Abstract
The recovery and recycling of nutrients (N&P) from wastewater are one of the major topics to save primary energy and resources, to raise the efficiency of wastewater treatment plants, and to foster a future circular economy. In the present study, the removal of ammonium (NH4+) and phosphate (PO43−) using natural and Ca-treated zeolite is investigated in detail. Special emphasis is put on the simultaneous removal of both species from model solutions followed by elaborate mineralogical analyses (XRD, EPMA, FEG-SEM) for zeolite characterization and in order to determine the type, structure, and crystal sizes of CaP-phases precipitating on the zeolites surface. The effectivity of the phosphate segregation and chemical composition and the crystalline structure of the CaP-phase precipitating on the surface of the zeolite depend on the physico chemical conditions in particular on pH, molar ratio of Ca and P (due to zeolite modification), and the presence of NH4+. Results of simultaneous removal experiments of N&P revealed that Ca pretreatment enhances P segregation and increases the obtainable P-loadings of Ca–zeolites. Maximum P-loadings of 25 mg g−1 Ca–zeolite in binary solutions containing both ammonium and phosphate were obtained. Simultaneous phosphate removal by surface precipitation of CaP-phases does not significantly influence ammonium ion exchange and the type of CaP-precipitates formed on the zeolite surface is assumed to be mainly brushite and apatite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the actual discussions of promoting renewable energy, biogas and sewage treatment plants try to enhance their capacity for anaerobic digestion to produce green fuels (e.g., biomethane). This leads to increasing amounts of liquid digestates and sludge liquor, which contain ascending concentrations of dissolved ammonium and phosphorous due to intensified co-substrate fermentation. Legal regulations (maximum permissible values) and technological issues (ammonium inhibition) necessitate the reduction of both dissolved species by certain wastewater treatment methods. Nowadays, NH4+ and PO43− are usually removed separately by biological and chemical processes. All of these standard processes are expensive and do not offer the possibility to recover, concentrate, and utilize NH4+ and PO43− for example as nutrients in fertilizers. In contrast to these enormous amounts of N and P that are lost via global wastewater treatment systems, the food supply of an increasing world population strongly depends on the availability of these nutrients for crop cultivation.
Phosphorous is one of the building blocks of life and essential for many cellular processes and DNA synthesis. Additionally, no chemical component can substitute P in the life cycle and therefore P is irreplaceable (Cordell et al., 2009). By now, nearly three times the amount of the natural P cycle is mined per year from phosphate rock for anthropogenic needs (e.g., for fertilizer in agriculture and chemical and pharmaceutical products) (Vučić et al., 2021). In this human-affected cycle, P is lost at all stages of the processing systems (Daneshgar et al., 2018). After Kok et al. (2018); 3.7 Mt a−1 of P deriving from food is discarded via wastewater, where it is currently lost.
A similar picture can be observed for nitrogen, but even at a larger scale: since the invention of the Haber Bosch process in the beginning of the twentieth century, which enables the synthesis of NH3 from natural gas and atmospheric N2, nitrogen is extensively used as fertilizer. The global nitrogen cycle accounts for ~ 400 Mt a−1, with approximately 50% thereof deriving from anthropogenic activities (Fowler et al., 2013).
Given these values, the recovery and utilization of NH4+ and PO43− are of great interest considering sustainability and closing of material cycles. As phosphorous is also one of the critical raw materials identified by the EU (European Commission, 2014), its recovery would contribute to a sustainable raw material supply. Currently, many countries throughout Europe promote the use of secondary phosphates (e.g., Switzerland and Germany) by making P-recovery mandatory for wastewater treatment plants that exceed defined sizes, e.g., > 50,000 population equivalent in Germany (Bundesministerium für Umwelt, Naturschutz und nukleare Sicherheit (BMU), 2017). Sweden and Austria are on the same path and committed themselves to put P-recovery into national legislation in the near future. Potential technologies for P-recovery comprise the precipitation of dissolved PO43− from the liquid phase as magnesium ammonium phosphates (MAP) or MAP/calcium phosphate minerals (Law & Pagilla, 2018) as well as recovery from sewage sludge ash after incineration. The latter approach has some serious constraints like the costly technological facilities needed for monocombustion, storage and ash processing, and the production of certain amounts of (toxic) by-products. Hence, technologies for liquid phase recovery of P (and N) are largely investigated, under which ion-exchange and adsorption processes on natural and modified zeolites have become an interesting topic (Yin & Kong, 2014; Zhang et al., 2011). In contrast to synthetic zeolites, natural zeolites present an attractive option for applications in water purification due to their high abundance, availability, and low costs (Alshameri, Ibrahim, et al., 2014; Guaya et al., 2015b; Malekian et al., 2011; Zhang et al., 2011).
So far, natural zeolites have been rarely used for anion sorption due to the overall negative charge on their surface, caused by their crystal structure (Jiang et al., 2013). For anion and especially phosphate removal, modification of the surface of the zeolite is necessary. This is usually done by incorporation or impregnation of metal oxides and hydroxides in the zeolite pore structure (e.g., Fe, Al, La, and other rare earth elements; Alshameri, Yan, et al., 2014; Jiménez-Cedillo et al., 2011; Sang et al., 2020; Simsek et al., 2013), the replacement of natural cations by the aforementioned metal ions via selective ion exchange (Guaya et al., 2015b), or the use of special, metal-incorporated zeolites synthesized from fly ashes (e.g., Hermassi et al., 2020; Xu et al., 2022). The process of phosphate removal is usually related to surface precipitation of various insoluble chemical species, like monodentate and bidentate complexes on incorporated iron hydroxides inside the zeolite micropores (Guaya et al., 2015b).
Several studies have been conducted for the simultaneous removal of NH4+ and PO43− from aqueous solutions by modified zeolites; many of them are based on synthetic zeolites, fly-ash zeolites, or mixtures of reactive sorbents (e.g., Gao et al., 2018; He et al., 2016; Hermassi et al., 2018; Salam et al., 2021; Zhang et al., 2007). Guaya et al. (2015b) modified natural clinoptilolite with Fe(III)-ions to promote the formation of hydroxyl groups (≅ Fe-OH). Although simultaneous removal of NH4+ and PO43− was found to be possible, the NH4+ exchange capacity was slightly decreased by the competing ions and surface precipitation of various MAP and CaP- minerals (blocking of pores). Furthermore, Guaya et al. (2015a) studied adsorption on a natural zeolite modified with hydrated aluminum oxide and increased PO43− sorption from 0.6 to 7 mg-P g−1 while NH4+ sorption capacity is slightly decreased from 33 to 30 mg-N g−1. In both studies, recovery of phosphate was found to be possible, but a reduction of phosphate uptake after several regeneration cycles was observed (Guaya et al., 2015a). Other studies show that simultaneous removal of NH4+ and PO43− with zeolites modified by Fe, Al, Mn, and La is possible for simulated wastewaters (e.g., Li et al., 2005; Ning et al., 2008; Li et al., 2009 and Huo et al., 2012). Performance depends on the competing ions in the applied wastewater and the pre-treatment. Simultaneous recovery of nitrogen and phosphorus was achieved by natural zeolites from sludge fermentation liquid in a study of Wan et al. (2017). Regeneration of expended zeolites can be carried out by H2SO4 (e.g., Choi et al., 2014) or NaOH-solutions (e.g., Guaya et al., 2015a). Generally, regeneration performance and products thereof depend on the type of zeolite surface modification and the phosphorous removal mechanism.
Some studies regarding P-removal with Ca-modified zeolites were published (e.g., Hermassi et al., 2016; Schick et al., 2012), but so far, Ca-modified zeolites were only rarely used for simultaneous N&P-removal from different media. Sun et al. (2011) made sorption experiments with CaCl2-modified zeolite in aqueous solution. Phosphate removal efficiencies were pH-dependent, with maximum values between 7 and 9. They stated that the mechanism of ammonium adsorption on the modified zeolite was ion exchange, whereas the removal of phosphate was a matter of chemical precipitation. You et al. (2017) investigated Ca- and Mg-modified zeolites for the removal of NH4+ and PO43− from simulated treated wastewater effluents. They measured maximum sorption capacities for a Ca-treated zeolite of 123.1 ± 9 mg NH4+ g−1 and 119.5 ± 7.5 mg PO43− g−1 in an ammonium/phosphate binary system. Sorption mechanisms involved ammonium ion exchange and precipitation of Ca and Mg phosphates or mixed ammonium-magnesium phosphate minerals like apatite, brushite (CaHPO4.H2O), and struvite (NH4MgPO4.6 H2O). Mitrogiannis et al. (2017) reported that Ca(OH)2-treated zeolite can be effectively used for PO4-P removal from aqueous solutions under low P concentrations (0.5–10 mg L−1) with the predominant mechanisms for P-removal being ligand exchange and Ca-P surface precipitation. In subsequent studies, they applied Ca(OH)2-treated zeolites for P-removal from urine (Mitrogiannis et al., 2018) and olive mill wastewater (Mitrogiannis et al., 2020), where maximum P removals of 73.9 and 85.9% for two different wastewater samples were achieved.
The present study further investigates the phosphate removal via natural and Ca-modified zeolites (clinoptilolite) by applying an elaborate set of chemical and mineralogical analyses for the liquid solutions as well as the solid materials. The type of P-phases formed on the applied zeolites is further investigated and sorption capacities in binary aqueous solutions containing NH4+ and PO43− are presented in order to improve simultaneous ammonium and phosphate removal and recovery from real effluents in the ILS process (Ellersdorfer, 2018) and other potential future applications.
2 Materials and Methods
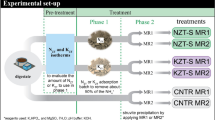
Sorption experiments for single and simultaneous phosphate segregation were conducted using natural and Ca-modified natural zeolite. For Ca-modification, natural zeolite from Eastern Slovakia was treated with CaCl2 solution (0.5 M; pH 10) for 24 h in columns using a volume of 20 L of treatment solution and 2.3 kg of natural zeolite (grain size 1.5–2 mm). Diameter of the column was 120 mm and flow rate 2.5 L min−1. To ensure a complete submersion of the zeolite, the modification process was run in up flow mode. After modification, the zeolite was flushed with deionized water until pH 7 was reached. The modified zeolite was dried at 105 °C and stored in hermetically sealed bottles until sorption experiments.
Screening and single phosphate sorption experiments were conducted in batch operation with dibasic sodium phosphate dodecahydrate solutions (Na3PO4.12 H2O) in a concentration range from 500 to 2500 mg PO43− L−1. For preliminary screening experiments, a concentration of 2500 mg PO43− L−1 was used. For simultaneous sorption from binary solutions (N&P), an additional amount of 1000 mg L−1 of NH4+ was added to each phosphate solution using ammonium sulfate ((NH4)2SO4)). If necessary, pH-values were adjusted with sulfuric acid (H2SO4; 96%). The batch system used for sorption experiments was the same for both experimental approaches: 500 mL of initial single/binary solution was treated with 20 g of natural and Ca-modified zeolite for 24 h in an overhead shaker at 20 °C respectively. In order to avoid abrasion of the material due to agitation, the zeolite was packed into cotton bags.
Liquid samples were taken before and after the sorption experiments and filtrated by syringe filters (0.22 µm). Ammonium was detected via Kjeldahl method using boric acid and HCl (0.1 M)/Tashiro’s indicator for titration. For phosphate analysis, the photometer method was used (Merck Spectroquant® 114848). The difference in PO43− and NH4+ concentration of the initial and final solution represents the amount of removed phosphate and ammonium and therefore the effective segregation capacity for different equilibrium concentrations which yields to the sorption isotherm under the given conditions.
For data interpretation, the equilibrium loadings (\({q}_{{PO}_{4}^{3-}/ {NH}_{4}^{+}})\) were evaluated by the amount of adsorbed phosphate/ammonium per unit mass of zeolite (in mg PO43− g−1 zeolite or mg NH4+ g−1 zeolite), calculated according to the following equation:
where C0 and Ce are the phosphate/ammonium concentrations (mg L−1) of the stock solutions at initial and final stage of the experiment, V is the total solution volume (L), and m is the mass of the zeolite (g).
Mineralogical investigations were done before and after the modification step and at the end of the segregation experiments using dried samples (105 °C), which were sealed in plastic containers and stored for subsequent solid analysis. The material was characterized by X-ray diffraction (XRD) as well as electron microprobe (EPMA) and scanning electron microscopy (FEG-SEM). XRD was conducted with a PANalytical X’Pert Pro, Cu Kα, λ = 1.541 nm at the Chair of Petroleum Geology, Montanuniversität Leoben. XRD patterns were evaluated with the software X’Pads with its attached ICDD databases. Electron micropobe technique was used for chemical mapping using a Superprobe Jeol JXA8200 instrument at the Eugen F. Stumpfl Microprobe Laboratory provided by UZAG at Montanuniversität Leoben. Element mappings were made from carbon-coated polished sections. Morphology and surface studies of the zeolite before and after the sorption experiments were undertaken using FEG-SEM technique (LEO 1525, Carl Zeiss SMT at Erich Schmid Institute of Material Science, Leoben). Zeolite grains were sticked on a carbon tab placed on an aluminum stub and coated with a carbon layer. BSE high resolution microphotographs of the zeolite were taken at an acceleration voltage of 5 kV.
3 Results and Discussion
3.1 Characterization of the Untreated and Treated Raw Material
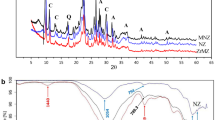
The XRD spectra represent the whole rock mineral composition of both the untreated and Ca-treated zeolitic rock (Fig. 1). The X-ray diffraction pattern of the untreated zeolite identifies a HEU-type zeolite (clinoptilolite) as the main mineralogical phase in the raw material. Minor phases include biotite (annite?), plagioclase (dominantly andesine), alkali feldspar, quartz, and cristobalite. Minor amounts of plagioclase and biotite differ slightly between the untreated and treated zeolite but do not indicate significant changes in the mineralogical composition of the different samples nor mineral transformation from pre-treatments (Vollprecht et al., 2019). The different cation loading after the treatment has no significant impact on the peak position of the clinoptilolite. A slight enhancement of the background signal between 20 and 30° 2θ may indicate minor amounts of amorphous vulcanic glass (Ostrooumov et al., 2012).
The mean chemical composition (n = 22) of the clinoptilolite obtained by EPMA and the cations per formula units were calculated based on 72 oxygens, deriving from the ideal formulas of HEU type zeolites (Heulandite (Na, K)Ca4(Al8Si28)O72.24 H2O and clinoptilolite (Na, K)6(Al6Si30)O72.20 H2O) and are listed in Table 1.
Si and Al are assigned to the tetrahedral site and Na, K, Ca, and Mg to the exchangeable cation sites. Spots for the WDS analysis were selected using back scattered electron (BSE) images and EDS spectra, in order to avoid contamination by iron hydroxides and to distinguish minor minerals (quartz and feldspar) from the zeolite matrix.
3.2 Sorption Experiments
Results from preliminary screening experiments for phosphate segregation show that the PO43−-loading on natural zeolite accounts for 12.3 mg PO43− g−1 zeolite (Table 2). The pH value of the initial phosphate solution (~ 2500 mg PO43− L−1) slightly decreases from 12.23 to 11.93 after 24 h of contact with the natural zeolite sample. Adjustment of the pH-value of the initial solution to 8.5 leads to a small increase of the phosphate removal capacity (14.5 mg PO43− g−1) and a larger decline in the pH-value during 24 h of PO43−−segregation (8.5 to 7.67) as a consequence of the logarithmic pH-scale. Phosphate loading of Ca-pretreated zeolite (6.5 mg PO43− g−1) is only half of the loading of natural zeolite at high pH-values (~ 12) but is more than doubled when the pH-value is adjusted to values < 8. With a value of 13.1 mg PO43− g−1, the final loading of Ca-pretreated zeolite after 24 h is higher than the value of untreated zeolite without pH-adjustment. These results emphasize the importance of the pH-value for phosphate removal by the formation of CaP-phases. In any of the conducted experiments, the pH-value of the loading solution is lower after phosphate segregation, which is a result of the acidic reactions responsible for the CaP-phase formation.
The single phosphate sorption experiments (Fig. 2; left) reveal a continuous increase of the phosphate loading with a maximum of around 20 mg PO43− g−1 at initial solution concentrations of 2500 mg PO43− L−1 for both natural and Ca-treated zeolites. Whereas natural zeolite P-loading is higher at solution concentrations < 250 mg L−1; Ca-treatment leads to higher phosphate removal capacities between 250 and 1000 mg PO43− L−1. Nevertheless, the observed maximum P-loadings are similar, which may be an effect of the establishment of an equilibrium between the dissolved cations in the solution (mostly Na+) and the exchangeable ions on the zeolite (mainly Ca2+; Table 1), which is limited by the amount of available cations in the solution.
The addition of ammonium changes the phosphate sorption capacities of natural zeolite and leads to significantly higher P-loadings up to a concentration of 1000 mg PO43− L−1 (Fig. 2; right). Nevertheless, the maximum P-loading of natural zeolite at 2500 mg PO43− L−1 in binary solution is similar to that of the single phosphate experiment (~ 20 mg PO43− g−1). The P-loadings of Ca-modified zeolite reveal significantly higher values over the whole concentration range with a maximum of 25 mg PO43− g−1 at 2500 mg PO43− L−1. These results indicate that the P-loading capacity of the natural zeolite is affected by Ca-limitation at higher P-concentration levels, where the natural zeolitic Ca2+-ions are already expended and the additional ammonium-ions in the loading solutions cannot deliberate extra Ca-ions. Ca pretreatment increases the available amounts of Ca2+ in the zeolite structure and cations like NH4+ can mobilize these additional Ca-ions in order to increase Ca-phosphate precipitation.
Ammonium sorption for natural and Ca-treated zeolite is between 12 and 16 mg NH4+ g−1 and tends to increase slightly with rising P-concentrations. As indicated by the similar values of NH4+-loading on both zeolites, the ammonium ion exchange is hardly influenced by the formation of CaP-phases, although a small decrease in the NH4+-loading can be observed for the maximum phosphate binding zeolite (Ca-treated zeolite at 2500 mg L−1 PO43−). In comparison to single ammonium sorption experiments (1000 mg NH4+ L−1 without PO43−-addition; dotted lines in Fig. 2), NH4+-uptake during simultaneous operation is in the same range as in single ion exchange experiments, which is another proof that ammonium exchange is not negatively influenced by CaP-precipitation (e.g. blockage of pores).
3.3 Identification of the Solid Phases After Precipitation
Several different techniques were applied in order to identify the precipitated mineral phase on the zeolites surface. Observation with FEG-SEM technique gives information about the morphological aspects like crystal form, shape, size, and grade of crystallinity. Analyses with EPMA technique provided information about the chemical composition of the precipitate. The formation of the precipitates was observed only in pH-adjusted solutions combined with the Ca-treated zeolite.
A BSE image of the cross section after sorption experiments presented in Fig. 3 shows the typical texture of a zeolitic tuff as it was used in this study. Coarser grained, partially idiomorphic mineral phases are embedded in a finer grained matrix. The main different components distinguishable by different gray scales were identified by EDS and consist of clinoptilolite, plagioclase, K-Feldspar, and minor amounts of quartz and biotite. Clinoptilolite occurs thereby as distinct grains filling pore spaces, extremely fine-grained matrix material, and clinoptilolite pseudomorphs of former glass shards representing its volcanic origin. This is consistent with the mineral composition obtained by XRD (Fig. 1).
Mapping of the elemental distribution by EPMA in the same area is shown in Fig. 4a–f. Aluminum and silicon are revealed in all regions of the zeolite grain as they are considered as the ions in the tetrahedral sites of zeolite crystal lattice. K, Na, and Ca as exchangeable cations of the zeolite are also distributed over the whole cross section. Distinct higher amounts of Ca, Na, and Al in some regions of the cross section can be explained by feldspar crystals which occur in the fine-grained matrix of the zeolite rock. High concentrations of calcium and phosphorous are less uniformly distributed. Notably, phosphorous is largely absent in the center of the grain but is enriched on the rim of the zeolite forming the precipitate together with calcium (Fig. 4e and f).
a–f Elemental distribution obtained by EPMA of the cross section of a representative zeolite grain after sorption experiments with 2500 mg L−1 PO43− for 24 h and adjusted pH to 7: a silicon map, b aluminum map, c sodium map, d potassium map, e calcium map, and f phosphorous map; brighter regions correspond to higher amounts of the respective element
BSE pictures from polished cross sections obtained during EPMA in Fig. 5 reveal details of zeolite grains after precipitation at higher magnification; an accumulation of fine-grained CaP-phases especially in small cavities and niches along the rim of the zeolite grain is particularly visible and shows two different types of crystal shapes occurring as described in detail below.
a–f Representative images of the CaP precipitates present on the surface of Ca-treated zeolite after the experimental approach in the study. Pictures a to c show acicular crystals growing on clinoptilolites crystals and pictures d and e show platy to tabular crystals of brushite(?), f: rose-like aggregate of brushite(?) crystals
Precipitates of calcium phosphate (CaP) produced during pH-adjusted screening experiments were observed using FEG-SEM technique following the protocol described in the “Materials and Methods” section. The CaP-crystals shown in Fig. 5 are representative of precipitates produced at pH 7 during 24 h sorption from a 2500-mg PO43− L−1 solution (Table 2). The experiments resulted in the formation of acicular crystals up to a grain size of 5 µm (Fig. 6a–c) and platy, radiating rose-like aggregates of tabular crystals up to a size of 100 µm (Fig. 6d–f). Due to crystal habit and crystal form, it can be assumed that these are tabular crystals of monoclinic brushite with perfect cleavage in [001] and [010]. Besides, brushite acicular crystals, especially contact twinned, can also be assumed as hydroxylapatite.
In summary, the simultaneous removal of ammonium and phosphate is possible both with natural and Ca-modified zeolites. The applied method of Ca pretreatment with CaCl2-solutions, which increases the Ca-loading and subsequently also the P-removal efficiency, can be executed by simple immersion of the zeolite grains in liquid CaCl2-solutions without several ion-exchange stages (e.g., Guaya et al., 2015a, b) subsequent drying, calcination, or other (thermal) treatments. This is of specific interest for the further development of the ILS-process, where columns filled with zeolite grains (1–3 mm) together with sodium hydroxide solutions are used for ammonium removal via acid scrubbing. CaCl2-solutions can be applied for a simple in-line treatment of zeolite grains to remove phosphate from wastewater in a subsequent extra-column, which is loaded with CaCl2 and recovered by acid flushing from time to time. Hence, the ILS-process itself is not changed but only expanded by an additional column used for P-removal, which enables simultaneous removal but selective recovery of N&P in order to recycle these valuable nutrients.
4 Conclusions
Results from the sorption experiments show that Ca-treated natural zeolite can be used to simultaneously remove ammonium and phosphate from aqueous solutions. Natural zeolitic calcium is exchanged with sodium ions in Na3PO4-solutions leading to the precipitation of free PO43− as CaP-phases on the zeolite. The same effect can be observed in presence of additional NH4+, which enhances CaP-precipitation due to higher cation concentrations and ammonium selectivity, which deliberates more Ca-ions, if they are available (Ca-treatment). For natural zeolites, Ca-limitation impedes CaP-precipitation at higher PO43−-concentrations; wherefore, the following conclusions can be drawn:
-
P segregation using zeolites is feasible and similar for natural and Ca-pretreated zeolites in phosphate solutions.
-
Ca pretreatment enhances P segregation and increases the obtainable P-loadings of Ca–zeolites.
-
P segregation is higher in solutions containing additional NH4+ (binary solutions) for both natural zeolites and Ca-treated zeolites as long as natural zeolitic Ca-ions are sufficiently available.
-
Ca-treatment significantly increases P-loadings in binary solutions with higher PO43−concentrations (> 100 mg L−1) and maximum P-loadings of 25 mg g−1 Ca–zeolite were measured.
-
CaP-phase formation does not significantly influence ammonium ion exchange in the experimental setup used for this study.
-
The type of CaP-precipitates formed on the zeolites surface is assumed to be brushite and apatite.
This leads to the assumption that the natural Ca-loading of the applied zeolite is not sufficient for a complete CaP-precipitation and P-loadings are limited by the available Ca-cations in the zeolite structure. The effectivity of the phosphate segregation as well as the chemical composition and crystalline structure of the CaP-phases precipitating on the surface of the zeolite depends on the physicochemical conditions in particular on pH, molar ratio of Ca and P (influenced by zeolite modification), and the presence of NH4+. For the effective recovery of phosphate from real effluents of wastewater treatment plants, pH-values and cation concentrations have to be considered due to their influence on the amount and mineralogical type of precipitates and therefore on the method used for selective P-recovery in the future. In view of a potential application of simultaneous N&P sorption in wastewater treatment, the fixation of the precipitated CaP-phases to the zeolites surface is a major benefit in order to selectively recover phosphorous and avoid the need for subsequent filtration of fine-grained precipitates.
Availability of Data and Materials
The authors declare that all data and materials support their published claims and comply with field standards.
Code Availability
Not applicable.
References
Alshameri, A., Ibrahim, A., Assabri, A., Lei, X., Wang, H., & Yan, C. (2014a). The investigation into the ammonium removal performance of Yemeni natural zeolite: Modification, ion exchange mechanism, and thermodynamics. Powder Technology, 258, 20–31. https://doi.org/10.1016/j.powtec.2014.02.063
Alshameri, A., Yan, C., & Lei, X. (2014b). Enhancement of phosphate removal from water by TiO2/Yemeni natural zeolite: Preparation, characterization and thermodynamic. Microporous and Mesoporous Materials, 196, 145–157. https://doi.org/10.1016/j.micromeso.2014.05.008
Bundesministerium für Umwelt, Naturschutz und nukleare Sicherheit BMU (2017). Verordnung zur Neuordnung der Klärschlammverwertung. AbfKlärV.
Choi, J.-W., Kwon, K.-S., Lee, S., An, B., Hong, S.-W., & Lee, S.-H. (2014). Pilot-scale test for a phosphate treatment using sulfate-coated zeolite at a sewage disposal facility. Water, Air, & Soil Pollution, 225,. https://doi.org/10.1007/s11270-013-1835-3
Cordell, D., Drangert, J.-O., & White, S. (2009). The story of phosphorus: Global food security and food for thought. Global Environmental Change, 19, 292–305. https://doi.org/10.1016/j.gloenvcha.2008.10.009
Daneshgar, S., Callegari, A., Capodaglio, A., & Vaccari, D. (2018). The potential phosphorus crisis: Resource conservation and possible escape technologies: A review. Resources, 7, 37. https://doi.org/10.3390/resources7020037
Ellersdorfer, M. (2018). The ion-exchanger-loop-stripping process: Ammonium recovery from sludge liquor using NaCl-treated clinoptilolite and simultaneous air stripping. Water Science and Technology, 77, 695–705. https://doi.org/10.2166/wst.2017.561
European Commission. (2014). Communication from the commission to the European parliament, the council, the European economic and social committee and the committee of the regions on the review of the list of critical raw materials for the EU and the implementation of the raw materials initiative.
Fowler, D., Coyle, M., Skiba, U., Sutton, M., Cape, J., & Reis, S., et al. (2013). The global nitrogen cycle in the twenty-first century. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 368, 20130164. https://doi.org/10.1098/rstb.2013.0164.
Gao, F., Xiao, L., & Zhang, H. (2018). Potential of using synthesized nano-zeolite for ammonium and phosphate immobilization in dairy wastewater. The Journal of Dairy Research, 85, 375–378. https://doi.org/10.1017/S0022029918000560
Guaya, D., Valderrama, C., Farran, A., Armijos, C., & Cortina, J. (2015a). Simultaneous phosphate and ammonium removal from aqueous solution by a hydrated aluminum oxide modified natural zeolite. Chemical Engineering Journal, 271, 204–213. https://doi.org/10.1016/j.cej.2015.03.003
Guaya, D., Valderrama, C., Farran, A., & Cortina, J. (2015b). Modification of a natural zeolite with Fe(III) for simultaneous phosphate and ammonium removal from aqueous solutions. Journal of Chemical Technology & Biotechnology, 91, 1737–1746. https://doi.org/10.1002/jctb.4763
He, Y., Lin, H., Dong, Y., Liu, Q., & Wang, L. (2016). Simultaneous removal of phosphate and ammonium using salt–thermal-activated and lanthanum-doped zeolite: Fixed-bed column and mechanism study. Desalination and Water Treatment, 57, 27279–27293. https://doi.org/10.1080/19443994.2016.1166459
Hermassi, M., Valderrama, C., Moreno, N., Font, O., Querol, X., Batis, N. H., & Cortina, J. L. (2016). Powdered Ca-activated zeolite for phosphate removal from treated waste-water. Journal of Chemical Technology & Biotechnology, 91, 1962–1971. https://doi.org/10.1002/jctb.4867
Hermassi, M., Dosta, J., Valderrama, C., Licon, E., Moreno, N., Querol, X., Batis, N. H., & Cortina, J. L. (2018). Simultaneous ammonium and phosphate recovery and stabilization from urban sewage sludge anaerobic digestates using reactive sorbents. The Science of the Total Environment, 630, 781–789. https://doi.org/10.1016/j.scitotenv.2018.02.243
Hermassi, M., Valderrama, C., Font, O., Moreno, N., Querol, X., Batis, N. H., & Cortina, J. L. (2020). Phosphate recovery from aqueous solution by K-zeolite synthesized from fly ash for subsequent valorisation as slow release fertilizer. The Science of the Total Environment, 731, 139002. https://doi.org/10.1016/j.scitotenv.2020.139002
Huo, H., Lin, H., Dong, Y., Cheng, H., Wang, H., & Cao, L. (2012). Ammonia-nitrogen and phosphates sorption from simulated reclaimed waters by modified clinoptilolite. Journal of Hazardous Materials, 229–230, 292–297. https://doi.org/10.1016/j.jhazmat.2012.06.001
Jiang, C., Jia, L., He, Y., Zhang, B., Kirumba, G., & Xie, J. (2013). Adsorptive removal of phosphorus from aqueous solution using sponge iron and zeolite. Journal of Colloid and Interface Science, 402, 246–252. https://doi.org/10.1016/j.jcis.2013.03.057
Jiménez-Cedillo, M., Olguín, M., Fall, C., & Colín, A. (2011). Adsorption capacity of iron- or iron–manganese-modified zeolite-rich tuffs for As(III) and As(V) water pollutants. Applied Clay Science, 54, 206–216. https://doi.org/10.1016/j.clay.2011.09.004
Kok, D.-J., Pande, S., van Lier, J., Ortigara, A., Savenije, H., & Uhlenbrook, S. (2018). Global phosphorus recovery from wastewater for agricultural reuse. Hydrology and Earth System Sciences, 22, 5781–5799. https://doi.org/10.5194/hess-22-5781-2018
Law, K., & Pagilla, K. (2018). Phosphorus recovery by methods beyond struvite precipitation. Water Environment Research, 90, 840–850. https://doi.org/10.2175/106143017X15131012188006
Li, B., Ning, P., Chen, Y.-B., Deng, C.-L., & Jiang, Z. (2005). Nitrogen and phosphate removal by activated zeolite with lanthana. Wuhan Ligong Daxue Xuebao/journal of Wuhan University of Technology, 27, 56–59.
Li, B., Lu, X., Ning, P., & Yang, Y. (2009). Nitrogen and phosphate removal by zeolite-rare earth adsorbents, 2009 International Conference on Environmental Science and Information Application Technology, ESIAT (pp. 599–602).
Malekian, R., Abedi-Koupai, J., Eslamian, S., Mousavi, S., Abbaspour, K., & Afyuni, M. (2011). Ion-exchange process for ammonium removal and release using natural Iranian zeolite. Applied Clay Science, 51, 323–329. https://doi.org/10.1016/j.clay.2010.12.020
Mitrogiannis, D., Psychoyou, M., Baziotis, I., Inglezakis, V., Koukouzas, N., Tsoukalas, N., Palles, D., Kamitsos, E., Oikonomou, G., & Markou, G. (2017). Removal of phosphate from aqueous solutions by adsorption onto Ca(OH)2 treated natural clinoptilolite. Chemical Engineering Journal, 320, 510–522. https://doi.org/10.1016/j.cej.2017.03.063
Mitrogiannis, D., Psychoyou, M., Koukouzas, N., Tsoukalas, N., Palles, D., Kamitsos, E., Pantazidis, A., Oikonomou, G., & Baziotis, I. (2018). Phosphate recovery from real fresh urine by Ca(OH)2 treated natural zeolite. Chemical Engineering Journal, 347, 618–630. https://doi.org/10.1016/j.cej.2018.04.102
Mitrogiannis, D., Psychoyou, M., Kornaros, M., Tsigkou, K., Brulé, M., Koukouzas, N., et al. (2020). Calcium-modified clinoptilolite as a recovery medium of phosphate and potassium from anaerobically digested olive mill wastewater. Environmental Science and Pollution Research International, 27, 2977–2991. https://doi.org/10.1007/s11356-019-07212-5
Ning, P., Bart, H.-J., Li, B., Lu, X., & Zhang, Y. (2008). Phosphate removal from wastewater by model-La(III) zeolite adsorbents. Journal of Environmental Sciences, 20, 670–674. https://doi.org/10.1016/S1001-0742(08)62111-7
Ostrooumov, M., Cappelletti, P., & de’Gennaro, R. (2012). Mineralogical study of zeolite from New Mexican deposits (Cuitzeo area, Michoacan, Mexico). Applied Clay Science, 55, 27–35. https://doi.org/10.1016/j.clay.2011.09.011
Salam, M., Mokhtar, M., Albukhari, S., Baamer, D., Palmisano, L., AlHammadi, A., & Abukhadra, M. R. (2021). Synthesis of zeolite/geopolymer composite for enhanced sequestration of phosphate (PO43-) and ammonium (NH4+) ions; Equilibrium properties and realistic study. Journal of Environmental Management, 300, 113723. https://doi.org/10.1016/j.jenvman.2021.113723
Sang, W., Mei, L., Hao, S., Li, D., Li, X., Zhang, Q., Jin, X., & Li, C. (2020). Na@La-modified zeolite particles for simultaneous removal of ammonia nitrogen and phosphate from rejected water: Performance and mechanism. Water Science and Technology: A Journal of the International Association on Water Pollution Research, 82, 2975–2989. https://doi.org/10.2166/wst.2020.541
Schick, J., Caullet, P., Paillaud, J.-L., Patarin, J., Freitag, S., & Mangold-Callarec, C. (2012). Phosphate uptake from water on a surfactant-modified zeolite and Ca-zeolites. Journal of Porous Materials, 19, 405–414. https://doi.org/10.1007/s10934-011-9488-3
Simsek, E., Özdemir, E., & Beker, U. (2013). Zeolite supported mono- and bimetallic oxides: Promising adsorbents for removal of As(V) in aqueous solutions. Chemical Engineering Journal, 220, 402–411. https://doi.org/10.1016/j.cej.2013.01.070
Sun, Y., Lin, J., Huang, H., Zhang, W., & Ma, D. (2011). Simultaneous removal of ammonium and phosphate from aqueous solution by calcium chloride-modified zeolite. Advanced Materials Research, 356–360, 1581–1585. https://doi.org/10.4028/www.scientific.net/AMR.356-360.1581
Vollprecht, D., Frühauf, S., Stocker, K., & Ellersdorfer, M. (2019). Ammonium sorption from landfill leachates using natural and modified zeolites: Pre-tests for a novel application of the ion exchanger loop stripping process. Minerals, 9, 471. https://doi.org/10.3390/min9080471
Vučić, V., Süring, C., Harms, H., Müller, S., & Günther, S. (2021). A framework for P-cycle assessment in wastewater treatment plants. Science of the Total Environment, 760, 143392. https://doi.org/10.1016/j.scitotenv.2020.143392
Wan, C., Ding, S., Zhang, C., Tan, X., Zou, W., Liu, X., & Yang, X. (2017). Simultaneous recovery of nitrogen and phosphorus from sludge fermentation liquid by zeolite adsorption: Mechanism and application. Separation and Purification Technology, 180, 1–12. https://doi.org/10.1016/j.seppur.2017.02.031
Xu, R., Lyu, T., Wang, L., Yuan, Y., Zhang, M., Cooper, M., Mortimer, R. J. G., Yang, Q., & Pan, G. (2022). Utilization of coal fly ash waste for effective recapture of phosphorus from waters. Chemosphere, 287, 132431. https://doi.org/10.1016/j.chemosphere.2021.132431
Yin, H., & Kong, M. (2014). Simultaneous removal of ammonium and phosphate from eutrophic waters using natural calcium-rich attapulgite-based versatile adsorbent. Desalination, 351, 128–137. https://doi.org/10.1016/j.desal.2014.07.029
You, X., Valderrama, C., & Cortina, J. (2017). Simultaneous recovery of ammonium and phosphate from simulated treated wastewater effluents by activated calcium and magnesium zeolites. Journal of Chemical Technology & Biotechnology, 92, 2400–2409. https://doi.org/10.1002/jctb.5249
Zhang, B., Wu, D., Wang, C., He, S., Zhang, Z., & Kong, H. (2007). Simultaneous removal of ammonium and phosphate by zeolite synthesized from coal fly ash as influenced by acid treatment. Journal of Environmental Sciences, 19, 540–545. https://doi.org/10.1016/S1001-0742(07)60090-4
Zhang, M., Zhang, H., Xu, D., Han, L., Niu, D., Tian, B., et al. (2011). Removal of ammonium from aqueous solutions using zeolite synthesized from fly ash by a fusion method. Desalination, 271, 111–121. https://doi.org/10.1016/j.desal.2010.12.021
Acknowledgements
The authors thank Maik Zimmermann for operational support during EPMA studies. Daniel Kiener is thanked for giving access to the FEG-SEM facilities at Erich Schmid Institute of Material Sciences. Johann G. Raith is gratefully acknowledged for his support.
Funding
Open access funding provided by Montanuniversität Leoben. This research was funded by the Austrian Research Promotion Agency (FFG), project “ReNOx 2.0,” grant number 864876.
Author information
Authors and Affiliations
Contributions
Conceptualization, K.S. and M.E.; methodology, K.S.; investigation, K.S.; resources, M.E.; writing-original draft preparation, K.S. and M.E.; writing-review and editing, M.E; visualization, K.S.; supervision and project administration, M.E.; funding acquisition, M.E. and K.S.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable. No animal or human participants.
Consent to Participate
Not applicable. No animal or client participants.
Consent for Publication
Not applicable. No individual person’s data in any form.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stocker, K., Ellersdorfer, M. Phosphate Fixation and P Mineralogy on Natural and Ca-Modified Zeolites During Simultaneous Nutrient Removal. Water Air Soil Pollut 233, 41 (2022). https://doi.org/10.1007/s11270-022-05509-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05509-9