Abstract
Despite the large number of scientific studies on the effects of antibiotics on soil microorganisms, little is known about the role played by soil organic matter (humus) in the interaction of antibiotics with microorganisms and plants, including the impacts on respiration and growth rate and the implications for nitrogen metabolism, which is an important factor in soil fertility The aim of this study was to analyze the effects of two widely used antibiotics, tetracycline and streptomycin, on microbiotic activity and plant growth in two soils with dissimilar organic carbon content, at the extremes of the fertility spectrum based on humus content. The study used humus-rich (Corg 5.4%) and humus-poor soils (Corg 1.5%) and measured basal respiration, substrate-induced respiration, nitric oxide emission, germination, and growth of white mustard 3 and 60 days after three progressively increasing doses of antibiotics were applied. Tetracycline was found to impair the ecological function of humus-rich soil by reducing denitrification and compromising soil microbial activity, while the effect of streptomycin on humus-poor soil was to reduce nitrification and soil fertility due to nitrogen escape. Both streptomycin and tetracycline increased the microbial biomass and suppressed the growth of white mustard seeds, which indicates an increase in the allelopathic activity of microorganisms in the soil conditions under the influence of antibiotics and their metabolites. Due to the low sorption of streptomycin in humus-poor soils, it poses a great danger to agricultural production, especially in areas of low fertility. In humus-rich soils, high concentrations of tetracycline caused numerous problems, including death of the crop plants. Thus, the effect of antibiotics as well as the more traditional soil pollutants, such as heavy metals, to a large extent, depends on the humus content of soils.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Ecotoxicologists have been studying the effects of antibiotics on ecosystem health since the 1970s, when pharmaceuticals were first found in soil (Bernhardt et al., 2017). Scientists quickly discovered that antibiotics affect the composition of soil microbiota and tamper with plant biomass production (Cycon et al., 2019). But the most concerning finding was the movement of antibiotics through agroecosystems, which can lead to the emergence of antibiotic-resistant microorganisms that pose a risk to human health (Manyi-Loh et al., 2018). Most antibiotics enter soil through animal excretions (Cycon et al., 2019), irrigation, and manure- or sewage sludge-based fertilization (Bondarczuk et al., 2016; Daghrir & Drogui, 2013; Singer et al., 2016; Wu et al., 2014).
Antibiotic have been reported to exert negative impact on public health and food safety, such as drug toxicity, immunopathological diseases, carcinogenicity, allergic reactions, and drug sensitization, amongst others (Baynes et al. (2016). These adverse impacts tend to be influenced by land use, contaminated water sources, policies (e.g., production, trade, animal health, food security), national and international trade, animal demography, and interactions between the human populations as well, as they are reported to vary considerably between regions and countries (WHO, 2012).
The effects of antibiotics on soil microorganisms range from insignificant to severe, as they affect microbial abundance (Akimenko et al., 2015; Pinna et al., 2012; Xu et al., 2016), overall microbial activity (Cui et al., 2014; Liu et al., 2015; Schmitt et al., 2004), and enzyme activity (Chen et al., 2013; Ma et al., 2016), as well as carbon mineralization and nitrogen cycling (Thiele-Bruhn & Beck, 2005; Rosendahl et al., 2012). Some antibiotics can also provide a carbon source for the nutrition of microorganisms, thus boosting microbial biomass (Thiele-Bruhn & Beck, 2005). Soil microorganisms are important because they contribute to agricultural crop productivity through their role in decomposing organic matter to release mineral elements for crop nutrition.
In recent decades, significant results have been obtained for the isolation and identification of plant and microorganism metabolites with allelopathic activity and their role in soil ecosystems. The allelopathic properties of plants and microorganisms, the main functions and mechanisms of action of allelochemicals, and their stability in soil ecosystems have been investigated (Barazani & Friedman, 2001; Lozano et al., 2014; Cheng & Cheng, 2015; Polyak & Sukcharevich, 2019). A particular focus has been the possibility of using allelopathy to combat phytopathogenic microorganisms (Aslam et al., 2017). However, the peculiarities of plant and microorganism allelopathic activity in a changing environment, and in response to anthropogenic impact, remain poorly understood.
The wide range of antibiotic effects on soil microorganisms is linked to the diversity of soil physicochemical properties and climatic conditions (temperature, precipitation, and humidity) in which they are found (Cycon et al., 2019). In addition, the uptake of antibiotics by soil organic matter pools (carbon fractions) can significantly prolong and thus enhance their action (Danilova et al., 2020).
The most accessible and affordable antibiotics — streptomycin and tetracycline — are also the most commonly found in the natural environment, including agroecosystems. They are the drugs of choice for treating human and animal infections and are also used in animal feed as growth promoters (Chopra & Roberts, 2001). Tetracycline exhibits a very long-lasting persistence in soil, with a half-life of 578 days (Walters et al., 2010), which is due to its high affinity and strong ability to bind to soil organic components, forming stable residues (Teixidó et al., 2012). Streptomycin is more hydrophilic than tetracycline and is more often found residing in the soil solution than being sorbed onto soil particles (Cycon et al., 2019).
The effects of antibiotics on soil microorganisms have been the subject of much scientific research. However, surprisingly, little is known about the role that soil organic matter (humus) plays in the interaction of antibiotics with microorganisms, including impacts on respiration and growth rate of microorganisms and plants as well as implications for nitrogen metabolism, which is an important factor in soil fertility. Therefore, the purpose of this study was to analyze the impacts of two widely used antibiotics (tetracycline and streptomycin) on the ecological functionality of two soils at the extremes of the fertility spectrum based on humus content. We believe that our results will be particularly relevant to help guide public policy and industry practice in this critical area.
2 Methods
Samples of the upper soil (0–20 cm depth) were obtained from two distinctly different locations. Humus-rich soil 1 was taken from a fallow where wheat was grown the previous summer (N 51.10°, E 40.31°, Voronezh region, Russian Federation), while humus-poor soil 2 was obtained from a rice field used for rice cultivation the previous year (N 47.83°, E 45.41°, the Bolshoi Tsaryn settlement, Republic of Kalmykia). Soil 1 contained 5.4% Corg, 3.9 mg/100 g NH4, and 12 mg/100 g P2O5. It had pH of 6.7 and water-holding capacity of 83%. Soil 2 contained 1.5% Corg, 0.72 mg/100 g NH4, and 17 mg/100 g P2O5. It had pH of 7.3 and water-holding capacity of 96%.
Tetracycline hydrochloride (C22H25ClN2O8) and streptomycin sulfate (C21H39N7O12) were purchased from the Biohimik company (Russian Federation, http://biohimik.ru/produktsiya). Tetracycline had octanol/water partition coefficient (pKow) of − 1.25 with the dissociation constant (pKa) of 3.29/7.32/9.11. Streptomycin had pKow of − 3.23 ± 1.04 and pKa of 11.09 (Halling-Sørensen, 2001).
For mesocosm experiments, the samples were freed from course plant fragments, sieved through a 5-mm sieve, and adjusted for moisture by distilled water to soil pressure of 300 kPa (pressure plate method). Duplicate 200 g soil samples were dosed with three concentrations of tetracycline hydrochloride and streptomycin sulfate (100, 600, and 1,200 mg kg−1 normalized by tetracycline and streptomycin weight). An antibiotic-free soil sample was used as reference. Soil samples of 200 g dry weight were placed in closed plastic containers. Soil moisture was adjusted during the experiment by watering every 2 days. Samples were incubated at 18–23 °C with pH at 6.5. Samples were analyzed after 3 days and 60 days for soil microorganisms, microbial biomass, soil basal respiration, and N2O emissions.
The carbon content of microbial biomass (Cmic) was determined by the substrate-induced respiration (SIR) method (Ananyeva et al., 2009). The method is based on the respiratory response of soil microorganisms to available organic substrate (glucose) in the soil, which is proportional to the content of microbial biomass. A soil sample (3 g) was placed into a glass vial (15 mL); glucose solution was added drop by drop (0.2 mL/g), to obtain a 10 mg/g concentration. The vial was sealed and incubated for 3 h at 22 °C. An air sample (0.5 mL) was then taken by syringe from the air phase of the vial and injected into a KristalLux 4000 M gas chromatograph, to measure the CO2 concentration. The incubation time of the soil with glucose was fixed. The SIR rate (μL CO2/ (g h)) was calculated, taking into account the CO2 concentration, the volume of the gas phase in the vial, and the weight and incubation time of the soil sample. The Cmic content (μg C/g soil) was determined by the equation: Cmic = SIR × 40.04 + 0.37 (Anderson & Domsh, 1993). Basal respiration of soil (BR) was measured in a similar way to SIR but with the application of distilled water to the soil instead of glucose solution. The incubation lasted 24 h at 22 °C, and the result was expressed as μg C-CO2/(g h).
The denitrification rate was estimated by the accumulation of N2O in the gas phase of the vial, in the presence of acetylene blocking the work of nitrous oxide reductase (Ananyeva et al., 2015). Glucose (6 mg/g soil) and, additionally, KNO3 (0.4 mg/g soil) were added to the soil (3 g dry weight equivalent in 15 mL hermetically sealed vials). Air was displaced from the vial with argon, after which 1 mL of acetylene was introduced by syringe. The vials were thoroughly shaken and incubated for a day at a temperature of 28 °C. The determination of N2O in the gas phase was performed with a Chromatec Crystal 5000.2 gas chromatograph with an electron capture detector. Nitrogen was used as a carrier gas.
Phytotoxicity assays were performed 3 days after the exposure of the samples to the antibiotics, according to the Phytotestkit protocol (manufactured by MicroBioTests Inc.) modified for the Russian Federation (according to ISO 18763). Each test plate had two identical compartments, upper and lower, for growing shoots and roots, respectively, measuring 13.5 × 8.5 × 0.8 cm (length × width × height). The lower compartment was fitted with filter paper impregnated with eluate and plant seeds were placed in it. Dicotyl white mustard (Sinapis alba L.) was used for test species, as is the international practice. The plates were incubated at 22–24 °C for 96 h. At the end of the exposure, the length of roots and shoots was recorded, and germination rate was calculated.
2.1 Data Analysis
Data were processed; standard deviation and correlation analyses were performed using the STATISTICA version 8 software package (Statsoft Inc.). ANOVA was used to analyze statistically significant differences within and between test groups. The degree of statistical significance of the results was calculated using the GraphPad Prism 9 software (intergroup statistical significance was fixed at p ≤ 0.05; Tukey and Šídák criteria were used).
3 Results and Discussion
3.1 Tetracycline
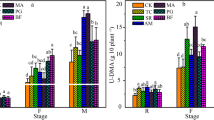
Since tetracycline is readily adsorbed on organic humus particles at low concentrations, it did not significantly affect soil microbiological parameters in the first phase of the observations, as confirmed by high Kd and KOC values (Cycon et al., 2019). At higher concentrations, however, it may have caused nutrient release into the soil solution due to exchange adsorption of the antibiotic and solid organic particles, thereby enhancing mustard root growth (by 75 and 110% at concentrations of 100 and 600 mg/kg, respectively) (Table 1). But at the maximum concentration of 1200 mg/kg, tetracycline triggered a significant increase in basal respiration and microbial biomass (by 50% and 35%, respectively) (Fig. 1a), while nitrogen oxide emission plummeted by 85%. The higher rate of nitrification by the expanding biomass, coupled with nitrate accumulation in the soil, resulted in the complete (100%) suppression of mustard germination (Table 1). Danilova (2020) observed similar effects of high concentrations of tetracycline on nitrification. The effect could be part of the self-protection mechanism of microorganisms against high levels of reactive oxygen species introduced into the soil by tetracycline, which is characteristic of heterotrophic nitrification.
Dose-related effects of tetracycline (T) and streptomycin (S) on basal respiration (BR), microbial biomass C (Cmic), and nitrous oxide emission in humus-rich soil 1 and humus-poor soil 2 on day 3 (a) and day 60 (b) of exposure. Mean ± standard error (n = 3) values are given; values with different letters vary significantly (p ≤ 0.05) for each parameter and Corg content (p ≤ 0.05, according to Tukey and Šídák criteria)
In the second phase of observations (60 days), characterized by prolonged release of tetracycline into the humus-rich soil solution, due to desorption from organic humus particles (Cycon et al., 2019), microbial biomass incrementally increased with every dose of antibiotic applied (by 35, 10, and 20%).
In humus-poor soils, the increase in microbial biomass in the first stage was less pronounced and occurred at lower tetracycline concentrations (23 and 21% at 100 and 600 mg/kg, respectively), while basal respiration and nitrogen emission remained unchanged. The low activity of tetracycline in humus-poor soils may be due to a weakly acidic environment, as antibiotic activity drops with decreasing pH (Kulshrestha et al., 2004).
However, during the second observation period (60 days), nitric oxide emission decreased at tetracycline concentrations of 100 and 600 mg/kg, which may be due to tetracycline accumulation in the humus-poor soil because of its typically long decomposition period (Cycon et al., 2019). At the same time, an increase in microbial biomass was only observed with the maximum dose of the antibiotic (Fig. 1b). It is possible that the increase in biomass was not caused by the antibiotic but by the glucose used in the SIR assay technique to measure microbial biomass. Glucose may have stimulated the growth of certain taxa of microorganisms that used tetracycline as a carbon source for nutrition (Liu et al., 2015).
In humus-poor soils, tetracycline not only suppressed white mustard germination, but also inhibited root and shoot growth in proportion to the increasing dose (Table 1). The antibiotic coating adjuvants, such as titanium dioxide, azorubine dye, and tropaeolin O dye, may have contributed to the inhibition of seed growth.
Thus, high concentrations of tetracycline in humus-rich soil initiate an increase in basal respiration, an explosive growth of microbial biomass, and a decrease in nitrification. The exact interaction of these processes is unpredictable, but the fact that sprouting of white mustard was completely suppressed by high doses of tetracycline suggests a negative synergy between the two. Moreover, the adsorption of tetracycline on organic particles of humus and the subsequent slow release into the soil solution over time exacerbate its negative effects.
In humus-poor soils, the activity of tetracycline, probably due to its slow decomposition, affects the microbial biomass, and the release of nitric oxide is continuous and more prolonged. At the same time, the effect of tetracycline leads to the suppression of both seed germination and roots and shoots growth.
3.2 Streptomycin
The effects of streptomycin were limited to humus-poor soils in our experiments. Thus, during the first observation period, basal respiration increased steadily with rising antibiotic concentrations (10, 40, and 80% at 100, 600, and 1200 mg/kg streptomycin, respectively), (Fig. 1a), while the biomass of microorganisms also experienced a significant spike. We believe that some taxa of microorganisms used streptomycin as an additional carbon source for their nutrition, similar to what occurred with tetracycline. Some of these taxa may have been denitrifiers converting nitrogen to gaseous forms, as evidenced by the elevated nitric oxide emissions (25 and 45%) at concentrations of 600 and 1200 mg/kg streptomycin, respectively.
Interestingly, nitric oxide emission increased steadily over time: by the end of the second observation period, it was almost three times higher than in the control at the maximum antibiotic dose of 1200 mg/kg (Fig. 1b). However, the biomass of microorganisms decreased sharply at this time point (by 25%), probably due to denitrifiers inhibiting other taxa of microorganisms as well as due to substrate depletion. Massive nitrogen emissions from the soil during the period of observations reduced fertility, inhibiting mustard seed germination (by 75 and 70% at 600 and 1200 mg/kg, respectively) and root growth (by 90% at the maximum dose).
In humus-rich soil, streptomycin had no effect on microorganisms, as it appears to be reliably taken up by soil organic matter, while somewhat inhibited mustard seed germination may be due to toxic adjuvants in the antibiotic coating. Mustard root growth (by 45, 90, and 90% with respective doses) was linked to the release of nutrients as a result of exchange adsorption of the antibiotic and solid organic matter.
Thus, the main threat to the fertility of humus-poor soils from streptomycin is related to the removal of nitrogen in gaseous form, while these soils are already poor in many nutrients. In contrast, organic matter in humus-rich soils can reliably adsorb streptomycin without affecting microbial biomass growth or nitrification.
4 Conclusions
The results of our experiments show that tetracycline and streptomycin have distinct and widespread effects on soils with different levels of humus. Streptomycin has no significant effect on the activity of microorganisms in humus-rich soils because it is reliably adsorbed by soil organic particles. However, streptomycin promotes the release of nutrients through adsorption exchange, which is a positive factor for mustard root growth. On the other hand, the toxic adjuvants in the antibiotic coating inhibit mustard germination.
Tetracycline is adsorbed less in humus-rich soils, and since a larger amount of the antibiotic remains available in the soil solution, this increases its negative effect. In particular, tetracycline boosts microbial biomass and basal respiration while reducing nitrogen release, resulting in complete inhibition of seed growth.
Both antibiotics continue to affect microbial biomass in both types of soil long after application, as they are gradually released into the soil solution following the breakdown of their sorption bonds.
However, in humus-poor soils, tetracycline activity is somewhat attenuated due to low soil pH, while streptomycin works at its full capacity, increasing basal respiration, boosting microbial biomass, and intensifying nitrogen emission, which increases in proportion to the applied concentration. As a result, seed growth is inhibited, and soil fertility is reduced due to nitrogen depletion. Moreover, at high concentrations of streptomycin, the release of gaseous nitrogen into the air continues for a long time after application.
In conclusion, tetracycline impairs the ecological functions of humus-rich soils by reducing denitrification and impairing microbial activity, while streptomycin impairs humus-poor soils by reducing nitrification and soil fertility due to nitrogen escape. At the same time, both antibiotics boost microbial biomass, thus suppressing the growth of white mustard seeds.
The negative influence of chemical plant protection products — herbicides, fungicides, and nematicides on allelopathic interactions between plants and microorganisms — is that they change the physicochemical properties of the soil and cause microbial imbalance in the soil. Antibacterial drugs are obviously no less active in modifying allelopathic interactions, which can lead to inhibition of plant growth. We see this in our experiments with antibiotics.
Soil organic matter reliably adsorbs streptomycin and, to a lesser extent, tetracycline. The longer antibiotics remain in the soil, the less predictable is their effect. Given the low sorption of streptomycin in soils poor in humus, our conclusion is that it poses a great danger to agricultural production, especially in areas of low fertility. However, in soils rich in humus, it is high concentrations of tetracycline that cause a number of serious problems, up to and including the death of crop plants.
The data obtained showed that the organic content of a soil can influence the impact of antibiotics on the functioning of living systems. Thus, the effect of antibiotics, which represent a group of poorly studied, unconventional soil pollutants (Ma et al., 2016; Thiele-Bruhn, 2003) as well as traditional soil pollutants, such as heavy metals, to a large extent, depends on the humus content of soils. In recent studies, this has been demonstrated in the assessment of plant development and microbial activity under the influence of heavy metals, in soils that differ in their Corg content (Terekhova et al., 2021a, b).
Since antibiotic use will obviously continue for a long time to come, we believe that further research is needed to identify the pathways, which minimize their negative effects. The research should focus primarily on the dynamic changes in the structure of microbial communities in soils, which are subjected to sustained antibiotic pressure.
The influence of natural and anthropogenic factors on the formation, stability, and activity of compounds with allelopathic properties in microbial-plant associations is of great practical importance (Lozano et al., 2014). It is known that chemical plant protection products (herbicides, fungicides, and nematicides) negatively affect allelopathic interactions between plants and microorganisms (Aslam et al., 2017). They change the physicochemical properties and cause a microbial imbalance in the soil (Cheng & Cheng, 2015). It is equally important to understand the effect of antimicrobial substances on the modification of allelopathic interactions, which can lead to changes in plant growth. As our results show, the effect of antibiotics depends not only on their chemical structure, but also on the abiotic conditions of the soil, in particular on the content of organic carbon.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Akimenko, Y. V., Kazeev, K. S., & Kolesnikov, S. I. (2015). Impact assessment of soil contamination with antibiotics (for example, an ordinary chernozem). American Journal of Applied Sciences, 12, 80–88. https://doi.org/10.3844/ajassp.2015.80.88
Aslam, F., Khaliq, A., Matloob, A., Asif Tanveer, A., Hussain, S., & Zahir, Z. A. (2017). Allelopathy in agro-ecosystems: A critical review of wheat allelopathy - Concepts and implications. Chemoecology, 27, 1–24. https://doi.org/10.1007/s00049-016-0225-x
Ananyeva, N. D., Susyan, E. A., Ryzhova, I. M., Bocharnikova, E. O., & Stolnikova, E. V. (2009). Microbial biomass carbon and the microbial carbon dioxide production by soddy-podzolic soils in postagrogenic biogeocenoses and in native spruce forests of the southern taiga (Kostroma oblast). Eurasian Soil Science, 42, 1029–1037. https://doi.org/10.1134/S1064229309090105
Ananyeva, N. D., Ivashchenko, K. V., Stolnikova, E. V., Stepanov, A. L., & Kudeyarov, V. N. (2015). Specific features of determination of the net production of nitrous oxide by soils. Eurasian Soil Science, 48(6), 608–619. https://doi.org/10.1134/S1064229315060022
Anderson, T., & Domsch, K. (1993). The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biology & Biochemistry, 25(3), 393–395. https://doi.org/10.1016/0038-0717(93)90140-7
Barazani, O., & Friedman, J. (2001) Allelopathic Bacteria and Their Impact on Higher Plants. Critical Reviews in Microbiology 27(1) 41–55. https://doi.org/10.1080/20014091096693
Baynes, R. E., Dedonder, K., Kissell, L., Mzyk, D., Marmulak, T., Smith, G., Tell, L., Gehring, R., Davis, J., & Riviere, J. E. (2016). Health concerns and management of select veterinary drug residues. Food and Chemical Toxicology, 88, 112–122. https://doi.org/10.1016/j.fct.2015.12.020
Bernhardt, E. S., Rosi, E. J., & Gessner, M. O. (2017). Synthetic chemicals as agents of global change. Frontiers in Ecology and the Environment, 15(2), 84–90. https://doi.org/10.1002/fee.1450
Bondarczuk, K., Markowicz, A., & Piotrowska-Seget, Z. (2016). The urgent need for risk assessment on the antibiotic resistance spread via sewage sludge land application. Environment International, 87, 49–55. https://doi.org/10.1016/j.envint.2015.11.011
Cheng, F., & Cheng, Z. (2015). Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Frontiers in Plant Science, 6, 1020. https://doi.org/10.3389/fpls.2015.01020
Chen, W., Liu, W., Pan, N., Jiao, W., & Wang, M. (2013). Oxytetracycline on functions and structure of soil microbial community. Journal of Soil Science and Plant Nutrition, 13, 967–975. https://doi.org/10.4067/S0718-95162013005000076
Chopra, I., & Roberts, M. (2001). Tetracycline antibiotics: Mode of action applications molecular biology and epidemiology of bacterial resistance. Microbiology and Molecular Biology Reviews, 65(2), 232–260. https://doi.org/10.1128/MMBR.65.2.232-260.2001
Cui, H., Wang, S.-P., Fu, J., Zhou, Z.-Q., Zhang, N., & Guo, L. (2014). Influence of ciprofloxacin on microbial community structure and function in soils. Biology and Fertility of Soils, 50, 939–947. https://doi.org/10.1007/s00374-014-0914-y
Cycon, M., Mrozik, A., & Piotrowska-Seget, Z. (2019). Antibiotics in the soil environment-degradation and their impact on microbial activity and diversity. Frontiers in Microbiology, 10, 338. https://doi.org/10.3389/fmicb.2019.00338
Daghrir, R., & Drogui, P. (2013). Tetracycline antibiotics in the environment: A review. Environmental Chemistry Letters, 11, 209–227. https://doi.org/10.1007/s10311-013-0404-8
Danilova N., Galitskaya P., & Selivanovskaya S. (2020). Veterinary antibiotic oxytetracycline’s effect on the soil microbial community. Journal of Ecology and Environment. Article number: 44. https://doi.org/10.1186/s41610-020-00154-x.
Halling-Sørensen, B. (2001). Inhibition of aerobic growth and nitrification of bacteria in sewage sludge by antibacterial agents. Archives of Environmental Contamination and Toxicology, 40, 451–460. https://doi.org/10.1007/s002440010197
Kulshrestha, P., Giese, R. F., & Aga, D. S. (2004). Investigating the molecular interactions of oxytetracycline in clay and organic matter: Insights on factors affecting its mobility in soil. Environmental Science and Technology, 38, 4097–4105. https://doi.org/10.1021/es034856q
Liu, B., Li, Y., Zhang, X., Wang, J., & Gao, M. (2015). Effects of chlortetracycline on soil microbial communities: Comparisons of enzyme activities to the functional diversity via Biolog EcoPlatesTM. European Journal of Soil Biology, 68, 69–76. https://doi.org/10.1016/j.ejsobi.2015.01.002
Lozano, Y. M., Hortal, S., Armas, C., & Pugnaire, F. I. (2014). Interactions among soil, plants, and microorganisms drive secondary succession in a dry environment. Soil Biology and Biochemistry, 78, 298–306. https://doi.org/10.1016/j.soilbio.2014.08.007
Ma, T., Pan, X., Chen, L., Liu, W., Christie, P., Luo, Y., et al. (2016). Effects of different concentrations and application frequencies of oxytetracycline on soil enzyme activities and microbial community diversity. European Journal of Soil Biology, 76, 53–60. https://doi.org/10.1016/j.ejsobi.2016.07.004
Manyi-Loh, C., Mamphweli, S., Meyer, E., & Okoh, A. (2018). Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules, 23(4), 795. https://doi.org/10.3390/molecules23040795
Polyak, Y. M., & Sukcharevich, V. I. (2019). Allelopathic interactions between plants and microorganisms in soil ecosystems. Biology Bulletin Reviews, 9, 562–574. https://doi.org/10.1134/S2079086419060033
Pinna, M. V., Castaldi, P., Deiana, P., Pusino, A., & Garau, G. (2012). Sorption behavior of sulfamethazine on unamended and manure-amended soils and short-term impact on soil microbial community. Ecotoxicology and Environmental Safety, 84, 234–242. https://doi.org/10.1016/j.ecoenv.2012.07.006
Rosendahl, I., Siemens, J., Kindler, R., Groeneweg, J., Zimmermann, J., Czerwinski, S., et al. (2012). Persistence of the fluoroquinolone antibiotic difloxacin in soil and lacking effects on nitrogen turnover. Journal of Environmental Quality, 41, 1275–1283. https://doi.org/10.2134/jeq2011.0459
Schmitt, H., van Beelen, P., Tolls, J., & van Leeuwen, C. L. (2004). Pollution-induced community tolerance of soil microbial communities caused by the antibiotic sulfachloropyridazine. Environmental Science & Technology, 38, 1148–1153. https://doi.org/10.1021/es034685p
Singer, H. P., Wossner, A. E., McArdell, C. S., & Fenner, K. (2016). Rapid screening for exposure to “non-target” pharmaceuticals from wastewater effluents by combining HRMS-based suspect screening and exposure modeling. Environmental Science and Technology, 50, 6698–6707. https://doi.org/10.1021/acs.est.5b03332
Terekhova, V. A., Prudnikova, E. V., Kulachkova, S. A., Gorlenko, M. V., Uchanov, P. V., Sushko, S. V., & Ananyeva, N. D. (2021a). Microbiological indicators of heavy metals and carbon-containing preparations applied to agrosoddy-podzolic soils differing in humus content. Eurasian Soil Science, 54(3), 448–458. https://doi.org/10.1134/S1064229321030157
Terekhova, V. A., Prudnikova, E. V., Kiryushina, A. P., Karpukhin, M. M., Plekhanova, I. O., & Yakimenko, O. S. (2021b). Phytotoxicity of heavy metals in contaminated podzolic soils of different fertility levels. Eurasian Soil Science., 54(6), 964–974. https://doi.org/10.1134/S1064229321060132
Teixidó, M., Granados, M., Prat, M. D., & Beltrán, J. L. (2012). Sorption of tetracyclines onto natural soils: Data analysis and prediction. Environmental Science and Pollution Research, 19, 3087–3095. https://doi.org/10.1007/s11356-012-0954-5
Thiele-Bruhn, S. (2003). Pharmaceutical antibiotic compounds in soils - A review. Journal of Plant Nutrition and Soil Science, 166, 145–167. https://doi.org/10.1002/jpln.200390023
Thiele-Bruhn, S., & Beck, I.-C. (2005). Effects of sulfonamide and tetracycline antibiotics on soil microbial activity and microbial biomass. Chemosphere, 59(4), 457–465. https://doi.org/10.1016/j.chemosphere.2005.01.023
Walters, E., McClellan, K., & Halden, R. U. (2010). Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids-soil mixtures in outdoor mesocosms. Water Research, 44, 6011–6020. https://doi.org/10.1016/j.watres.2010.07.051
World Health Organization (WHO). (2012). The evolving threat of antimicrobial resistance: Options for action. World Health Organization. ISBN: 978 92 4 150318 1.
Wu, X.-L., Xiang, L., Yan, Q.-Y., Jiang, Y.-N., Li, Y.-W., Huang, X.-P., et al. (2014). Distribution and risk assessment of quinolone antibiotics in the soils from organic vegetable farms of a subtropical city Southern China. Science of the Total Environment, 487, 399–406. https://doi.org/10.1016/j.scitotenv.2014.04.015
Xu, Y., Yu, W., Ma, Q., Wang, J., Zhou, H., & Jiang, C. (2016). The combined effect of sulfadiazine and copper on soil microbial activity and community structure. Ecotoxicology and Environmental Safety, 134, 43–52. https://doi.org/10.1016/j.ecoenv.2016.06.041
Acknowledgements
The authors would like to thank Pavel Uchanov and Anastasya Paramonova for technical support during the experiments and Andrei Tchourakov for structuring and editing the article.
Funding
The authors are grateful to the Russian Foundation for Basic Research for financial support (№18–44-920007p_a) and the Russian Science Foundation (№21–14-00076). This research was performed according to the development program of the Interdisciplinary Scientific and Educational School of M.V. Lomonosov Moscow State University “The future of the planet and global environmental change.”
Author information
Authors and Affiliations
Contributions
KA, investigation, analysis, and interpretation of results and writing the original draft; PA, data collection, analysis, and interpretation of results; KK, writing — review and editing; BL, validation, formal analysis, and visualization; PL, data collection and methodology; MN, methodology and writing — review and editing; BM, writing — review and English editing; TV, study conception and design, funding acquisition, and project administration.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bloor, M.C., Kiryushina, A., Kydralieva, K. et al. Divergent Effects of Antibiotics on Plants and Microbiota in Soils with Contrasting Humus Content. Water Air Soil Pollut 232, 518 (2021). https://doi.org/10.1007/s11270-021-05459-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05459-8