Abstract
Boreal lakes with soft water and low buffering capacity are susceptible to excess ion loading resulting from metal mining. The impact of two Finish mining sites in downstream lakes was assessed with a chronic sediment toxicity test using a laboratory-reared freshwater Lumbriculus variegatus (Oligochaeta). The test organisms were exposed to mining-contaminated natural lake sediments and hypolimnion water (HLW) or artificial freshwater (AFW) as overlying water in two independent experimental setups. In both test setups, growth and reproduction of L. variegatus were lower in sediments from the lakes receiving high amount of mining effluents from the mines nearby. In the biomining site, the main contaminants in the recipient lakes were the ore metals Ni and Zn, while in the lakes affected by the conventional underground mine, they were Cu and Zn. These metals accumulated in L. variegatus especially in the setup with natural HLW above the sediment. Growth and reproduction were lower in the HLW than in the AFW setup. The mining-contaminated sediments did not support optimum growth or reproduction of L. variegatus in comparison to the local reference sediments. Decline of pH in the unbuffered natural sediments brought up challenges in the assessment of metal-contaminated lake sediments with high sulfur content, and a need to develop new tools for their risk assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mining activities in boreal regions often take place near freshwater reservoirs and pose a serious threat to aquatic ecosystem health (Kreutzweiser et al., 2013; Thienpont et al., 2016; Väänänen et al., 2016). Metals are released from active mining sites through accidents and draining of diluted wastewaters (e.g., Heikkinen & Väisänen, 2007; Kauppi et al., 2013; Leppänen et al., 2017). Lakes and streams with soft water typically have low buffering capacity, and are thus vulnerable to excess ion loading (Birceanu et al., 2008; Väänänen et al., 2016). Seasonal changes in water chemistry such as oxygen depletion in hypolimnion during wintertime during ice-cover period or low pH during spring flood increase risk for adverse effects of metals by affecting the metal speciation (Väänänen et al., 2016).
Intentions to exploit low-grade ores resulted in employing non-conventional mining technologies besides conventional mining (Rawlings & Johnson, 2007). Among the non-conventional mining technologies, biomining consists in using bacteria to leach valuable metals from low-grade ore. Biomining is announced as more environmentally friendly than conventional mining, because it reduces toxic emissions, tailings, and acid mine drainage (Jerez, 2017). However, biomining has not always been able to overcome environmental impacts. For example, in Europe, in the largest mine utilizing biomining technique for nickel (Ni) production (Terrafame mine, Talvivaara, Finland), accidental discharges, leading to severe contamination of the receiving water systems, have been reported; yet, only a few studies addressed the environmental effects on the boreal lakes and lake sediments, such as alterations in the sediment chemistry or benthic communities (Kauppi et al., 2013; Leppänen et al., 2017; Väänänen et al., 2016).
With a vastly growing need for the extraction of new metals, there is also a need for environmentally realistic toxicity tests when estimating metal-rich sediment toxicity (Simpson & Batley, 2007). While field monitoring and on-site experiments are often laborious, expensive, and difficult or even impossible to put into action (Chapman et al., 1998), standardized laboratory toxicity tests present simple and cost-effective procedures to assess the toxicity of contaminated lake sediments. Yet, laboratory testing has also drawbacks, such as a short equilibrium time after sediment spiking or focusing only on the dissolved metals (Poteat & Buchwalter, 2014; Simpson & Batley, 2007; Vandegehuchte et al., 2013). In addition, adjusting and buffering the pH of exposure media to achieve circumneutral pH in laboratory tests can result in unrealistic exposure conditions, particularly for most of the boreal lakes with naturally slightly acidic pH and low buffering capacity.
Although the abundance of benthic invertebrates, their growth, and body burden of metals can serve as indicators of pollution by mining effluents (Cadmus et al., 2016; Camusso et al., 2012; Chapman, 2001), this information have been less frequently applied in the risk assessment of metal-contaminated sediments to ecosystem health (Chapman, 2001; Kreutzweiser et al., 2013; Norwood et al., 2003). Benthic invertebrates are an essential part of aquatic ecosystems being as a source of food to higher trophic levels (Cadmus et al., 2016; Camusso et al., 2012; Chapman, 2001; Rainbow, 2002; Suter & Cormier, 2015). Potential responses to metal exposures include changes in the community structure of benthic invertebrates that may have numerous effects on ecosystem health, as, for example, on nutrient dynamics and fish stocks (Cadmus et al., 2016; Rainbow, 2002; Suter & Cormier, 2015). Several species have been used in sediment toxicity testing and commonly used organisms include amphipods, midges, cladocerans, and oligochaetes (Burton, 2010). Ideal test organisms for sediment testing are relatively easily cultured and handled, ecologically relevant, exposed to contaminants via both sediment and water, tolerant to a variety of sediment characteristics, and have constant and predictable responses to contaminants (Chapman, 2001; Giesy & Hoke, 1989). The freshwater Oligochaeta Lumbriculus variegatus used in our study serves as a good representative of benthic invertebrates in toxicity testing because it is widely distributed globally, is tolerant to sediment properties, and is exposed to contaminants through multiple sources, namely, from sediment particles, interstitial water, and overlaying water (Camusso et al., 2012; Chapman, 2001; Phipps et al., 1993).
Sediment-water toxicity tests of L. variegatus were used to characterize the toxicity of several lake sediments downstream from two different boreal metal mines: a Ni and zinc (Zn) open-pit mine using biomining technology and an underground mine using flotation technique to extract copper (Cu) and Zn. Our specific objectives were to assess (a) toxic effects on the growth and reproduction of L. variegatus using either hypolimnion (HLW) or artificial freshwater (AFW) as overlying water, so that the role of exposure from the sediment and overlying water could be assess, and (b) the role of metal-contaminated sediments as a source of metals to L. variegatus by evaluating 28-day bioaccumulation.
2 Materials and Methods
2.1 Study Areas and Sites
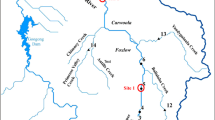
The two study areas were located in northeastern and Central Finland downstream of two different metal mines (Fig. 1). The Terrafame mine in northeastern Finland, which was previously known as Talvivaara mine (hereupon referred as TV mine), is an open-pit mine which employs biomining technique to recover metals from the ore, and it has been in operation since 2008 (Riekkola-Vanhanen, 2013). The main products are Ni, Zn, Cu, and cobalt (Co). Several accidental spills of mining effluents have been reported in TV mine. The largest gypsum pond leakage occurred in November 2012 (Kauppi et al., 2013; Salmelin et al., 2017). TV mine is located at the border of the watersheds of Oulujoki and Vuoksi, and the mining effluents were discharged into both watersheds. The TV study area included 7 lakes with 9 sampling sites in these two watersheds from northwest (Oulujoki) and southeast (Vuoksi) to the mining district (Fig. 1). Waters from the mining area flow from Lake Kalliojärvi to Lake Kolmisoppi, via Lake Jormasjärvi, and finally to Lake Nuasjärvi in the Oulujoki watershed and from Lake Ylä-Lumijärvi to Lake Kivijärvi and finally to Lake Laakajärvi in the Vuoksi watershed. A reference sampling site was located in the unaffected Lake Kiantajärvi.
The Pyhäsalmi (PS; left box) and Talvivaara (TV; right box), study area (pink boxes) and the catchment area (black lines in map of Finland/dashed line in terrain map) geographical location in Finland. Different sampling sites are indicated with colored dots. Finnish names for the lakes (e.g., L. Kolmisoppi) are given (S: south; N: north). Map data: General map of Finland, ©National Land Survey of Finland 10/2016; Catchment areas and Talvivaara mining district, National Database of Regional Land Use Plans ©Finnish Environment Institute (SYKE), 6/2017; Pyhäsalmi mining district, mining concession, ©Finnish Safety and Chemicals Agency (Tukes) 6/2017; Maps were constructed with ArcGIS® v. 10.3.1 (ESRI Inc., Redlands, CA)
The Pyhäsalmi (PS) mine in Central Finland is a conventional underground mine which uses conventional flotation process to recover metals, and it has been operating since 1962. Cu, Zn, and pyrite are the mine’s main products. The PS study area included two separate basins: Lake Pyhäjärvi and Lake Junttiselkä (Fig. 1). In the PS study area, the most common outfall direction is towards north from the mine, but during peak discharge there is a backflow towards south. Historically the loading has been greatest during the early 1970s and effluents were also run towards south during the early years (Mäkinen, 2011). The PS study area had included three contaminated sampling sites: two in the Junttiselkä basin and one in Lake Pyhäjärvi (Fig. 1). A reference site was located in the unaffected Lake Parkkimanjärvi.
2.2 Water and Sediment Sampling
Water and sediment samples were collected during the ice-cover period in spring 2015 (TV on March 24–27 and PS on April 8 and 9). Water samples were taken with a Limnos water sampler from epilimnion and from hypolimnion (1 m above the lake bottom) for the toxicity tests with L. variegatus. Surface sediment samples (0–5 cm) were taken with a Sandman gravity corer for the toxicity tests. Eight to nine replicate sediment cores per site were mixed to obtain one homogenous bulk sample immediately after the sediment retrieval. All sediments for the toxicity tests were stored (at + 4 °C in darkness) up to 4 weeks (toxicity test with HLW) or up to 7 months (toxicity test with AFW). Separated subsamples from the bulk sediment samples were frozen (− 20 °C) for element analysis.
2.3 On-site Measurements and Chemical Analyses
Water depth, temperature, oxygen concentration and saturation, specific conductivity, and pH were measured from epilimnion and hypolimnion on-site with a multi-parameter water quality sonde (YSI V2 6600). Total inorganic carbon, dissolved organic carbon, and sulfate (SO42−) were analyzed in field-collected water samples. Sediment dry weight (SDW%), loss-on-ignition (LOI%), and organic carbon (SOC%) were determined in the field-collected sediments and in the artificial control sediment (excluding SOC in the artificial sediment). Sediment pH was measured with a portable pH (VWR pH 100).
Sediment, water, and L. variegatus samples were analyzed for element (Na, S, Cu, Ni, Zn, and Mn) (Table S1 in the supplementary material) concentrations. Elements were measured from both the field-collected water (hypolimnion) and sediment samples and samples (overlying water and sediment) from the toxicity tests with L. variegatus. In the HLW setup, overlying water samples (15 mL) were taken at 0 and 28 days. In the AFW setup, water samples were taken at the end of the test only. Sediment samples in both test setups were taken from the bulk sample (approximately 40 g) for each exposure and reference sediments at the beginning of the test (0 day) and from each replicate (approximately 8 g) at the end of the test (28 day). For element analysis, the water samples from the field and the toxicity tests were filtered (0.45 μm Whatman® GD/X or GD/XP) and acidified with Suprapur 65% nitric acid (HNO3; Sigma-Aldrich®) immediately after sampling in the field and during the toxicity tests at the laboratory. Sediment and L. variegatus samples were vacuum freeze dried (Christ Alpha) and weighed. Approximately 0.5 g of dried sediment or 6 to 22 mg L. variegatus was weighed into 50-mL or 15-mL plastic tubes, respectively. Two drops of ultrapure water (conductivity < 0.056 μS/cm, Elga) were applied to the L. variegatus samples. For digestion, 5 mL or 1.5 mL of aqua regia (HNO3:HCl, 1:3 v:v) was added to the sediment and the L. variegatus sample tubes. Samples were sonicated (ELMA Transonic 820/H or Bandelin Sonorex RK 512/H) three to four times for 3 min at 50 °C. After sonication, the sediment samples were filtered (Whatman no. 41) and the extract was diluted to 50 mL with ultrapure water. Digested L. variegatus samples were diluted to 10 mL with ultrapure water. All samples were analyzed by inductively coupled plasma–optical emission spectrometry (ICP–OES; PerkinElmer Optima 8300). Results below the detection limit (Table S1 in the supplementary material) or with relative standard deviation (RSD) above 10% were discarded from data analysis.
SO42− ions were determined by ion chromatography (Dionex ICS-1000) in the accredited laboratory of Finnish Environment Institute (SYKE). After filtration in the laboratory, dissolved organic carbon was determined by IR-detection (TOC-L, Total Organic Carbon Analyzer; Shimadzu) once per sample.
SDW and LOI were determined according to SFS standard protocol 3008 (2000) on three or two measurement replicates of each field-collected sediment sample and of artificial control sediment, respectively, by drying the samples at 105 °C and by ignition at 550 °C for 2.5 h. Organic carbon of the field-collected sediments was analyzed in two to three measurement replicates of acid-treated (1 M HCl) samples by a thermal conductivity detector (Elementar Vario EL III elemental analyzer) using acetanilide as a reference compound. The SOC of artificial control sediment was estimated with equation y = 0.435x − 0.847, where x = LOI (%) and y = SOC (%) (Pajunen, 2004).
2.4 Site Characterization and Classification According to Contamination Gradient
In the TV area, the sampling sites were grouped according to their contamination level in four groups: reference, low, medium, and high hazardous groups (Fig. 1), and these groups were used in the statistical analyses. The main parameters in the mining effluent reported by Kauppi et al. (2013) were used to characterize the lakes. They were SO42− concentration and specific conductivity in the hypolimnetic waters, and concentrations of Na, Mn, S, and Ni in the sediments. The site groups were determined in the following procedure: Firstly, concentrations of each variable were divided into quartiles and the 1st, 2nd, 3rd, and 4th quartiles were assigned as reference, low, medium, and high hazardous groups, respectively. The limits for the 1st, 2nd, 3rd, and 4th quartiles were as follows: SO42− mg/L: < 98, 98–140, 140–280, and > 280; specific conductivity μS/cm: < 250, 250–403, 403–1031, and > 1031; Na mg/kg: < 464, 464–688, 688–3708, and > 3708; S mg/kg DW: < 5246, 5246–6155, 6155–26764, and > 26764; Ni mg/kg DW: > 43, 43–105, 105–130, and > 130; Mn mg/kg DW: < 1913, 1913–2228, 2228–2995, and > 2995. The quartile group that included the high number of variables defined the site group; e.g., if 5 out of total 6 variables had concentration belonging to the 4th quartile, the site is classified as high hazardous group. If different variables were ranked equally to both low and high hazardous groups, the final group was defined as medium. Lake Kolmisoppi was defined as a medium hazardous group due to its overall contaminant loading and vicinity to TV mine, because it did not rank directly into any group. Each hazardous group included three sampling sites, except reference, which included only one site in Lake Kiantajärvi. Lake Jormasjärvi N and Lake Laakajärvi N and S were assigned to the low hazardous group; Lake Nuasjärvi FM12, Lake Jormasjärvi Mid, and Lake Kolmisoppi were the medium hazardous group; and Lake Kalliojärvi, Lake Kivijärvi, and Lake Ylä-Lumijärvi were assigned to the high hazardous group (Fig. 1).
In the PS area, the mining-affected sites (Junttiselkä S, Junttiselkä N, and Pyhäjärvi KS) and one reference site in Lake Parkkimanjärvi were compared in the statistical analysis. The northernmost area of Junttiselkä has been the most loaded site.
2.5 Test Organisms
The stock of L. variegatus used as test organisms came from the University of Jyväskylä (the Department of Biological and Environmental Science) and was originated from Ann Arbor, Michigan (Kukkonen & Landrum, 1994). The Oligochaeta worms were cultured in aerated 20-L glass aquaria containing AFW (Ca + Mg hardness 1.0 mM, pH 8.7) (ISO, 2012) at 20 °C (SD = 1) in 16:8 h light:dark cycle (illuminance < 500 lx at water surface). Shredded soft-paper towels, presoaked 24 h in ultrapure water, were used as a substrate in the aquaria. The worms were fed three times a week with Tetramin® (TetraWerke, Melle, Germany) fish food ad libitum. Most of the overlaying water volume was replaced twice a week. There was a 24-h gut purging time for L. variegatus in the AFW prior to the tests. Only active and medium-sized worms (approximate DW of worms varied between 0.9 and 1.0 mg per individual, estimated from the initial biomass) were chosen for the test with no recent signs of body fragmentation.
2.6 Toxicity Tests
Two separate chronic toxicity tests were conducted with the field-collected sediments (Fig. 2). The same reference and mining-contaminated sediments were used in both test setups, and their toxicity to L. variegatus were assessed according to OECD 225 (2007) at + 19.4 °C (SD = 0.3) with the following modifications. The first HLW test setup included 10 worms per replicate and the field-collected HLW without pH adjustment. The purpose of the HLW test design was to take the combined exposure from the sediment and water in the lake bottom into account. The second AFW test setup included 20 worms per replicate and the AFW as overlying water. The number of L. variegatus in the AFW setup was doubled to achieve adequate biomass for the elemental analyses. Both HLW and AFW test setups were conducted in an adequate worm-to-SOC ratio (range 1:56 to 1:679, w/w) and density (Table S2 in the supplementary material) (Leppänen & Kukkonen, 1998a, 1998b; OECD, 2007).
The composition of the artificial control sediment is described in the OECD 225 standard protocol (2007) and supplementary information (Table S1 in the supplementary material). The final pH of the artificial control sediment was adjusted to 6.0, as described in the standard (OECD, 2007). The same AFW (hardness 1 mM, pH range 6.8–7.2) used in the laboratory culture of L. variegatus was used as overlying water with artificial control sediment. The test was carried out in 250-mL beakers (height 120 mm; diameter 60 mm) with three replicates for each field-collected sediment and 12 (HLW setup) or six replicates (AFW setup) for the controls. Unsieved sediments were mixed manually with plastic spoon (polystyrene) prior to the test. Approximately 45 mL of sediments was placed into the beakers, which corresponds to 39 g (SD = 5) of field-collected sediment and 53 g (SD = 7) of control sediment as wet weight (WW) (Table S2 in the supplementary material). The sediment to overlying water ratio was 1:4 (v:v) and the sediments were allowed to settle 2 days before the introduction of the organisms. Aeration of the overlying water through a glass pasteur pipette was turned on 1 day prior to introducing the L. variegatus to the beakers and they were aerated thereafter throughout the test. The aeration may change the speciation of metal and increase their availability in overlying water and sediment (Teuchies et al., 2011) and our tests can be interpreted as worst-case scenario tests. The L. variegatus burrowed within 15 min in the sediments, and no escape behavior was recorded. Three subsamples of 10 or 20 laboratory-cultured L. variegatus were weighed (Mettler Toledo XP56) to determine their initial total biomass (DW after the vacuum freeze-drying), which were 8.9 (SD = 2.6) mg DW and 19.5 (SD = 2.8) mg DW in the HLW and AFW setup at the beginning of the test, respectively. Evaporated water was compensated with ultrapure water on demand. Temperature and pH (VWR pH100), oxygen saturation (YSI proODO), and total ammonia (Tetra NH3/NH4 test or Thermo Fischer Scientific OrionTM HP Ammonia Electrode) from water were monitored during the tests. Sediment pH (VWR pH 100) was measured at the beginning and at the end of the test.
After 28 days of exposure, the L. variegatus were removed from the sediment with a 250-μm sieve. Individuals were counted, rinsed twice in AFW on a Petri dish, blotted dry on tissue paper as a group (approximately for 10 s), and weighed WW (Mettler Toledo XP56) immediately after the test. DW of L. variegatus was measured after the vacuum freeze-drying. When dead worms were found, they were omitted from the weighing sample and subsequent metal analyses. Growth was defined as a relative change in biomass (%, WW and DW) during the exposure period. Reproduction rate (RR) was defined by a change in the number of L. variegatus at 28 days in comparison to 0 day. The mean reproduction rate of L. variegatus was 2.3 (SD = 0.2) and 2.3 (SD = 0.3) in the artificial control sediment of HLW and AFW setup, respectively. Thus, the OECD 225 standard (OECD, 2007) requirements for valid control were achieved in the artificial control sediment in both test setups. Ammonium concentration increased during the toxicity tests but yet remained below its toxicity threshold under the exposure conditions for L. variegatus (Schubaur-Berigan et al., 1995). All the used equipment were acid washed (10% HNO3) prior to test and rinsed with ultrapure water prior to use, excluding the sieve and spatula which was used to collect the worms from the sieve.
2.7 Data Analyses
The results are expressed as mean and standard deviation (SD) unless otherwise stated. A replicate beaker was omitted from the data of one low hazardous site (Lake Jormasjärvi N) because one chironomid larva was found alive at the end of the test. Non-parametric Spearman rank correlation was used to examine association between elemental concentrations in the sediments and L. variegatus separately in the HLW and the AFW setup. One-way analysis of variance (ANOVA) was conducted to study the effect of the sediment from the differently hazardous sites (TV: reference, low, medium, and high) or the sampling sites (PS: reference, Junttiselkä N, Junttiselkä S, and Pyhäjärvi KS) on the concentrations of Cu, Ni, Zn, and Mn of L. variegatus in the sediments of TV and PS mining sites separately. If statistical differences were observed by ANOVA (p < 0.05), the least significant difference (LSD) procedure was used for multiple comparisons. The element concentrations were log10-transformed before ANOVA in order to reduce the heteroscedasticity of variances.
Changes in L. variegatus reproduction (reproduction rate (RR)) and growth (change rate in DW and WW) were calculated according to the equation (LN(x0) − LN(x28)) / 28, where x0 and x28 are the number of the L. variegatus (i.e., 10 or 20 individuals) or their total DW or total WW at the beginning and end of the test, respectively. ANOVA was conducted to study the effect of the sediment from the different sampling sites (PS: reference, Junttiselkä N, Junttiselkä S, and Pyhäjärvi KS) on the RR and change rate in DW and WW of L. variegatus. If statistical differences were observed by ANOVA (p < 0.05), the LSD procedure was used for multiple comparisons. In the TV area, due to the heteroscedasticity of variances, the Kruskal-Wallis analysis was conducted to study the effect of the sediment from the differently hazardous sites (TV: reference, low, medium, and high) on the RR and change rate in DW and WW of L. variegatus. If statistical differences were observed by the Kruskal-Wallis analysis (p < 0.05), Conover procedure was used for multiple comparisons. The reference sediments were from Lake Kiantajärvi for TV and from Lake Parkkimanjärvi for PS. All the statistical tests were performed with SPSS version 24 (IBM 2013).
3 Results
3.1 Site Characteristics
In the TV area, water quality of the high hazardous lakes was especially deteriorated and the hypolimnion of two stratified lakes (Kalliojärvi and Kivijärvi) in this group was saline. Hypoxic (< 5 mg O2/L) conditions were observed in one lake of the low hazardous group (Laakajärvi) and two lakes of the high hazardous group (Kalliojärvi, Kivijärvi), in all PS sampling sites (Junttiselkä N and S, and Pyhäjärvi KS) and in both reference lakes during the ice-cover period in late spring 2015 (Table S3 in the supplementary material). Both in the TV and PS area, the element concentrations in the sediments of the study sites reflected the long-term mining-affected contamination (Table S4 in the supplementary material).
3.2 Element Concentrations in Sediments and Overlying Water in the Test Setups
The concentrations of ore elements, Cu, Ni, Zn, and Mn, were elevated in the sediment of the mining-contaminated lake sediments of TV compared with the reference lake (Table 1). In the high hazardous lake group, the mean concentrations of these ore elements were generally the highest in both test setups except in the AFW setup where the Mn concentration of the high hazardous group was lower than the concentration in other lake groups or the reference lake. Similarly, the mean Ni, Zn, and Mn concentrations of overlying water in the sediment tests were higher in the mining-contaminated lakes than in the reference lake (Table 1). In the PS sediments, the concentrations of Cu and Zn were also higher in the sediments of the mining-contaminated sites than in the sediment of the reference lake (Table 2). The Mn concentrations were low in the Junttiselkä basin, whereas in the Pyhäjärvi KS, the Mn concentrations were higher than the concentrations in the reference lake. In the overlying water, the Cu, Zn, and Mn concentrations were in general higher in the mining-contaminated lakes than in the reference lake. An exception was the AFW setup where the Mn concentration was mostly lower than the Mn in the reference lake. Ni concentration was at the same level in the mining-contaminated sites and the reference lake. Oxygen saturation, ammonium concentration, and pH during the exposures are presented in Table S5 in the supplementary material.
3.3 Element Concentrations and Sediment Toxicity to Lumbriculus variegatus
In the HLW setup of TV, there were statistically significant differences in Cu, Ni, and Zn concentrations of L. variegatus between the TV sediments (ANOVA results; Table 3). The sediments of the high hazardous lake group had the highest concentrations of these three elements (multiple comparison by LSD procedure; Table 3). The Mn concentration did not differ statistically significantly between TV sediments. In the AFW setup, Cu, Zn, and Mn concentrations of L. variegatus exposed in the mining-contaminated sediments were at the same level as the concentrations of L. variegatus in the reference sediment. Only the Cu concentration in the medium hazardous group and Mn concentration in the low hazardous group were lower than in the other hazardous groups but did not differ statistically significantly from the reference sediment (Table 3). Ni concentration of L. variegatus was above the detection limit only in Lake Ylä-Lumijärvi, which was the closest lake to the TV mining site and was grouped to the high hazardous lake group. In the HLW setup, the mean Cu, Ni, Zn, and Mn concentrations of L. variegatus were relatively higher than those concentrations in AFW setups in TV sediments.
In the HLW setup of TV, the concentration of Ni, Zn, and Mn in the sediments correlated positively with the concentration of Ni (rs = 0.610, p = 0.007), Zn (rs = 0.536, p = 0.003), and Mn (rs = 0.533, p = 0.003) in L. variegatus. In the AFW setup, a significant correlation between Zn concentrations of the sediment and L. variegatus (rs = 0.772, p < 0.001) only was observed.
In the HLW and AFW setups of PS, the Cu and Zn concentrations of L. variegatus were statistically significantly affected by the PS sites (Table 4). Both Cu and Zn concentrations were higher in the mining-contaminated lakes than the concentrations of L. variegatus exposed in the sediment of the reference lake (Table 4). Similarly to TV sediments, the Mn concentrations of L. variegatus had divergent gradient in the PS area: in the Pyhäjärvi KS sediment, the Mn concentrations were in both setups higher than in the reference sediment but the Mn concentrations of L. variegatus exposed in the sediment of two sites in the Junttiselkä basin were lower than the concentrations in the reference or Pyhäselkä KS sediment.
In the HLW setup of PS, the concentrations of Cu, Zn, and Mn in the sediments correlated positively with the Cu (rs = 0.909, p < 0.001), Zn (rs = 0.790, p = 0.002), and Mn (rs = 0.790, p = 0.002) concentrations of L. variegatus. In the AFW setup, the same significant positive correlations between Cu (rs = 0.818, p = 0.001), Zn (rs = 0.678, p = 0.015), and Mn (rs = 0.706, p = 0.010) concentrations of the sediment and L. variegatus were observed.
The RR and change in both DW and WW of L. variegatus were statistically significantly affected by the TV sediments in the HLW setup (Table 5). The RR and change in DW of the reference sediment were statistically significantly different from the low, medium, and high hazardous sediments (Fig. 3). In the AFW setup, RR did not differ between the test sediment groups and only statistically significant difference was observed in the change of DW of L. variegatus between the low, high, and medium hazardous groups (Fig. 3).
Boxplots (min, 1st quartile, median, 3rd quartile, max) of Lumbriculus variegatus reproduction rates (RR) and biomass change (dry weight, DW) percentage (%) after a 28-days exposure to the sediments from the TV study area. On the left, the results of the hypolimnion water (HLW) setup (sediment + HLW) and on the right the artificial water (AFW) setup (sediment + AFW) are represented. Horizontal line is the reference for L. variegatus proportional count or biomass at the beginning of the tests. Letters denote statistical difference in multiple comparisons by the Conover procedure (p < 0.05) between sediments and open circle and asterisk outliers > 1.5 and > 3 times the interquartile range, respectively
At the PS mining site, the RR and change in DW and WW were highest in the sediments of the reference Lake Parkkimanjärvi and RR and change in DW and WW were statistically significantly affected by the sampling site in both the HLW and AFW setups (Fig. 4; Table 5). In the HLM setup, the RR and change in DW and WW were significantly different in the lake sites receiving effluents from the outlet ditch (Junttiselkä N and S) in comparison with the reference site (LSD multiple comparison, p < 0.05). In the AFW setup, the RR and change in WW of two sites in the Junttiselkä basin differed statistically significantly from the reference site, but the growth in DW only in the Junttiselkä S site (LSD multiple comparison, p < 0.05; Fig. 4).
Boxplots (min, 1st quartile, median, 3rd quartile, max) of Lumbriculus variegatus reproduction rate (RR) and biomass change (dry weight, DW) percentage (%) after a 28-day exposure in the sediments from the PS study area. On the left, the results of the hypolimnion water (HLW) setup (sediment + HLW) and on the right the artificial water (AFW) setup (sediment + AFW) are represented. Horizontal line is the reference for L. variegatus proportional count or biomass at the beginning of the tests. Letters denote statistical difference in multiple comparisons by the least significant difference procedure (p < 0.05) between sampling sites
4 Discussion
4.1 Element Concentrations in Water, Sediments, and Lumbriculus variegatus
In our study areas, metal contamination in the lakes nearest to the mining sites and a decreasing contamination gradient were observed downstream of both mines. The metal concentrations in the sediment studied were elevated in the study lakes receiving waters from the mining sites in comparison to the median background lake sediment concentrations in Finland reported by Mäkinen and Lerssi (2007). In the sediments of the medium and high hazardous lakes in the TV, Ni concentrations were 4 to 37 times higher than the background concentration in Finnish lakes (20 mg/kg DW). However, the TV area is situated in the black shale area, which has naturally elevated background concentrations of Ni and Zn (Loukola-Ruskeeniemi et al., 1998). The Ni concentration of the sediment in Lake Kolmisoppi being situated in the black shale area is only 1.3 times higher than the Ni concentration of 65 mg/kg DW typical to black shale sediments in the area (Loukola-Ruskeeniemi et al., 1998). The concentration gradient was not as clear for Zn with concentrations of up to 7-fold in the sediments downstream of both the TV and PS mines in comparison to median background concentrations (115 mg/kg DW) in Finland (Mäkinen & Lerssi, 2007). Cu concentrations were 3 to 8 times the background concentration (25 mg/kg DW) in the sediment in the Junttiselkä and Pyhäjärvi KS in the PS area.
Ore metals Cu, Zn, and Ni showed the accumulation potential to benthic invertebrates in the mining-contaminated sediments. The Cu, Zn, and Ni concentrations increased accordingly with sediment concentrations especially in the HLW setup, and the element concentrations in L. variegatus correlated positively with the element concentrations in the sediment. Cu, Ni, and Zn accumulated more in the HLW than in the AFW setup. Overlying water concentrations of dissolved elements in the HLW setup were slightly higher and the pH lower (supplementary material S5) than in the AFW setup and the metals were likely more available from the HLW. Element concentrations in AFW were low especially in the high hazardous group in comparison with the HLW setup. However, the elements dissolved partially from the sediment to the overlying AFW during the exposure period; hence, the uptake from the overlying water in the AFW setup was possible. Overall, the use of AFW as overlying water underestimated the metal accumulation potential in the laboratory toxicity tests in contrast to natural conditions in the recipient where both the hypolimnion and sediment of the field sites are polluted. Due to the different storage time of the HLW and AFW sediments, the comparisons between HLW and AFW setups are indicative.
Although the biomass of L. variegatus at 28 days for the element analyses was higher in the AFW setup in all treatments, Ni was not detected at all from the body of L. variegatus in AFW setup in contrast to HLW setup. L. variegatus exposed to Lake Ylä-Lumijärvi sediment were the only exception, and it was the most polluted lake in the TV area. Sediment pore-water has been reported to be the main accumulation route for Ni in L. variegatus (Camusso et al., 2012) and similarly in our tests, Ni uptake from the water layer above sediment seems to be a more important accumulation route for L. variegatus than uptake from the sediment.
In contrast to Ni, the accumulation of Cu and Zn from the sediment was notable. Cu and Zn concentrations of L. variegatus exposed to mining-contaminated sediments were on average only 25% and 31% less in the AFW setup than in the HLW setup, respectively. Cu and Zn concentrations of L. variegatus were generally also higher in the mining-contaminated sediments than in the local reference sediments. In our experiments, both overlying water and sediment were important exposure routes of metals for L. variegatus. Camusso et al. (2012) reported that, for As, Cd, Cu, and Zn, diet is the main exposure route for L. variegatus in natural sediments, and Méndez-Fernández et al., 2017) observed also that As, Cu, Hg, Ni, and Zn accumulate from sediment to aquatic oligochaetes. Zn body burdens of aquatic oligochaetes have been linked to chronic sediment toxicity but increased Cu body burdens have not been connected to toxic effects (Méndez-Fernández et al., 2017).
It is worth to note that gut purging of L. variegatus was not carried out after the exposure periods before the element analyses. We did not aim to model the element kinetics in our study, but the total element concentration of L. variegatus reflected the availability of elements from the differently hazardous sediments. The gut content of L. variegatus varies from 5 to 18% of the total mass of worms (Brooke et al., 1996; Dawson et al., 2003) and the total element concentrations were likely higher than the tissue concentrations of L. variegatus. In the high hazardous sediments, the positive bias was likely higher than in the low hazardous sediments but the comparison between sediment concentrations and the total element concentrations of L. variegatus, however, clearly showed accumulation of the studied elements. Cain et al., 1995concluded earlier that the metals associated with gut content of four aquatic invertebrates in natural river sediments did not alter the interpretations of site metal contamination based on the analysis of whole-body concentrations. Thus, the total concentrations including the gut content reflected both the metal bioaccumulation and differences between sites.
The Mn concentrations and thus also accumulation potential of the studied sediments between the lakes were different from the other metals. The high accumulation in L. variegatus was observed from the sediments of Lake Pyhäjärvi KS only, and in the Junttiselkä basin, the concentrations of L. variegatus were lower than in the Lake Pyhäjärvi or reference sediments. Mn concentration in the Pyhäjärvi KS sediment was high and the reference sediment of the PS area was also rich in Mn. The Mn accumulation in TV sediments was generally at the same level as in the reference sediments. Mn has been observed to accumulate to chironomids (Woelfl et al., 2006) and perch (Perca fluviatilis) (Karjalainen et al., 2020) in metal-contaminated lakes and also to whitefish (Coregonus lavaretus) eggs and larvae under experimental conditions (Arola et al., 2017). In our study, Mn concentrations of L. variegatus correlated positively with the sediment concentrations although in some test sediments (e.g., in the HLW setup of the high hazardous group in the TV) the accumulation was surprisingly low in relation to the sediment concentration. Sediment-water fluxes of metals are determined by adsorption, precipitation, and coprecipitation with organic matter, Mn and Fe oxyhydroxides, and metal sulfides which are all processes dependent on oxygen condition, redox potential and pH of sediment, sediment pore-water, and overlying water (Teuchies et al., 2011). In natural sediments, small differences in sediment-water conditions may change the metal availability. Oxygen-rich conditions may lead to decreasing Mn concentrations of overlaying water and precipitation in the sediment (Teuchies et al., 2011) and decrease the Mn availability for aquatic organisms. Although the kinetics of metals in sediments and L. variegatus clearly need further studies and modeling approaches, our data from the exposure conditions did not make possible to closely interpret the fate of metals in different sediments.
4.2 Toxicity of Mining-Contaminated Sediments
The toxicity tests revealed that the environmental quality of the water bodies near both mining sites did not support optimum growth of the benthic oligochaetes. In the TV mining site, although the growth of L. variegatus was reduced in all hazardous groups in comparison to reference, there were no differences between low and medium hazardous groups. This can be due to different characteristics of the individual lake sediments and different sedimentation patterns of the contaminants. In the PS mining site, the growth was decreased both in the southern and northern parts of the Lake Junttiselkä (Junttiselkä S and N) sediments which had higher major ion contamination than the sediment of Lake Pyhäjärvi KS.
Growth and reproduction were equally sensitive endpoints in our toxicity tests. On the contrary, Nikkilä et al., (2001) have stated that growth was more sensitive when L. variegatus was exposed to retene-spiked sediments. HLW setup induced more distinct response to both endpoints used in our study. In the reference sites, growth and reproduction were smaller compared to those in the artificial control sediment. This discrepancy between reference and control sediments has been observed earlier with several species (Wolfram et al., 2012). Reproduction and growth of L. variegatus were likely impaired in the field-collected sediments in comparison to artificial control sediment, because the quality of food may not have been at the same level in the natural lake sediments as in the artificial control. In addition to peat, 0.5% of Urtica dioica was provided for the worms in artificial control sediment according to the OECD 225 standard (OECD, 2007) but not in the field-collected sediments. The requirement for reproduction of L. variegatus by factor of at least 1.8 in the control sediment described in the standard may be unrealistically high for most of the field-collected sediments. It is relatively challenging to find a reference sediment that would meet the standard requirements (OECD, 2007) and support the sufficient growth of the test species (Burton, 2010; Wolfram et al., 2012). This was the case also in our experiments.
L. variegatus tolerates and survives in various environmental conditions (Chapman, 2001; Phipps et al., 1993). L. variegatus is a versatile test organism that can be used in several types of toxicity tests and for several types of contaminants and it is able to tolerate at low pH between 4 and 5 (Veltz et al., 1996). Sulfide- and metal-rich sediments are easily acidified when disturbed impeding their risk assessment, but nevertheless, mining-contaminated sediments have been aimed to use in laboratory-conducted toxicity testing (Burton, 2013; Norwood et al., 2003). Typically, tests are conducted with buffering and renewal of the overlying artificial water. Thus, stabilization and renewal of the overlying water are recommended in spiked sediment toxicity tests in general, but during the metal toxicity tests, no renewal of the overlying water was performed in order to prevent metal clearance from the sediments due to leaching from the sediment (Vandegehuchte et al., 2013). Also in our toxicity tests, the major challenge in both test setups was the decrease in pH during exposure. The pH decreased similarly both in the reference and mining-affected sediments (Table S5, supplementary material). We attempted to compensate the increase in acidity in the AFW setup by using AFW above the test sediment instead of HLW, but the sediment pH declined below 5. Only the artificial control sediment and the on-site limed Lake Ylä-Lumijärvi sediment of the high mining effect group were able to sustain the pH recommended in the OECD 225 standard (2007). Because the optimum pH of overlying water is from 6 to 9 in the OECD standard 225 (2007), the large decrease in pH may affect the test results. Buffering of the overlying water alone may not be adequate to support circumneutral exposure conditions in toxicity tests of metal- and sulfide-rich sediments (Väänänen, 2017). Large pH shifts in natural sediments indicated that initiation of acid-generating processes in sediments (e.g., oxidation of sulfides) is possible and this may lead to acidification of overlaying water if the environmental conditions change. Acidification of the lake water in Lake Junttiselkä has been reported at least in 2006, when the pH of Lake Junttiselkä dropped around 5 during the spring turnover period (Heikkinen & Väisänen, 2007). Oxidation of the hypolimnion after winter can result into mobilization and increased bioavailability of many metals leading to environmental conditions, where metals can be released to overlying water from the sediment (De Jonge et al., 2012).
Because of the prolonged storage time of sediments before the AFW setup, comparison between HLW and AFW was not straightforward. Sediments should be used soon after their retrieval from the field, because storage time can have an effect on the sediment toxicity (e.g., Becker & Ginn, 1995; De Lange et al., 2008; Moore et al., 1995). However, not all studies have verified the effects of storage time on sediment toxicity. For example, DeFoe and Ankley (1998) observed that sediment storage up to 101 weeks did not have an effect on the biological responses of Hyalella azteca or Chironomus tentans, when the responses were expressed proportional to concurrent control. Our reference sediments went through the same storage period as the mining-contaminated sediments. Overall, the mining-contaminated sediments were less harmful in relation to corresponding reference sediments with AFW above the sediment after the storage period, but the role of AFW in metal toxicity tests of field-collected sediments needs further assessment.
5 Conclusions
The toxicity assessment of boreal lake sediments revealed that mining had affected most of the sites nearest to the TV mining site and Junttiselkä N and S near the PS mining site. Both biomining and conventional mining activities seem to have similar effects on the receiving water bodies. Especially in the lakes of high hazardous group, the water quality (e.g., oxygen depletion, increased conductivity) was impaired in addition to the observed sediment metal contamination. Influence of mining was also seen in the low hazardous lakes, which indicated that contaminants were transported far away from the main effluent discharge point. In the recipient lakes, the main contaminants were the main products of both the studied mining sites. Besides the metals that were extracted from the ore, mining released elements that were locally abundant in the ground and the bedrock.
Metal contamination of the mining-affected boreal lake sediments decreased the growth and reproduction of L. variegatus. The main ore metals were available for bioaccumulation to L. variegatus relative to their concentrations in the sediments and overlying water. Sediment contamination and multi-metal accumulation affected the toxicity endpoints especially in the high hazardous sediments. Toxicity test with aerated natural HLW as overlying water represented the worst-case scenario in the nature, for example, after winter stratification period when the overlying water is oxidized. The present study brought up the challenges of assessing contaminated lake sediments together with hypoxic hypolimnion as they are. Although the test setups were influenced by a considerable decrease in the pH of the sediment and water, toxicity appeared along the contamination gradient. Standard toxicity tests with L. variegatus (OECD, 2007) could be applied with success to natural boreal sediments if the pH remains at least at 5 which seems to be the tolerance limit of L. variegatus. Suitable natural reference sediment should be used together with artificial control sediment. If only artificial sediment is used as control, the effect of mining on sediment toxicity might be overestimated.
References
Arola, H. E., Karjalainen, J., Vehniäinen, E.-R., Väisänen, A., Kukkonen, J. V. K., & Karjalainen, A. K. (2017). Tolerance of whitefish (Coregonus lavaretus) early life stages to manganese sulfate is affected by the parents. Environmental Toxicology and Chemistry, 36, 1343–1353. https://doi.org/10.1002/etc.3667.
Becker, D. S., & Ginn, T. C. (1995). Effects of storage time on toxicity of sediments from Puget Sound, Washington. Environmental Toxicology and Chemistry, 14, 829–835. https://doi.org/10.1002/etc.5620140513.
Birceanu, O., Chowdhury, M. J., Gillis, P. L., McGeer, J. C., Wood, C. M., & Wilkie, M. P. (2008). Modes of metal toxicity and impaired branchial ionoregulation in rainbow trout exposed to mixtures of Pb and Cd in soft water. Aquatic Toxicology, 89, 222–231. https://doi.org/10.1016/j.aquatox.2008.07.007.
Brooke, L. T., Ankley, G. T., Call, D. J., & Cook, P. M. (1996). Gut content weight and clearance rate for three species of freshwater invertebrates. Environmental Toxicology and Chemistry, 15, 223–228.
Burton, G. A. (2010). Metal bioavailability and toxicity in sediments. Critical Reviews in Environmental Science and Technology, 40, 852–907. https://doi.org/10.1080/10643380802501567.
Burton, G. A. (2013). Assessing sediment toxicity: Past, present, and future. Environmental Toxicology and Chemistry, 32, 1438–1440. https://doi.org/10.1002/etc.2250.
Cadmus, P., Clements, W. H., Williamson, J. L., Ranville, J. F., Meyer, J. S., & Gutiérrez Ginés, M. J. (2016). The use of field and mesocosm experiments to quantify effects of physical and chemical stressors in mining-contaminated streams. Environmental Science and Technology, 50, 7825–7833. https://doi.org/10.1021/acs.est.6b01911.
Cain, D. J., Luoma, S. N., & Axtmann, E. V. (1995). Influence of gut content in immature aquatic insects on assessments of environmental metal contamination. Canadian Journal of Fisheries and Aquatic Sciences, 52, 2736–2746. https://doi.org/10.1139/f95-862.
Camusso, M., Polesello, S., Valsecchi, S., & Vignati, D. A. L. (2012). Importance of dietary uptake of trace elements in the benthic deposit-feeding Lumbriculus variegatus. TrAC Trends in Analytical Chemistry, 36, 103–112. https://doi.org/10.1016/j.trac.2012.02.010.
Chapman, P. M. (2001). Utility and relevance of aquatic oligochaetes in ecological risk assessment. Hydrobiologia, 463, 149–169. https://doi.org/10.1023/A:1013103708250.
Chapman, P. M., Wang, F., Janssen, C., Persoone, G., & Allen, H. E. (1998). Ecotoxicology of metals in aquatic sediments: Binding and release, bioavailability, risk assessment, and remediation. Canadian Journal of Fisheries and Aquatic Sciences, 55, 2221–2243. https://doi.org/10.1139/f98-145.
Dawson, T. D., Lott, K. G., Lonard, E. N., & Mount, D. R. (2003). Time course of metal loss in Lumbriculus variegatus following sediment exposure. Environmental Toxicology and Chemistry, 22, 886–889.
De Jonge, M., Teuchies, J., Meire, P., Blust, R., & Bervoets, L. (2012). The impact of increased oxygen conditions on metal-contaminated sediments part I: Effects on redox status, sediment geochemistry and metal bioavailability. Water Research, 46, 2205–2214. https://doi.org/10.1016/j.watres.2012.01.052.
De Lange, H. J., Van Griethuysen, C., & Koelmans, A. A. (2008). Sampling method, storage and pretreatment of sediment affect AVS concentrations with consequences for bioassay responses. Environmental Pollutation, 151, 243–251. https://doi.org/10.1016/j.envpol.2007.01.052.
DeFoe, D. L., & Ankley, G. T. (1998). Influence of storage time on toxicity of freshwater sediments to benthic macroinvertebrates. Environmental Pollutation, 99, 123–131. https://doi.org/10.1016/S0269-7491(97).00159-0.
Giesy, J. P., & Hoke, R. A. (1989). Freshwater sediment toxicity bioassessment: Rationale for species selection and test design. Journal of Great Lakes Research, 15, 539–569.
Heikkinen, M.-L., & Väisänen, T. (2007). Pyhäjärven Junttiselän tila ja kunnostusmahdollisuudet. Reports of environment centre of Northern Ostrobothnia, 7, 78 (In Finnish).
ISO. (2012). Water quality - Determination of the inhibition of the mobility of Daphnia magna Straus (Cladocera, Crustacea) - Acute toxicity test. International Organization for Standardization, 6341, 2012, 22 p.
Jerez, C. A. (2017). Biomining of metals: How to access and exploit natural resource sustainably. Microbial Biotechnology, 10, 1191–1193. https://doi.org/10.1111/1751-7915.12792.
Karjalainen, J., Arola, H. E., Wallin, J., Väisänen, A., & Karjalainen, A. K. (2020). Condition and sperm characteristics of perch (Perca fluviatilis) inhabiting boreal lakes receiving metal mining effluents. Archives of Environmental Contamination and Toxicology, 79, 270–281. https://doi.org/10.1007/s00244-020-00752-9.
Kauppi, S., Mannio, J., Hellsten, S., Nysten, T., Jouttijärvi, T., Huttunen, M., Ekholm, P., Tuominen, S., Porvari, P., Karjalainen, A. K., Sara-Aho, T., Saukkoriipi, J., & Maunula, M. (2013). Assessment of the potential impacts on water environment caused by the gypsum pond leakage at the Talvivaara mine (Arvio Talvivaaran kaivoksen kipsisakka-altaan vuodon haitoista ja riskeistä vesiympäristölle). Reports of Finnish Environment Institute, 11, 90 (In Finnish).
Kreutzweiser, D., Beall, F., Webster, K., Thompson, D., & Creed, I. (2013). Impacts and prognosis of natural resource development on aquatic biodiversity in Canada’s boreal zone. Environmental Reviews, 21, 227–259. https://doi.org/10.1139/er-2013-0044.
Kukkonen, J., & Landrum, P. (1994). Toxicokinetics and toxicity of sediment-associated pyrene to Lumbriculus variegatus (Oligochaeta). Environmental Toxicology and Chemistry, 13, 1457–1468. https://doi.org/10.1002/etc.5620130909.
Leppänen, M. T., & Kukkonen, J. V. K. (1998a). Factors affecting feeding rate, reproduction and growth of an oligochaete Lumbriculus variegatus (Müller). Hydrobiologia, 377, 183–194. https://doi.org/10.1023/A:1003252520704.
Leppänen, M. T., & Kukkonen, J. V. K. (1998b). Relationship between reproduction, sediment type, and feeding activity of Lumbriculus variegatus (Müller): Implications for sediment toxicity testing. Environmental Toxicology and Chemistry, 17, 2196–2202. https://doi.org/10.1002/etc.5620171109.
Leppänen, J. J., Weckström, J., & Korhola, A. (2017). Multiple mining impacts induce widespread changes in ecosystem dynamics in a boreal lake. Scientific Reports, 7, 10581. https://doi.org/10.1038/s41598-017-11421-8.
Loukola-Ruskeeniemi, K., Uutela, A., Tenhola, M., & Paukola, T. (1998). Environmental impact of metalliferous black shales at Talvivaara in Finland, with indication of lake acidification 9000 years ago. Journal of Geochemical Exploration, 64, 395–407. https://doi.org/10.1016/S0375-6742(98)00047-8.
Mäkinen, J. (2011). Lake Pyhäjärvi sedimentation investigations in Pyhäsalmi 2006–2010. Geological Survey of Finland. Report of Investigation, 7, 52.
Mäkinen, J., & Lerssi, J. (2007). Characteristics and Seasonal variation of sediments in Lake Junttiselkä, Pyhäsalmi, Finland. Mine Water and the Environment, 26, 217–228. https://doi.org/10.1007/s10230-007-0015-3.
Méndez-Fernández, L., Martinez-Madrid, M., Pardo, I., & Rodriguez, P. (2017). Baseline tissue concentrations of metal in aquatic oligochaetes: Field and laboratory approaches. Environmental Pollutation, 223, 636–643. https://doi.org/10.1016/j.envpol.2017.01.070.
Moore, D. W., Dillon, T. M., & Gamble, E. W. (1995). Long-term storage of sediments: Implications for sediment toxicity testing. Environmental Pollutation, 89, 147–154. https://doi.org/10.1016/0269-7491(94)00058-L.
Nikkilä, A., Kukkonen, J. V. K., & Oikari, A. (2001). Bioavailability of sediment-associated retene to an oligochaete worm Lumbriculus variegatus. Journal of Soils and Sediments, 1, 137–145. https://doi.org/10.1007/BF02986476.
Norwood, W. P., Borgmann, U., Dixon, D. G., & Wallace, A. (2003). Effects of metal mixtures on aquatic biota: A review of observations and methods. Human and Ecological Risk Assessment: An International Journal, 9, 795–811. https://doi.org/10.1080/713610010.
OECD, (2007). Test No. 225: Sediment-water Lumbriculus toxicity test using spiked sediment. OECD Guidelines for the testing of chemicals. p 31.
Pajunen, H. (2004). Lake sediments as a store of dry matter and carbon (Järvisedimentit kuiva-aineen ja hiilen varastona). Geological Survey of Finland. Report of Investigation, 160, 314 (In Finnish).
Phipps, G. L., Ankley, G. T., Benoit, D. A., & Mattson, V. R. (1993). Use of the aquatic oligochaete Lumbriculus variegatus for assessing the toxicity and bioaccumulation of sediment-associated contaminants. Environmental Toxicology and Chemistry, 12, 269–279. https://doi.org/10.1002/etc.5620120210.
Poteat, M. D., & Buchwalter, D. B. (2014). Four reasons why traditional metal toxicity testing with aquatic insects is irrelevant. Environmental Science & Technology, 48, 887–888. https://doi.org/10.1021/es405529n.
Rainbow, P. (2002). Trace metal concentrations in aquatic invertebrates: Why and so what? Environmental Pollutation, 120, 497–507. https://doi.org/10.1016/S0269-7491(02)00238-5.
Rawlings, D. E., & Johnson, B. D. (2007). Biomining (314 p). Springer-Verlag.
Riekkola-Vanhanen, M. (2013). Talvivaara mining company – From a project to a mine. Minerals Engineering, 48, 2–9. https://doi.org/10.1016/J.MINENG.2013.04.018.
Salmelin, J., Leppänen, M. T., Karjalainen, A. K., Hämäläinen, H., Leppänen, M. T., Kiviranta, H., Kukkonen, J. V. K., & Vuori, K.-M. (2017). Assessing ecotoxicity of biomining effluents in stream ecosystems by in situ invertebrate bioassays: A case study in Talvivaara, Finland. Environmental Toxicology and Chemistry, 36, 147–155. https://doi.org/10.1002/etc.3511.
Schubaur-Berigan, M. K., Monson, P. D., West, C. W., & Ankley, G. T. (1995). Influence of pH on the toxicity of ammonia to Chironomus tentans and Lumbriculus variegatus. Environmental Toxicology and Chemistry, 14, 713–717. https://doi.org/10.1002/etc.5620140419.
Simpson, S. L., & Batley, G. E. (2007). Predicting metal toxicity in sediments: A critique of current approaches. Integrated Environmental Assessment and Management, 3, 18–31. https://doi.org/10.1002/ieam.5630030103.
Suter, G. W., & Cormier, S. M. (2015). Why care about aquatic insects: Uses, benefits, and services. Integrated Environmental Assessment and Management, 11, 188–194. https://doi.org/10.1002/ieam.1600.
Teuchies, J., Bervoets, L., Cox, J. S., Meire, P., & de Deckere, E. (2011). The effect of waste water treatment on river metal concentrations: Removal or enrichment? Journal of Soils and Sediments, 11, 364–372. https://doi.org/10.1007/s11368-010-0321-4.
Thienpont, J. R., Korosi, J. B., Hargan, K. E., Williams, T., Eickmeyer, D. C., Kimpe, L. E., Palmer, M. J., Smol, J. P., & Blais, J. M. (2016). Multi-trophic level response to extreme metal contamination from gold mining in a subarctic lake. Proceedings of the Royal Society B: Biological Sciences, 283, 20161125.
Väänänen, K. (2017). Adverse effects of metal mining on boreal lakes: Metal bioavailability and ecological risk assessment. Publication of University of Eastern Finland. Dissertions. Forest. Natural Science, 284 http://urn.fi/URN:ISBN:978-952-61-2615-9.
Väänänen, K., Kauppila, T., Mäkinen, J., Leppänen, M. T., Lyytikäinen, M., & Akkanen, J. (2016). Ecological risk assessment of boreal sediments affected by metal mining: Metal geochemistry, seasonality, and comparison of several risk assessment methods. Integrated Environmental Assessment and Management, 12, 759–771. https://doi.org/10.1002/ieam.1751.
Vandegehuchte, M. B., Nguyen, L. T. H., De Laender, F., Muyssen, B. T. A., & Janssen, C. R. (2013). Whole sediment toxicity tests for metal risk assessments: On the importance of equilibration and test design to increase ecological relevance. Environmental Toxicology and Chemistry, 32, 1048–1059. https://doi.org/10.1002/etc.2156.
Veltz, I., Arsac, F., Biagianti-Risbourg, S., Habets, F., Lechenault, H., & Vernet, G. (1996). Effects of platinum (Pt 4+) on Lumbriculus variegatus Müller (Annelida, Oligochaeta): Acute toxicity and bioaccumulation. Archives of Environmental Contamination and Toxicology, 67, 63–67. https://doi.org/10.1007/BF00203908.
Woelfl, S., Mages, M., & Geller, W. (2006). Determination of heavy metals in macrozoobenthos from the rivers Tisza and Szamos by total reflection X-ray fluorescence spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy, 61, 1153–1157. https://doi.org/10.1016/J.SAB.2006.09.015.
Wolfram, G., Höss, S., Orendt, C., Schmitt, C., Adámek, Z., Bandow, N., Großschartner, M., Kukkonen, J. V. K., Leloup, V., López Doval, J. C., Muñoz, I., Traunspurger, W., Tuikka, A., Van Liefferinge, C., von der Ohe, P. C., & de Deckere, E. (2012). Assessing the impact of chemical pollution on benthic invertebrates from three different European rivers using a weight-of-evidence approach. Science of the Total Environment, 438, 498–509. https://doi.org/10.1016/j.scitotenv.2012.07.065.
Acknowledgements
We would like to thank O. Nousiainen for his help in the field and N. Honkanen, M. Koistinen, and L. Siitonen for their help in the laboratory and in both mines for assistance in sampling. We also thank M. Leppänen from the Finnish Environment Institute for arranging part of the water measurements and Prof. J. Kukkonen for his advices during the research.
Availability of Data and Material
Data available from the authors by inquiry.
Code Availability
Not applicable.
Funding
Open access funding provided by University of Jyväskylä (JYU). This work was supported by the Maj and Tor Nessling Foundation and the Academy of Finland (281800).
Author information
Authors and Affiliations
Contributions
JW: planning, methodology, experimental and field work, data collection and management, data analysis and statistics, writing, reviewing. JK: planning, data analysis and statistics, writing, reviewing, editing, supervision. AV: planning, supervision, methodology, metal analyses, writing, reviewing. AK: supervision, project administration, funding acquisition, planning, methodology, field work, quality control, writing, reviewing.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(XLSX 41 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wallin, J., Karjalainen, J., Väisänen, A. et al. Toxicity of Mining-Contaminated Lake Sediments to Lumbriculus variegatus. Water Air Soil Pollut 232, 202 (2021). https://doi.org/10.1007/s11270-021-05157-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05157-5