amounts of energy. Biological treatment is also used despite causing secondary pollution that requires further treatment and consumes time (Deng et al., 2020; Ewis et al., 2021). Compared to the current state of produced water treatment technologies, adsorption is envisaged as a promising technique due to its simplicity, ease of operation, and high removal capability (Elmobarak & Almomani, 2021; Khan et al., 2021). Nevertheless, the main drawback associated with adsorption technology is the adsorbent high cost, which in turn elevates the cost of the treatment process. Therefore, it is important to develop adsorbents with high efficiency, low cost, great selectivity, and excellent recyclability.
Traditional adsorbents such as carbon nanotubes, activated carbon, zeolite, polymers, clay minerals, and silica have been utilized for wastewater treatment; however, they are difficult to recover and regenerate (Tran et al., 2020). Among clay minerals, Bentonite is available abundantly and inexpensive, contains wide interlayer spacing, attains ion exchange capacity, and has high specific surface area (Apriandanu & Yulizar, 2021; Sakr et al., 2020). Even though bentonite has high dispersity in aqueous solution that renders its ability to be recovered and regenerated, it can be combined with other adsorbents to facilitate its separation from aqueous solution. For instance, the addition of iron oxide nanoparticles (Fe3O4 NPs) to bentonite enables the composite separation by external magnate due to Fe3O4 NPs magnetic property. In addition, Fe3O4 NPs have the affinity to interact with oil and subsequently separate it from aqueous solution, which enhances the overall removal efficiency. The removal of diesel oil from emulsified solution using Fe3O4 NPs and Fe3O4/Bentonite composite was studied in our previous work (Ewis et al., 2020). The study showed that Fe3O4 NPs attained 28% removal, whereas Fe3O4/Bentonite composite attained around 70% oil removal. Besides, the composite attained superparamagnetic magnetic property, which facilitated its separation from aqueous solution using an external magnet. Furthermore, carbonaceous adsorbents including activated carbon, activated carbon fiber, and carbon nanotubes are known for high adsorption capacity, surface reactivity, and high surface area (Marrakchi et al., 2020). More recently, the development of clay/carbon composites has received attention due to their enhanced properties including high recyclability, adsorption capacity, surface area, and porosity compared to the composite individual components. Liang et al. (Liang et al., 2017) fabricated carbon/bentonite composite for alkaline industrial wastewater treatment. The composite attained around 91% removal for initial COD value of 79,834 mg/L. In addition, the composite showed a high removal efficiency for 9 regeneration cycles. Yet, there is a growing focus on the development of low cost and sustainable adsorbents that possess high adsorption ability for wide range of contaminants.
In this work, new magnetic bentonite/reduced graphene oxide (Fe3O4/Bent/rGO) and magnetic Bentonite/multiwall carbon nanotube (Fe3O4/Bent/MWCNTs) composites were synthesized through co-precipitation method for produced water treatment. Water-in-oil emulsion solution was used as a representative of produced water concentration. The composites were characterized to elucidate their structural, thermal, and morphological properties using SEM, EDX, TEM, XRD, FTIR, TGA, and BET. Batch experiments were performed to study the influence of initial oil concentration and contact time on the removal efficiency. Moreover, the adsorption isotherms and kinetics models were carried out, and the models were statistically compared. Finally, the possible adsorption mechanism of both adsorbents for produced water was suggested. To the best of the authors’ knowledge, the application of such magnetic bentonite-based adsorbent combined with different carbon sources for the treatment of produced water has not been reported in the open literature. Therefore, this work can provide significant contribution to the pool of knowledge in produced water treatment research.
2 Materials and Methods
2.1 Materials and Chemicals
Ferric chloride (FeCl3.6H2O) (99%, purity), graphite powder (C) (99%, purity), sodium nitrate (NaNO3) (99.5%, purity), potassium permanganate (KMnO4) (99%, purity), hydrogen peroxide (H2O2) (30%, purity), hydrochloric acid (HCl) (35–38%, purity), sulfuric acid (H2SO4) (98%, purity), ferrous chloride (FeCl2.4H2O) (99%, purity), and ammonia 25% (NH4OH) were supported from Research-Lab, India. Diesel oil was purchased from local petrol station in Doha, Qatar. MWCNTs were obtained from NanoKarbon Co., Ltd, Korea. Ethylenediamine-tetrakis-tetrol (≈100%, purity) (non-ionic surfactant) and raw bentonite were purchased from Sigma-Aldrich.
2.2 Preparation of Graphene Oxide
Graphene oxide was prepared using modified Hammers method (Balog et al., 2010; Moshari et al., 2021). In brief, 5 g of graphite and 2.5 g of NaNO3 were dissolved in 115-mL H2SO4, and the mixture was stirred for 2 h at room temperature. After that, 20 g of KMnO4 was added to the solution in an ice bath to maintain the overall temperature at 15 ℃. The mixture was left under stirring for 2 more hours during which the color of the solution gradually turned from black to greenish black. Then, the temperature of mixture was raised to 35 ℃ and stirred for an additional hour. Afterwards, 230 mL of distilled water was slowly added to the solution under continuous mixing while maintaining the temperature at 90 ℃. Finally, the solution is diluted with 250 mL followed by the addition of 10 mL of 30% H2O2 that caused the solution color to turn into yellow. The product was then washed with 5% HCl to removed imputers and left overnight to settle down. Finally, the produced contents were washed several times till the pH reaches near neutral, centrifuged, and freeze dried to obtain graphene oxide powder.
2.3 Preparation of Fe3O4/Bent/rGO and Fe3O4/Bent/MWCNT Nanocomposites
Fe3O4/Bent/rGO was fabricated using co-precipitation method. Solution I was prepared by dissolving 2.5 g of (FeCl2.4H2O) and 1.5 g of (FeCl3.6H2O) in 100 mL of distilled water, under stirring at 60 ℃. Solution II was prepared by dispersing 0.2 g and 0.1 g of GO and bentonite, respectively, in 100-mL distilled water for 30 min at 50 ℃ by the application of ultrasound. Then, solution II was added to solution I under stirring. The temperature of the solution was raised up to 80 ℃. Then, 20 mL of ammonia (25%) was added to the mixture. The mixture was left for 3 h for the reaction to occur, and the final product was separated by a magnet and washed several times with distilled water and ethanol. Finally, the obtained nanocomposite was dried under vacuum at 70 ℃. Similarly, Fe3O4/Bent/MWCNTs was fabricated using the same procedure expect dispersing commercial MWCNTs instead of GO in solution II.
2.4 Adsorbent Characterization
The surface morphology was visualized via scanning electron microscopy (SEM) (Nova Nano SEM 450, USA) coupled with an energy-dispersive spectrometer (EDS). The morphology was observed using transmission electron microscope (TEM) (TECNAI G2 TEM, TF20). The surface functional group was detected by Fourier Transform Infrared Spectroscopy (FTIR) using Perkin Elmer spectrum one analyzer. X-ray diffraction (XRD) patterns were obtained using Rigaku MiniFlex-600. Thermogravimetric analysis (TGA) was conducted using TGA Q500 TA instrument (USA). The samples were heated from 3 to 850℃ at a heating rate of 10 ℃/min. The BET surface area and pore volume were conducted using Brunauer Emmett Teller (BET, Micromeritics, Tristar II series).
2.5 Water-in-Oil Emulsion Solution Preparation
Water-in-oil emulsion solution was prepared by mixing deionized water with a surfactant followed by adding diesel oil droplets. The solution was homogenized for 20 min at temperature of 296 K. The concentration of oil was varied from 20 to 160 mg/L (Abd El-Gawad, 2014). For every 100 ppm of oil, 0.1 g of surfactants was added and accordingly, the addition of surfactants was varied between 0.02 and 0.16 g depending on the desired oil concentrations.
2.6 Adsorption Experiments
The adsorption experiments were conducted by varying initial oil concertation and contact time at 200 rpm rotational speed, temperature of 303 K, normal solution pH (6.5), and 0.1 g of the adsorbent. Shimadzu TOC 5000 analyzer was used to measure the samples concentration.
The adsorbent adsorption capacity in mg oil/g adsorbent was calculated using Eq. 1:
where C0 is the initial concentration (mg/L) and Ce is the equilibrium concentration (mg/L). m is the mass of the nanocomposite (g), and V is the water-in-oil solution volume (L).
The removal efficiency was evaluated using Equation 2.
3 Results and Discussion
3.1 Characterization
The XRDs pattern for Fe3O4 NPs, raw bentonite, Fe3O4/Bent/rGO, and Fe3O4/Bent/MWCNTs are presented in Figure 1. For raw bentonite, montmorillonite is the main phase existing with characteristic peaks 2θ = 7.26°, 19.75° ,21.87°, 29°, 35°, 55°, and 62.9°. In addition, other phases including plagioclase, quartz, gypsum, kaolinite, and illite present as impurities (Andrini et al., 2017; Khatamian et al., 2019). The absorption peaks of Fe3O4 NPs are in 2θ = 30.24°, 35.62° ,43.26°, 53.56°, 57.12°, and 62.78°. These values corresponds to (220), (311), (311), (400), (422), (511), and (440) planes. The observed XRD pattern indicates that Fe3O4 NPs possess face-centered cubic lattice (fcc) crystal structure (Raghu et al., 2017). After loading bentonite with Fe3O4 NPs and RG, the XRD pattern shows obvious peaks at 2θ= 7.26°, 19.75°, and 21.87°, which are for bentonite, while the absorption peaks at 2θ= 30.24°, 35.62°, 43.3°, 53.56°, 57.5°, and 62.9° for Fe3O4 NPs (Mahmud et al., 2020). The absorption peak at 2θ= 26.25° corresponds to the presence of reduced graphene oxide (Saleem et al., 2017). These results affirm the successful synthesis of Fe3O4/Bent/rGO composite. For Fe3O4/Bent/MWCNTs, the XRD pattern shows peaks similar to Fe3O4/Bent/rGO that correspond to the existence of Fe3O4 NPs and bentonite, but with less intensity. The peaks observed at 2θ= 28.8° indicate the presence of MWCNTS (Andrews et al., 2001). The slight spikes of Fe3O4/Bent/rGO sample are observed due to the agglomeration of many Fe3O4 particles on the surface of rGO, which caused a growth on these particles. However, few smaller particles of Fe3O4 with less agglomeration are attached on CNT surface, which had no effect on their overall size observed in TEM and EDX analysis. Overall, the lattice strain was one of the major factors affecting nanoparticle synthesis and crystal growth.
The thermal stability of the adsorbents was investigated using TGA. As shown in Fig. 2, both adsorbents attained different thermal behaviors. The thermogram of Fe3O4/Bent/rGO showed continuous decrease weight loss (19%) over temperature range 30–770℃ followed by slightly constant thermal stability. This could be attributed to the dehydration and the removal of oxygen present on rGO surface at temperature below 200 ℃. Above 200 ℃, the weight loss is attributed to the gasification of carbonaceous materials as a result of rGO oxidation and the transformation of Fe3O4 to Fe2O3 Fe(OH)3. The sudden decrease in weight loss at 435 ℃ might be due to the degradation of carbon material intercalated with bentonite (Saraf et al., 2017). The thermogram of Fe3O4/Bent/MWCNTs displayed a dehydration weight loss (7%) in temperature range 30–100 ℃ followed by a slightly constant thermal stability up to 600 ℃. Finally, a gradual weight loss (2%) continued up to 850 ℃, which could be due to the transformation of Fe3O4 to Fe2O3 Fe(OH)3. Overall, Fe3O4/Bent/rGO showed a better thermal stability over temperature range 30–250 ℃ (Umar et al., 2020). From these results, it can be concluded that the combination of Fe3O4 NPs and CNT leads to positive synergism causing them to exhibit higher temperature stability compared to combination Fe3O4 NPs and rGO platelets (Sadegh et al., 2014).
The SEM images are used to observe the surface structure, morphology, uniformity, and distribution of the samples. Figure 3 shows the SEM images for rGO1, bentonite, Fe3O4/Bent/rGO, and Fe3O4/Bent/MWCNTs at different magnitudes and with the elemental analysis on the surface presented in Table 1. It can be observed that both adsorbents contain the same elements, but in different quantities. Carbon (C), oxygen (O), and iron (Fe) are the main elements existing in both adsorbents. The formation of iron oxide nanoparticles (Fe3O4 NPs) is indicated by the existence of elements O and Fe. Moreover, the carbonaceous material on bentonite surface is indicated by elemental carbon. The EDX analysis also indicates the existence of silica (Si), aluminum (Al), and magnesium (Mg) in both samples, which are related to bentonite. The SEM image shown in Fig. 3a indicates that rGO sheets are thin and possess wrinkled surface structure with distinct edges. On the other hand, bentonite is rock-like structure with a rough surface (Fig. 3b). The SEM image of Fe3O4/Bent/rGO shows the formation of Fe3O4 NPs and bentonite surface (Fig. 3c and 3d), whereas, rGO is believed to be formed on the surface as indicated by the wrinkled edge structure and/or intercalated into bentonite layers (Xu et al., 2019). It is worth noting that rGO sheet is formed due to the application of high temperature during Fe3O4/Bent/rGO synthesis, which caused the transformation of graphene oxide (GO) into rGO sheet (Ersan et al., 2017; Moshari et al., 2021). Similarly, the SEM images of Fe3O4/Bent/MWCNTs indicate that bentonite acts as a support for Fe3O4 NPs and MWCNTs (Fig. 3e and 3f). In addition, it can be observed that MWCNTs are distributed over bentonite surface without any agglomeration. The SEM images of adsorbents (Figs. 3c, 3d, 3e, and 3f) show that Fe3O4 NPs were formed randomly on rGO, bentonite, and MWCNT surface with obvious agglomerations, which is also confirmed by the TEM images shown in Fig. 4. Moreover, the TEM images confirm the agglomeration of Fe3O4 NPs in both adsorbents (Fig. 4), which is due to the high surface energy of the particles (Ewis et al., 2020). The size of Fe3O4 NPs over Fe3O4/Bent/rGO and Fe3O4/Bent/CNTs were 11.6 nm and 8.83 nm, respectively. These values are smaller than Fe3O4 NP size formed over Fe3O4/Bentonite composite that attained an average size of 13.55 nm (Ewis et al., 2020). Although the same amount of precursors is used for the preparation of both adsorbents, the amount of Fe3O4 NPs existing on Fe3O4/Bent/MWCNT surface is less than the amount present on Fe3O4/Bent/rGO surface. This fact is indicated by the amount of Fe and O in EDX analysis (Table 1) and further observed in the TEM images as well (Fig. 4).
The BET analysis for Fe3O4/Bent/rGO and Fe3O4/Bent/MWCNT is shown in Table 2. It is observed that Fe3O4/Bent/MWCNT attained higher BET surface area and pore volume compared to Fe3O4/Bent/rGO, which is expected to yield an enhancement in oil uptake. Generally, surface area and pore volume are important factors that influence the adsorbent uptake capacity. This is because surface area determines the number of effective collisions between the adsorbent and the contaminant (Muhammad, Rahman, et al., 2019; Muhammad, Rashid, et al., 2019), whereas higher pore volume allows to trap more contaminants within its pores. In addition, both adsorbents attained higher surface area compared to Fe3O4/Bentonite composite reported by Khatamiana et al. (Khatamian et al., 2019), which indicates that both adsorbents are expected to attain better adsorption performance. The nitrogen adsorption–desorption isotherm shown in Figure S1 and Figure S2 for Fe3O4/Bent/rGO and Fe3O4/Bent/MWCNTs, respectively, indicates type IV isotherm for mesoporous materials.
3.2 Isotherm
The influence of initial oil concentration on the adsorption capacities of Fe3O4/Bent/rGO and Fe3O4/Bent/MWCNTs is shown in Fig. 5. The figure indicates that the removal capability of both adsorbents increased as the initial concentration increased from 15 to 90 ppm. This increase is attributed to the presence of greater amount of oil molecules at high concentrations that interact with the adsorbent’s active sites due to the increase in the concentration gradient between the solution and the adsorbent. After that, the removal percentage remained roughly constant when initial oil concentration increased up to 150 ppm, which indicated that the saturation of oil molecules on the adsorbents and the active sites are fully occupied. Moreover, Fe3O4/Bent/MWCNTs showed similar removal capability to Fe3O4/Bent/rGO at concentration lower than 40 ppm. However, at higher initial oil concentration, Fe3O4/Bent/MWCNTs attained higher removal capability. This behavior could be due to the presence of more binding sites on Fe3O4/Bent/MWCNTs surface compared to that of Fe3O4/Bent/rGO. The higher BET surface area and average pore volume that Fe3O4/Bent/MWCNTs adsorbent (Table2) attains are expected to be responsible for the increase in oil removal compared to Fe3O4/Bent/rGO. This allows Fe3O4/Bent/MWCNTs to trap oil molecules within its structure and increase the oil molecule contact with the adsorbent functional groups, which increases the overall removal efficiency. Moreover, high specific surface area and lower pore radius are expected to be the main parameters that determine the enhancement in the adsorption capacity. Despite that Fe3O4/Bent/MWCNTs attained higher average pore radius and specific surface area, it showed a higher removal capability (Mu'azu et al., 2018). Consequently, pore volume has a vital role in determining the adsorbent removal capability.
The data displayed in Fig. 5 were used to fit equilibrium isotherm models to have insights into the adsorbent surface properties, adsorption affinity, and adsorption mechanism. The data were fitted to three common isotherm models, Langmuir, Freundlich, and Sips models.
Langmuir model describes the equilibrium distribution of oil molecules between the liquid and solid phase (Langmuir, 1917; Muthu Kumara Pandian et al., 2021). The model assumes a monolayer adsorption of adsorbate on the adsorbent surface in which no further adsorption occurs. The Langmuir isotherm model is described by Eq. 3.
where KL (L/mg) is the Langmuir constant and qm (mg/g) is the maximum adsorption capacity.
Freundlich model assumes a heterogeneous adsorption with a non-uniform distribution of adsorption heat and adsorbate molecules (Freundlich, 1906; Sarojini et al., 2022). The model is valid to describe the adsorption data over a restricted adsorbate concentration range. The model is described by Eq. 4.
where n is associated with the adsorption efficiency and Kf (Ln mg1/n/g)n) is related to the adsorption capacity.
Sips isotherm model is a combination of Langmuir and Freundlich model that is applied for describing the adsorption on a heterogeneous adsorbent surface and eliminate the restriction of limited adsorbate concentration range implied by Freundlich mode (Sips, 1948). At low concentration range, the model reduces to Freundlich model, while at higher concentrations, sips model predicts Langmuir monolayer adsorption (Alkurdi et al., 2020). The model is described by Eq. 5.
where \({q}_{m}\) (mg/g) is the maximum adsorption capacity, \(b\) (L/mg) is related to energy of adsorption, and \(n\) is sips isotherm constants.
The experimental adsorption data were fitted to Langmuir, Freundlich, and sips models using non-linear regression by employing Marquardt–Levenberg algorithm to find the minimum function that is a sum of squares of non-linear functions (Yan et al., 2015). The fitting of adsorption oil data to the isotherm models using Fe3O4/Bent/rGO and Fe3O4/Bent/MWCNTs is represented in Figs. 6 and 7, respectively. The isotherm parameters are displayed in Table 3. It can be observed that at low equilibrium concentration, the isotherms’ curves of both adsorbents exhibit higher slope due to the availability of active sites (Figs. 7–8).
A comparison of the isotherm models was performed based on the sum of square errors (SSE), correlation coefficient (R2), and Akaike Information Criterion (AIC) method (Akaike, 1998). AIC method compares between different models and identifies the best model that fits the experimental data. The best model attains the lowest AIC value and it is expressed as (El-Naas et al., 2017; Turner et al., 2015):
where p and N are the degree of freedom and sample size, respectively. Small sample size (N/P < 40) implies the use of second-order AIC (\({AIC}_{c}\)), which is defined as (El-Naas et al., 2017):
The model that attains the most statistical confidence is the one that have the highest value of information criterion weight (\({w}_{i}\)) and it is defined as (El-Naas et al., 2017):
where \(\Delta {AIC}_{c}\) is the difference between the information criterion value of i model relative to the best candidate model and it is expressed as:
Tables 4 and 5 show the SSE, R2, \(AIC\), \({AIC}_{c}\), and \({w}_{i}\) values of the three isotherm models for Fe3O4/Bent/rGO and Fe3O4/Bent/MWCNTs, respectively. The model that best fits the experimental data showed attain the lowest SSE and \(AIC\), while it should attain the highest R2, and \({w}_{i}\) considering that the sum of \({w}_{i}\) is equal to 1. The results indicate that Sips model best fits the experimental data for the adsorption of oil onto Fe3O4/Bentonite/rGO with R2 = 0.997 and \({w}_{i}\)= 0.9919. Similarly, the adsorption of oil onto Fe3O4/Bentonite/MWCNTs is best described by sips model with R2 = 0.9926 and \({w}_{i}\)= 0.9967. These results confirm the adsorption followed Freundlich model at lower concentrations and Langmuir model when the oil concentrations were increased, which indicate the involvement of both physical and chemical adsorption process. The maximum oil adsorption capacity were 81.65 mg/g and 77.12 mg/g using Fe3O4/Bentonite/rGO and Fe3O4/Bent/MWCNTs, respectively.
3.3 Kinetics
The influence of contact time between the composites and the oil solution is shown in Fig. 8. The figure shows a rapid adsorption within the first 30 min followed by a gradual decrease until equilibrium was reached. This could be attributed to the availability of binding sites for adsorption in the first 30 min; after that, the sites become fully occupied and the adsorption starts to decrease. Moreover, it can be observed that Fe3O4/Bent/MWCNTs reached equilibrium faster than Fe3O4/Bent/rGO, which could be attributed to the higher pore volume that the composite possesses. The maximum oil removal using Fe3O4/Bent/MWCNTs and Fe3O4/Bent/rGO were 97.7% and 92.4%, respectively.
Adsorption kinetics predicts the rate at which the adsorbate is removed from aqueous solution by the adsorbent. In addition, they provide insights into the uptake mechanism that controls the adsorption process and the type of interaction between the adsorbate and the adsorbent (Wang et al., 2020). Thus, in order to understand the adsorption mechanism and the interaction involved in the adsorption process, the experimental data presented in Fig. 8 were fitted to pseudo-first order (PFO) (Lagergren, 1898), pseudo-second-order (PSO) (Ho and McKay, 1999), and intraparticle diffusion model (Weber and Morris, 1963) as described by Eqs. 10–12, respectively.
where t is time in minutes, \({k}_{1}\) is the first-order rate constant in min−1, and k2 is the second-order rate constant in g.mg−1.min, while the terms ki and C represent the rate constant and intercept for a single stage associated with intraparticle diffusion model, respectively.
The fitted experimental data to the three kinetics models using both adsorbents are shown in Figs. 9 and 10, and the kinetics parameter calculated from the related plots is shown in Table 4. The high R2 values indicate that PSO model describes the oil adsorption kinetic well compared to PFO model for both adsorbents. This implies that oil adsorption onto both adsorbents involves chemisorption process, which involves electrostatic attraction and chemical bonding between the adsorbent and the adsorbate (Shan et al., 2015). In addition, the kinetics data were fitted to intraparticle diffusion model to have an insight into the adsorption rate controlling steps and the mechanism (Figs. 5C and 6C). For both adsorbents, the intraparticle diffusion model shows a multistage behavior. The first stage is the instantaneous adsorption of oil molecules represented by the sharp rise in qt with t0.5. In this stage, the adsorption is controlled by external mass transfer. The second stage is a curved portion, which shows a slow adsorption rate controlled by intraparticle diffusion. The third stage represents the equilibrium stage where internal diffusion occurs within the adsorbents’ pores. Moreover, the value of k1 is greater than k2 for both adsorbents (Table 4), which indicates that the rate limiting step is the interior pore-diffusion (Liu et al., 2019). The intercept (C) describes the boundary layer thickness. The Larger the value of C, the greater the contribution of the surface adsorption in the rate controlling step. Finally, the intraparticle diffusion model plots for both adsorbents did not pass through the origin, which indicate that intraparticle diffusion is not the only rate controlling step (Table 6).
3.4 Adsorption Mechanism
The FTIR spectra shown in Fig. 11 indicate the surface functional groups for both adsorbents before and after diesel oil adsorption. The figure clearly indicates that both adsorbents attain similar absorption peaks except the absorption peak at 687 cm−1 that is observed for Fe3O4/Bent/MWCNTs, which is related to the strong bending of C = C bond. Moreover, the peaks observed at 3640 cm−1 and 1040 cm−1 are related to O–H and C–O bond, respectively (Yu et al., 2020). The bond stretching vibration of CO observed at 1107 cm−1 is related to the carbon attached to the epoxy groups, while the absorption peak at 1639 cm−1 is attributed to the C = O bond (Parastar et al., 2021). The peak observed at 1430 cm−1 is ascribed to the stretching vibration of CO3−2 anion associated in dolomite that presents in bentonite as impurity (Xu et al., 2019). The broad peak at 1038 cm−1 indicates the presence of Si–O–Si groups of the tetrahedral sheet. The peaks observed at 521 cm−1 and 467 cm−1 correspond to Al–O–Si and Si–O–Si, respectively. The stretching vibration in the region between 800 cm−1 and 930 cm−1 are related to C–H bond. The peak observed between 500 cm−1 and 600 cm−1 are related to the presence of Fe–O bond (Khatamian et al., 2019). In addition, the stretching vibration in the region between 3300 cm−1 and 3600 cm−1 corresponds to the hydroxyl and carboxyl groups presented in GO (Halpegama et al., 2021). The non-existence of absorption peaks at 1724 cm−1 confirm the successful reduction of GO into rGO (Halpegama et al., 2021). After adsorption, the peak intensity in this region was reduced, and new peaks at 2928 cm−1 and 2857 cm−1 were observed. These peaks indicate the presence of C–H bond, which indicate the chemical interaction between the adsorbents and the diesel oil molecules. The vibration region between 2309 cm−1 and 2371 cm−1observed in Fig. 7d for Fe3O4/Bent/MWCNTs after observed is indication of C = O = C bond.
Based on these results, it can be assumed that the removal of oil molecules by the fabricated adsorbents is based on a chemical interaction as observed by the FTIR spectra represented in Fig. 11. Moreover, the hydrophobic interaction between the oil molecules and the adsorbent hydrophobic surface facilitated oil removal from the aqueous solution. The hydrophobic nature of the adsorbent is determined by the presence of active functional groups with a lone pair of electrons such as O–H and C–O groups that interact with the hydrophobic oil molecules. It can be observed from Fig. 7c and d that the intensity of these groups was reduced after adsorption, which confirms the hydrophobic interaction. In addition, π-π interaction caused by the presence of graphene sheets and MWCNTs has primary role in oil molecule adsorption and accounts for the increase in oil adsorption compared to Fe3O4/Bentonite composite. Moreover, the existence of positively charged element such as Mg and Al (as indicated by the EDX analysis, Table 1) results in electrostatic interaction between the positively charged elements and the additives presents the water-in-oil emulsion solution resulting in physical interaction. The possible adsorption pathway between the as-synthesized adsorbents and oil molecules is illustrated in Fig. 12.
3.5 Comparison of Adsorbents
Even though the sips isotherm model showed that Fe3O4/Bent/rGO attained slightly higher qm, the batch adsorption experiments showed that Fe3O4/Bent/MWCNTs attained slightly higher removal capability compared to Fe3O4/Bent/rGO at higher oil concentration and contact time. This could be explained by the observed higher pore volume in Fe3O4/Bent/MWCNTs compared to Fe3O4/Bent/rGO. Moreover, the amount of Fe3O4 NPs in Fe3O4/Bent/MWCNTs was less than their amount in Fe3O4/Bent/rGO adsorbent as indicated by the EDX analysis and TEM and SEM images despite using the same amount of Fe3O4 precursors. This also contributed to more surface availability on Fe3O4/Bent/MWCNTs as observed in BET analysis (Table 2). Consequently, slightly higher absorption of oil was observed in the Fe3O4/Bent/MWCNT composite compared to the Fe3O4/Bent/rGO composite. The observed maximum adsorption capacities of emulsified oil onto the prepared adsorbents were further compared to the ones reported in the literature as shown in Table 5. The results clearly show that both adsorbents exhibit superior adsorption capability and outperform all the reported adsorbents. These observations further affirm the potential of the Fe3O4/Bent/MWCNTs and Fe3O4/Bent/rGO composites to be utilized for oil removal (Table 7).
4 Conclusion
In this study, new magnetic bentonite-based adsorbents combined with two different carbon sources material (reduced graphene and multiwall carbon nanotubes) were fabricated via conventional co-precipitation method and were utilized for oil removal from water-in-oil emulsion. The characterization analysis showed that Fe3O4/Bent/MWCNTs attained higher BET surface area and pore volume. The adsorption experiments revealed that at low initial oil concentration, both adsorbents attained similar removal capability, whereas at higher oil concentration, Fe3O4/Bent/MWCNTs have higher removal capability. The adsorption of oil onto both adsorbents best fitted to Sips isotherm model. The maximum oil adsorption capacity were 81.65 mg/g and 77.12 mg/g using Fe3O4/Bent/rGO and Fe3O4/Bent/MWCNTs, respectively. Furthermore, the oil adsorption kinetics using both adsorbents was well described by pseudo-second-order kinetic model indicating a chemisorption process. The adsorption of oil onto both adsorbents was found to be controlled by electrostatic, π-π, and hydrophobic interaction. The composites reported in this study outperform other adsorbents that were utilized for oil removal from emulsified oil. Overall, the data reported in this study have demonstrated the potential application of the reported adsorbents for the effective removal of oil from water-in-oil emulsion.
Data Availability
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
References
Abd El-Gawad, H. S. (2014). Oil and Grease Removal from Industrial Wastewater Using New Utility Approach. Advances in Environmental Chemistry, 2014, 1–6.
Akaike, H. (1998). Information theory and an extension of the maximum likelihood principle (pp. 199–213). Springer.
Albatrni, H., Qiblawey, H., Almomani, F., Adham, S., & Khraisheh, M. (2019). Polymeric adsorbents for oil removal from water. Chemosphere, 233, 809–817.
Alkurdi, S. S. A., Al-Juboori, R. A., Bundschuh, J., Bowtell, L. & Marchuk, A. (2020). Inorganic arsenic species removal from water using bone char: A detailed study on adsorption kinetic and isotherm models using error functions analysis. Journal of Hazardous Materials, 405, 124112.
Andrews, R., Jacques, D., Qian, D., & Dickey, E. C. (2001). Purification and structural annealing of multiwalled carbon nanotubes at graphitization temperatures. Carbon, 39, 1681–1687.
Andrini, L., Moreira Toja, R., Gauna, M. R., Conconi, M. S., Requejo, F. G., & Rendtorff, N. M. (2017). Extended and local structural characterization of a natural and 800°C fired Na-montmorillonite–Patagonian bentonite by XRD and Al/Si XANES. Applied Clay Science, 137, 233–240.
Apriandanu, D. O. B., & Yulizar, Y. (2021). CuO-bentonite-gold nanocomposites: Facile green preparation and their characterization. Materials Letters, 284, 128911.
Balog, R., Jørgensen, B., Nilsson, L., Andersen, M., Rienks, E., Bianchi, M., Fanetti, M., Lægsgaard, E., Baraldi, A., Lizzit, S., Sljivancanin, Z., Besenbacher, F., Hammer, B., Pedersen, T. G., Hofmann, P., & Hornekær, L. (2010). Bandgap opening in graphene induced by patterned hydrogen adsorption. Nature Materials, 9, 315–319.
Chao, W., Wang, S., Li, Y., Cao, G., Zhao, Y., Sun, X., Wang, C., & Ho, S.-H. (2020). Natural sponge-like wood-derived aerogel for solar-assisted adsorption and recovery of high-viscous crude oil. Chemical Engineering Journal, 400, 125865.
Deng, Y., Peng, C., Dai, M., Lin, D., Ali, I., Alhewairini, S. S., Zheng, X., Chen, G., Li, J., & Naz, I. (2020). Recent development of super-wettable materials and their applications in oil-water separation. Journal of Cleaner Production, 266, 121624.
Ejeta, D. D., Tan, F.-H., Mathivathanan, A., Juang, T.-Y., Abu-Omar, M. M., Wang, C.-F., Lin, C.-H., & Lai, J.-Y. (2022). Preparation of fluorine- and nanoparticle-free superwetting polybenzoxazine/cellulose composites for efficient oil/water separations. Separation and Purification Technology, 288, 120675.
El-Naas, M. H., Alhaija, M. A., Al-Zuhair, S. J. E. S. & Research, P. (2017). Evaluation of an activated carbon packed bed for the adsorption of phenols from petroleum refinery wastewater. 24, 7511–7520.
Elmobarak, W. F., & Almomani, F. (2021). Application of Fe3O4 magnetite nanoparticles grafted in silica (SiO2) for oil recovery from oil in water emulsions. Chemosphere, 265, 129054.
Emam, E. (2013). Modified Activated Carbon and Bentonite Used to Adsorb Petroleum Hydrocarbons Emulsified in Aqueous Solution. American Journal of Environmental Protection, 2, 161.
Ersan, G., Apul, O. G., Perreault, F., & Karanfil, T. (2017). Adsorption of organic contaminants by graphene nanosheets: A review. Water Research, 126, 385–398.
Ewis, D., Benamor, A., Ba-Abbad, M. M., Nasser, M., El-Naas, M., & Qiblawey, H. (2020). Removal of Oil Content from Oil-Water Emulsions Using Iron Oxide/Bentonite Nano Adsorbents. Journal of Water Process Engineering, 38, 101583.
Ewis, D., Ismail, N. A., Hafiz, M., Benamor, A. & Hawari, A. H. (2021). Nanoparticles functionalized ceramic membranes: fabrication, surface modification, and performance. Environmental Science and Pollution Research. 28, 12256–12281.
Freundlich, H. J. J. P. C. (1906). Over the adsorption in solution. Journal of Physical Chemistry. 57, 1100–1107.
Halpegama, J. U., Bandara, P. M. C. J., Jayarathna, L., Bandara, A., Yeh, C.-Y., Chen, J.-Y., Kuss, C., Dahanayake, U., Herath, A. C., Weragoda, S. K., Chen, X., & Weerasooriya, R. (2021). Facile fabrication of nano zerovalent iron – Reduced graphene oxide composites for nitrate reduction in water. Environmental Advances, 3, 100024.
Ho, Y.-S. & McKay, G. J. P. b. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34, 451–465.
Khan, S., Anjum, R., & Bilal, M. (2021). Revealing chemical speciation behaviors in aqueous solutions for uranium (VI) and europium (III) adsorption on zeolite. Environmental Technology & Innovation, 22, 101503.
Khatamian, M., Divband, B., & Shahi, R. (2019). Ultrasound assisted co-precipitation synthesis of Fe3O4/ bentonite nanocomposite: Performance for nitrate, BOD and COD water treatment. Journal of Water Process Engineering, 31, 100870.
Lagergren, S. K. J. S. V. H. (1898). About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar,, 24, 1–39.
Langmuir, I. (1917). The constitution and fundamental properties of solids and liquids. Journal of the Franklin Institute, 183, 102–105.
Liang, X., Lu, Y., Li, Z., Yang, C., Niu, C., & Su, X. (2017). Bentonite/carbon composite as highly recyclable adsorbents for alkaline wastewater treatment and organic dye removal. Microporous and Mesoporous Materials, 241, 107–114.
Liu, J., Wang, N., Zhang, H., & Baeyens, J. (2019). Adsorption of Congo red dye on FexCo3-xO4 nanoparticles. Journal of Environmental Management, 238, 473–483.
Liu, S., Wang, S., Wang, H., Lv, C., Miao, Y., Chen, L., & Yang, S. (2021). Gold nanoparticles modified graphene foam with superhydrophobicity and superoleophilicity for oil-water separation. Science of the Total Environment, 758, 143660.
Mahmud, N., Benamor, A., Nasser, M. S., Ba-Abbad, M. M., El-Naas, M. H., & Mohammad, A. W. (2021). Effective Heterogeneous Fenton-Like degradation of Malachite Green Dye Using the Core-Shell Fe3O4@SiO2 Nano-Catalyst. ChemistrySelect, 6, 865–875.
Mahmud, N., Nasser, M. S., El-Naas, M. H., Ba-Abbad, M. M., Mohammad, A. W., Mansour, S. & Benamor, A. (2020). Synthesis and characterization of fe3o4 nanoparticles using different experimental methods. IOP Conference Series: Materials Science and Engineering, 778, 012028.
Marrakchi, F., Hameed, B. H., & Hummadi, E. H. (2020). Mesoporous biohybrid epichlorohydrin crosslinked chitosan/carbon–clay adsorbent for effective cationic and anionic dyes adsorption. International Journal of Biological Macromolecules, 163, 1079–1086.
Mofokeng, M., Nthunya, L. N., Gutierrez, L., Matabola, P., Mishra, S., & Nxumalo, E. N. (2020). Perflurooctyltriethoxy silane and carbon nanotubes-modified PVDF superoleophilic nanofibre membrane for oil-in-water adsorption and recovery. Journal of Environmental Chemical Engineering, 8, 104497.
Moshari, M., Mehrehjedy, A., Heidari-Golafzania, M., Rabbani, M., & Farhadi, S. (2021). Adsorption study of lead ions onto sulfur/reduced graphene oxide composite. Chemical Data Collections, 31, 100627.
Mu’azu, N. D., Jarrah, N., Kazeem, T. S., Zubair, M., & Al-Harthi, M. (2018). Bentonite-layered double hydroxide composite for enhanced aqueous adsorption of Eriochrome Black T. Applied Clay Science, 161, 23–34.
Muhammad, Y., Rahman, A. U., Rashid, H. U., Sahibzada, M., Subhan, S., & Tong, Z. (2019). Hydrodesulfurization of dibenzothiophene using Pd-promoted Co–Mo/Al2O3 and Ni–Mo/Al2O3 catalysts coupled with ionic liquids at ambient operating conditions. RSC Advances, 9, 10371–10385.
Muhammad, Y., Rashid, H. U., Subhan, S., Rahman, A. U., Sahibzada, M., & Tong, Z. (2019). Boosting the hydrodesulfurization of dibenzothiophene efficiency of Mn decorated (Co/Ni)-Mo/Al2O3 catalysts at mild temperature and pressure by coupling with phosphonium based ionic liquids. Chemical Engineering Journal, 375, 121957.
MuthuKumaraPandian, A., Rajasimman, M., Rajamohan, N., Varjani, S., & Karthikeyan, C. (2021). Anaerobic mixed consortium (AMC) mediated enhanced biosynthesis of silver nano particles (AgNPs) and its application for the removal of phenol. Journal of Hazardous Materials, 416, 125717.
Olajire, A. A. (2020). Recent advances on the treatment technology of oil and gas produced water for sustainable energy industry-mechanistic aspects and process chemistry perspectives. Chemical Engineering Journal Advances, 4, 100049.
Parastar, M., Sheshmani, S., & Shokrollahzadeh, S. (2021). Cross-linked chitosan into graphene oxide-iron(III) oxide hydroxide as nano-biosorbent for Pd(II) and Cd(II) removal. International Journal of Biological Macromolecules, 166, 229–237.
Raghu, M. S., Yogesh Kumar, K., Prashanth, M. K., Prasanna, B. P., Vinuth, R., & Pradeep Kumar, C. B. (2017). Adsorption and antimicrobial studies of chemically bonded magnetic graphene oxide-Fe3O4 nanocomposite for water purification. Journal of Water Process Engineering, 17, 22–31.
Sadegh, H., Shahryari-ghoshekandi, R., & Kazemi, M. (2014). Study in synthesis and characterization of carbon nanotubes decorated by magnetic iron oxide nanoparticles. International Nano Letters, 4, 129–135.
Sakr, M. A., Mohamed, M. G. A., Wu, R., Shin, S. R., Kim, D., Kim, K., & Siddiqua, S. (2020). Development of bentonite-gelatin nanocomposite hybrid hydrogels for tissue engineering. Applied Clay Science, 199, 105860.
Saleem, H., Haneef, M. & Abbasi, H. (2017). Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Materials Chemistry and Physics 204. 1–7.
Saraf, M., Natarajan, K., & Mobin, S. M. (2017). Microwave assisted fabrication of a nanostructured reduced graphene oxide (rGO)/Fe2O3 composite as a promising next generation energy storage material. RSC Advances, 7, 309–317.
Sarojini, G., Venkatesh Babu, S., Rajamohan, N., & Rajasimman, M. (2022). Performance evaluation of polymer-marine biomass based bionanocomposite for the adsorptive removal of malachite green from synthetic wastewater. Environmental Research, 204, 112132.
Shan, R.-R., Yan, L.-G., Yang, K., Hao, Y.-F., & Du, B. (2015). Adsorption of Cd(II) by Mg–Al–CO3- and magnetic Fe3O4/Mg–Al–CO3-layered double hydroxides: Kinetic, isothermal, thermodynamic and mechanistic studies. Journal of Hazardous Materials, 299, 42–49.
Sips, R. (1948). On the Structure of a Catalyst Surface. The Journal of Chemical Physics, 16, 490–495.
Tran, H. V., Hoang, L. T., & Huynh, C. D. (2020). An investigation on kinetic and thermodynamic parameters of methylene blue adsorption onto graphene-based nanocomposite. Chemical Physics, 535, 110793.
Turner, B., Henley, B., Sleap, S., Sloan, S. J. I. j. o. e. s. & technology (2015). Kinetic model selection and the Hill model in geochemistry. International Journal of Environmental Science and Technology, 12, 2545–2558.
Umar, A. A., Abdul Patah, M. F., Abnisa, F. & Daud, W. M. A. W. (2020). Preparation of magnetized iron oxide grafted on graphene oxide for hyperthermia application. Reviews in Chemical Engineering, 2020, 000010151520200001.
Wang, Q., Gao, T., Hao, L., Guo, Y., Liu, W., Guo, L., Wang, C., Wang, Z., & Wu, Q. (2020). Advances in magnetic porous organic frameworks for analysis and adsorption applications. TrAC Trends in Analytical Chemistry, 132, 116048.
Weber, W. J. & Morris, J. C. J. J. o. t. s. e. d. (1963). Kinetics of adsorption on carbon from solution. Journal of the sanitary engineering, 89, 31–60.
Xu, W., Chen, Y., Zhang, W., & Li, B. (2019). Fabrication of graphene oxide/bentonite composites with excellent adsorption performances for toluidine blue removal from aqueous solution. Advanced Powder Technology, 30, 493–501.
Yan, F., Chu, Y., Zhang, K., Zhang, F., Bhandari, N., Ruan, G., Dai, Z., Liu, Y., Zhang, Z., Kan, A. T., & Tomson, M. B. (2015). Determination of adsorption isotherm parameters with correlated errors by measurement error models. Chemical Engineering Journal, 281, 921–930.
Yu, C., Shao, J., Sun, W., & Yu, X. (2020). Treatment of lead contaminated water using synthesized nano-iron supported with bentonite/graphene oxide. Arabian Journal of Chemistry, 13, 3474–3483.
Zamora-Ledezma, C., Negrete-Bolagay, D., Figueroa, F., Zamora-Ledezma, E., Ni, M., Alexis, F., & Guerrero, V. H. (2021). Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environmental Technology & Innovation, 22, 101504.
Zhang, T., Zhang, C., Zhao, G., Li, C., Liu, L., Yu, J., & Jiao, F. (2020). Electrospun composite membrane with superhydrophobic-superoleophilic for efficient water-in-oil emulsion separation and oil adsorption. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 602, 125158.
Acknowledgements
This publication was made possible by an Award [GSRA6-2-0516-19029] from Qatar National Research Fund (a member of Qatar Foundation). Open Access funding provided by Qatar National Library. The contents herein are solely the responsibility of the author[s]. The authors would like to acknowledge the technical support of CLU at Qatar University in performing SEM and TEM analysis.
Author information
Authors and Affiliations
Contributions
Dina Ewis: conceptualization, literature search, methodology, data analysis and writing manuscript. Nafis Mahmud: methodology, writing-review and edition. Abdelbaki Benamor: formulation of idea and review/editing. Muneer M. Ba-Abbad: conceptualization, methodology, formulation of idea and review/editing. Mustafa Nasser: formulation of idea and review/editing. Muftah El-Naas: formulation of idea and review/editing.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ewis, D., Mahmud, N., Benamor, A. et al. Enhanced Removal of Diesel Oil Using New Magnetic Bentonite-Based Adsorbents Combined with Different Carbon Sources. Water Air Soil Pollut 233, 195 (2022). https://doi.org/10.1007/s11270-022-05641-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05641-6