Abstract
The article highlights sorptive properties of poultry litter. Preliminary studies on sorption of Cd2+, Cu2+, Ni2+, Pb2+, Zn2+ ions at the concentration range of 1–1000 mg L-1 were carried out. Langmuir and Freundlich models were used to determine the potential capacities of the poultry litter. Sorption parameter outcome from isotherms exhibited the following order: Pb > Cd > Cu > Zn > Ni. During the batch adsorption experiment, a decrease of the absorbance of solutions in the UV-Vis range was observed, proportional to the increase in the concentration of metals in the solutions. This phenomenon was associated with the precipitation of complexes of fulvic/humic-like compounds and uric acid with metals. The decrease in dissolved organic carbon (DOC) concentration confirmed previous assumptions. The FT-IR spectroscopy showed significant role of uric acid in Cd2+, Pb2+, Zn2+ sorption. FT-IR measurements allowed determining the type of active sites involved in sorption, especially carboxylic groups. The findings should be helpful in soil reclamation practices with poultry litter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metal contamination is a severe health and environmental problem. Pollutants arising from human activity are the cause of direct soil and water contamination, which results in their further movement into groundwater and up to the trophic chain (Kabata-Pendias and Mukherjee 2007). According to the data provided by Panagos et al. (2013), heavy metal contamination accounts for 34.8% and 30.8% of the total pollution load of soil and groundwater contaminants in the European Union, respectively. Metal contaminants have a significant impact on human health and may contribute to many diseases such as cancer, gastrointestinal and nervous disorders (Kabata-Pendias and Mukherjee 2007). Contamination with Cu, Ni, Cd, Zn, Pb is one of the most common heavy metal contaminations in the environment, which is the most frequently discussed topic of scientific communities (Mohammed et al. 2011). Despite significant progress in reducing heavy metal emissions to the environment, the problem of already degraded sites remains. In the European Union alone, the number of such sites is estimated at 2.5 million (EU-39), of which heavy metal contamination is about 35% (Panagos et al. 2013). Therefore, there is a need to create appropriate methods to minimize the harmful impact that polluted sites have on the environment. One of the ways is the use of materials based on waste from the agri-food industry (Park et al. 2011) such as plant waste or organic fertilizers. Poultry manure can be used, among others, as a raw material for the production of components supporting land remediation (Kucharski and Bialecka 2019). The incorporation of poultry manure into the soil, depending on the conditions of research, may cause immobilization (Liu et al. 2009; Chen et al. 2010; Galende et al. 2014; Zhang et al. 2015; Barakat et al. 2016; Shokalu et al. 2016; Saleem et al. 2016; Rocco et al. 2018; Haroon et al. 2019) or mobilization (Li and Shuman 1997; Zhou et al. 2005; Jalali and Khanboluki 2007; Jalali and Jalili 2010; Sabir et al. 2013; Jalali and Latifi 2017) of heavy metals in the investigated systems. However, the studies referred to lack the link between recorded experimental data and the specific mechanisms of heavy metal immobilization/mobilization. Scientific reports deal with mechanisms affecting the immobilization of heavy metals as an effect of the use of poultry manure is scarce, yet the following mechanisms can be distinguished on its basis: ion exchange by phosphate groups of organic and inorganic derivatives (Katoh et al. 2013; Miller and Karathanasis 2014; Katoh et al. 2016), complexation by dissolved organic matter (DOM) (Karathanasis et al. 2005; Karathanasis and Johnson 2006; Miller and Karathanasis 2014; Zhou et al. 2017), ion exchange by carboxyl groups of organic matter (Martinho et al. 2015), including fulvic and humic acids (Santos et al. 2018; Park et al. 2019), co-precipitation (Park et al. 2011). The purpose of this paper is an attempt to explain the sorption properties of poultry manure, supported by data recovered from UV-Vis and FT-IR techniques.
2 Material and methods
The poultry manure (PM) used in the research came from a poultry farm near Mikołów (Silesian Voivodeship). The farm specializes in providing young chickens to egg producers. Raw poultry manure was collected at week 16 of the breeding cycle and then stored at < 5 ° C. Next, the poultry manure was dried at 70 °C for 48 h, ground to 0.2 mm, and stored at the temperature of < 5 °C. The material was assayed according to the following methods (in accordance to): total organic carbon (TOC), total inorganic carbon (TIC), sulfur (S) (PN-EN 13137:2004; ELTRA CS, ELTRA CHS), total nitrogen (N) (internal procedure), and pH and conductivity (cond) were measured at a ratio of sample:water (deionized), respectively, 1:5 and 1:10, ash content (internal procedure; at 815 °C), water content (PN-EN 15934:2013), chemical composition (PN-EN 15309:2010; WD-XRF, Rigaku ZSX Primus II) after ashing the sample at 815 °C. The content of heavy metals was determined in accordance with PN-EN 13657:2006 and PN-EN ISO 11885:2009 (ICP-OES, Perkin Elmer) after the samples were mineralized in aqua regia. Selected chemical properties of poultry manure (PM) and aqueous extracts are provided in Table 1.
According to the data collected in an earlier publication (Kucharski and Bialecka 2019), the chemical composition, in most cases, does not differ from the literature data. The only exceptions are the nitrogen and phosphorus contents within the upper limit of the range. A poultry manure sample whose active sites were substituted with a hydrogen atom (PMPr) was prepared in two stages. In the first stage, uric acid derivatives and dissolved organic matter (DOM) were washed out. 0.2 g of the sample was pre-shaken with 50 ml of deionized water for 2 h. The sample was then centrifuged and then eluted for 8 h in a solution of 0.1 M nitric acid (V). The resulting suspension was centrifuged and then dried at 60 °C for 12 h.
Glassware and plasticware used in the study were soaked before use in a 1:1 solution of HNO3:H2O (1:1) and then in deionized water. The solutions intended for testing were prepared from metal nitrate salts (analytical grade, CHEMPUR) in 1 L of deionized water (ASTM Type I). Solutions with metal concentrations of 1–800 mg l-1 were prepared each time before testing. For the sorption experiment, 0.2 ± 0.002 g of sample (PM) was weighed into 50 ml PP tubes, and then, 50 ml of metal solutions was added. An initial pH value was measured for each suspension. The suspensions were shaken on an overhead shaker (GFL 3040; 20 rpm) at the temperature of 20 ± 2 °C for 24 h and the pH was measured again. Next, the suspensions were centrifuged (3938 rpm) for 10 min and then filtered through a GVS 0.45 μm nitrocellulose filter. The resulting solutions were divided into two parts. First part was used to obtain UV-Vis spectra and for the determination of dissolved organic carbon (DOC). Second part was used for measuring the concentration of soluble phosphorus and metals with the use of ICP-OES method. UV-Vis measurement was performed with SPEKOL 1300 in the range (190-1100 nm). Metals and phosphorus solutions were appropriately diluted and protected by the addition of 0.6 ml nitric acid (V) solution (HNO3: H2O; 1:1). The measurement was made by using a Perkin Elmer OPTIMA 5300DV spectrometer. The solid phase, remaining after filtration, was dried at 60 °C for 12 h after which they were tested by infrared spectrometry over a mid-infrared range 400–4000 cm-1 in transmission mode. Spectra were collected using a Nicolet iS50 spectrometer purged with nitrogen, equipped with a DLaTGS (deuterated l-alanine doped triglycenesulphate) detector and a KBr beam splitter. The number of scans was set at 64 with a resolution of 4 cm-1. The aperture was set on 80 and the scan velocity on 0.4747. Samples were prepared by grinding with KBr in agate mortar and pressed using small hydraulic press Specac, then measured as KBr mini-pellets with a concentration of 1%wt. Spectra were collected using a spectrum of pure KBr as a background; any correction was applied to spectra. All spectra were processed using the Origin software (Origin Pro 8.1).
3 Results and discussion
3.1 UV-Vis measurement of water extracts.
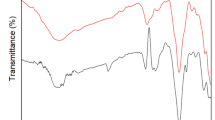
The spectra obtained (Fig. 1) differed from those found in the scientific literature of animal manure samples. According to He et al. (2009), the spectrum of UV-Vis, water extracts, and animal manure is characterized by a monotonic decrease in intensity with the increase in wavelength, very similar to the spectra of humic compounds. In the same work, a band around 280 nm was observed in the spectrum of a water extract made from cattle manure. In later works on organic matter waste (He et al. 2013, 2015), the 200–290 nm band was associated with absorption resulting from the electron transition p-p* of aromatic compounds. The presence of a band around 280 nm was found in samples of humus extracts of organic waste (Rupiasih and Vidyasagar 2009) and assigned to carbohydrates. The presence of a band in the range of 260–280 nm was observed during research on the composting process of poultry manure. Bouzid and Djadi (2015) noted the presence of a clear band in the 260–280 nm range in raw poultry manure samples. The band was assigned to nitrogen-containing compounds (proteins, RNA, DNA, amino acids). The recorded band disappeared after the composting process. Due to the metal nitrate solutions used in sorption studies, the possibility of occurrence of bands in the 190–260 nm of the p-p* electron transition and 260–350 nm of the p-p* transition should also be taken into account (Otten et al. 2007). This problem was solved by measuring the absorbance of metal nitrate solutions with the maximum possible concentration resulting from the dilution of the samples before measurement. The spectra showed the existence of a band in the 190–240 nm range and the absence of a band in the 260–350 nm range characteristic for diluted nitrate ion solutions (Otten et al. 2007). The obtained UV-Vis spectrum is similar to the spectra of uric acid and its salts (Moawad 2002). Results of Mowrer et al. (2013), including the determination of uric acid in 118 poultry manure samples, indicated its content in the range of 45–53,742 mg kg-1day m. The high content of uric acid derivatives in the tested samples forced a 10-fold dilution of the sample before UV-Vis measurement. It is noteworthy that the band intensity disappears in the 220–350 nm range and the 190–220 nm band intensity increases in samples of water extracts (sample 0) exposed to solar radiation for 48 h. In the case of high uric acid content in manure samples, the presence of mineralization products, urea, and glyoxylic acid was taken into account (Mowrer et al. 2014). Due to their structure, both compounds can form soluble complexes with metals, which may be associated with a decrease in the sorption capacity of poultry manure. The spectrum of glyoxylic acid is characterized by the absorption band of the carbonyl group with a maximum at 276 nm (Eugene et al. 2016). In the case of urea, the prepared UV-Vis spectrum had a broad band in the 190–250 nm range. At this stage of the work, it was impossible to confirm the presence of both compounds 220–260 nm band remains a debatable issue.
The UV-Vis spectra of the extracts were divided into 3 parts: the band representing the electronic transition p-p*of nitrate (V) ions and uric acid (190–220 nm), the band characteristic for uric acid (220–260 nm), and the band characteristic for uric acid and electron transition p-p* of other aromatic compounds in the sample (260–320 nm). The dissolved organic carbon (DOC) content was used as a marker for the total dissolved organic matter (DOM). The effect of DOM on the sorption process was examined for selected wavelengths 375, 365, 300, 290, 280, 254, and 240 nm, respectively. Correlation analysis (STATISTICA 12) of DOC content and total dissolved phosphorus (DTPhos) in extracts in relation to the absorbance value for selected wavelengths showed correlations for DOC 0.979 (A300), 0.955(A254), 0.920(A280), 0.904(A240), 0.894 (A290), 0.855 (A365), 0.835 (A375), 0.827 (DTPhos). As previously considered, the values for A300, A280, A240, and A290 are in the uric acid absorption bands. These parameters correlated very well with the DOC content in the solution. The quoted correlations, in comparison with the data from Table 1, may indicate a considerable share of uric acid in the sorption of Cd2+, Pb2+, Zn2+ ions. In the case of Cu2+, Ni2+ ions, no significant decreases in DOC content were observed with increasing concentration of ions in solutions. For DTPhos, large correlations with DOC content were noted.
Absorbance values at A375, A365, and A254 were found to be free from influences from uric acid absorption bands. According to He et al. (2016), they can be used for determining the average molecular weight (A254/A365) and the degree of aromaticity of the A254 part of the DOM not containing uric acid derivatives. Data collected in Table 2 may suggest a decrease in “aromaticity” and average molecular weight of DOM depending on the concentration of Cd2+, Pb2+, and Zn2+ ions. In the case of Cu2+, Ni2+ ions which increased A254/A365 values would indicate an unlikely increase in the average molecular weight of the DOM.
3.2 FT-IR measurement of solid-phase.
Averaged solid phase spectra obtained after the adsorption process and the spectrogram of the initial sample are presented in Figs. 2 and 3. The spectra were characterized by a wide band in the range 3500–3100 cm-1 identified as originating from vibrations stretching O–H bonds of phenyl, carboxyl, carbohydrate, amino, and amide (Merlin et al. 2014; Huang et al. 2017). In the area of 2926 and 2855 cm-1, bands originating from C–H binding vibrations of CH2 and CH3 groups were found (He et al. 2016). Within the 1080-1,1040 cm-1, and 1030 cm-1 area, bands derived from C–O bonds of polysaccharides were found (Eugene et al. 2016; He et al. 2016). Vibrations from phosphate groups were identified as a wide band with a maximum of around 1040 cm-1 (Uchimiya et al. 2010) which was revealed as a result of the impact of 0.1 M nitric acid (V) on the sample. In contrast, bands from the 900–690 cm-1 area were identified as derived from C–H bonds of aromatic groups. In the spectrum of the poultry manure (PM) sample and solid samples after the metal sorption process, no bands characteristic of glyoxylic acid derivatives (Redington and Liang 1984) and urea (Onija et al. 2012) were found. This fact does not exclude the presence of both compounds in the tested samples but only the lack of optional insoluble derivatives of both compounds. For the other bands of the FT-IR spectrum, similarly, as in the UV-Vis spectrum, two groups can be outlined. In the first group, there were no bands derived from the uric acid spectrum. However, changes in the spectra associated with ion exchange sorption of carboxyl groups can be noted. For sample 0, a weak band around 1740 cm-1 was attributed to the C = O stretching vibration of carboxyl groups (Murphy et al. 2007) disappearing after treatment of the samples by metal ions solutions. In the range 1674–1625 cm-1, there is a strong band attributed to the stretching vibration of C = O bonds of COO groups (Eugene et al. 2016) and amide groups. The increase in band intensity is evident in all considered cases, suggesting the participation of carboxyl groups in the process of metal ion sorption (Merlin et al. 2014). Confirmation of this fact is the decrease in the intensity of the 1248 cm-1 band identified as C–O bond vibration of the COOH group CO in favor of the appearance of bands in the area of 1427–1413 cm-1 and 1384 cm-1 assigned to asymmetric vibrations stretching the C–O bonds of COO group (Eugene et al. 2016; Merlin et al. 2014). The presence of carboxyl groups in the water-insoluble phase was confirmed by FT-IR measurement of a poultry manure sample treated with 0.1 M nitric (V) acid (PMPr). The protonation effect of carboxyl groups in the PMPr sample was evident in the form of an increase in band intensity in the area of 1740 cm-1 and 1248 cm-1. There was also a significant reduction in band intensity in the range of 1674–1625 cm-1 compared to the corresponding intensities of the spectrum bands of poultry manure (PM) and manure after leaching with deionized water. Murphy et al. (2007) obtained similar results in their research on biomass. Spectra with clear peaks from carboxyl groups were obtained as a result of protonation. Bands in the range 1550–1516 cm-1 were attributed to vibrations originating from aromatic rings (He et al. 2016). In the second group (Fig. 4), in addition to the characteristic bands derived from carboxyl groups, a clear effect of uric acid on the spectra was noted. The following bands were found around 3014 cm-1 (vibrations of NH bond), 1590 cm-1 (vibrations of C = O bond of amide groups), 1437 cm-1 (stretching vibrations of C = C bond), 1304 cm-1 (O–H deformation), 1123 cm-1 and 1029 cm-1(vibrations of the amide group C–N bonds), (Sekkoum et al. 2016), and 1009–708 cm-1 (vibrations of the OH bonds), (Moawad 2002). In the case of samples treated with Cu2+, Ni2+ ion spectra, in particular in the area of the so-called fingerprint, do not differ significantly from the reference spectrum (sample 0) which may indicate uric acid remaining in the solutions.
3.3 Batch adsorption experiment
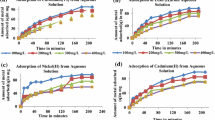
The tests were carried out in order to determine the maximum sorption capacity of poultry manure. Despite the limitations, adsorption isotherms (Freundlich and Langmuir models) are readily used for characterizing the sorption process on organic materials (biosorption), (Michalak et al. 2013). Data revealed that the Langmuir model was characterized by the highest correlation coefficients of the model with experimental data. The unit of maximum sorption capacity most commonly found in the literature on biosorption is the mass of metal ions (mg) per unit of sorbent mass (g), and this unit was adopted for comparison with literature values.
Sorption characteristics of the tested poultry manure are presented accordingly (b, Langmuir constant l × mg-1; qm, maximum sorption capacity g mg-1; R2 model correlation coefficient), Pb2+ (0,16; 169,9; 0,99), Cd2+ (0.028; 50.8; 0.93), Cu2+ (0.061; 29.3; 0.97), Zn2+ (0.124; 17.9; 0.72), and Ni2+ (0.027; 8.54, 5; 0.74). According to De Gisi et al. (2016), the maximum sorption capacities of biosorbents are in the ranges, respectively, Ni2+ (18.86–52 mg g-1), Zn2+ (7.84–80 mg g-1), Pb2+ (7.84–167 mg g-1), Cd2+ (5.71–125.63 mg g-1), and Cu2+ (0.39–289 mg g-1).The cited data shows that the investigated poultry manure has relatively good sorption properties.
Based on the obtained data, it is assumed that two mechanisms participated in the metal ion sorption process. The most important of these is the exchange sorption of carboxyl groups present on the solid phase surface of manure.
The results of FT-IR measurements support the significant share of this mechanism. In the case of the manure, it was assumed that the carboxyl groups are partially substituted with ions (K+, Na+, Mg2+, Ca2+). This can be evidenced by the increase in the concentration of these ions in solutions after the sorption process. This thesis is also supported by the results of FT-IR tests of PM, 0, and PMPr samples. For the PM sample, one broad band with a maximum of 1668 cm-1 was obtained. The process of washing with distilled water (sample 0) and protonating carboxyl groups (sample PMPr) caused a gradual separation of the band into two parts, including an increase in band intensity with the maximum expected for carboxyl groups. A relatively small change in pH during the sorption process can also prove the presence of the alkali-substituted carboxyl groups. In all the cases considered, the number of hydrogen ions was lower than the amount of adsorbed metal. A similar relationship was noted in the case of poultry manure and its pyrolysis products (Uchimiya et al. 2010).
Another mechanism is the formation of uric acid complexes. Data obtained using UV-ViS and FT-IR techniques support this process. In the case of Cd2+ Zn2+, and Pb2+ ion sorption, the uric acid content is very visible. For Cu2+, Ni2+ ions, no insoluble uric acid salts were found in the dry residue after sorption. The presence of uric acid and its mineralization products can be a problem. In open systems, partially soluble metal-uric acid complexes can increase their mobility. Increased mobility of Zn and Cd as a result of washing with aqueous poultry manure extract was confirmed by Jalali and Khanboluki (2007). It was proved in the work that the aqueous extract from poultry manure can effectively elute Zn and Cd ions from columns filled with sandy soil. However, the study does not identify any compounds that may affect the mobility of these ions in the investigated system.
The last identified factors are metal ion hydrolysis reactions. This phenomenon is particularly visible in the case of copper ion solutions. In the case of sorption with solutions in the range of 150–1000 mg l-1 of Cu2+ ions, a significant decrease in the pH of solutions was noted, disproportionate to the amount of sorbed ions. A similar situation was noted for lead and cadmium ions in the range of 400–1000 mg l-1. Due to the dependence of the sorption process on the pH, the occurrence of the phenomenon of metal ion hydrolysis caused a decrease in the sorption process efficiency. However, the degree of dependence varies concerning individual metal ions (Fig. 4). The conducted tests showed no possibility of precipitation of metal ions in the form of hydroxides in the tested system. The hydroxide precipitation occurred at pH 7.0 (Zn2+), 8.08 (Ni2+), 7.42 (Cd2+), and 7.0 (Pb2+). The exception was Cu2+ ion solutions precipitating at pH 5.2. The initial pH of the systems was 6.29, so in most of the considered cases, it did not allow the formation of insoluble metal hydroxides. Uchmiya et al. (2010) came to similar conclusions. The study showed that in the case of ions with a concentration of 1.5 mM/l and the initial pH of the tested systems at the level of 8.0, precipitation of metal hydroxides does not occur.
4 Conclusions
Studies have shown a significant effect of uric acid on the sorption of metal ions such as Pb2+, Cd2+, Zn2+, Cu2+, and Ni2+. Uric acid may affect the mobility of metals in open systems. It is advisable to include its content in poultry manure samples in relation to the test results or to modify the manure to reduce uric acid mobility. Analysis of extract samples in the UV-Vis range may be an appropriate screening test for uric acid. The significant role of carboxyl groups in the sorption process was also confirmed. Due to the dependence of the biosorption process on the pH, measurements of maximum sorption capacity should be carried out, taking into account pH changes.
References
Barakat, M. A., Ismail, S. M., & Ehsan, M. (2016). Immobilization of Ni and Zn in Soil by Cow and Chicken Manure. International Journal of Waste Resources, 6, 228. https://doi.org/10.4172/2252-5211.1000228.
Bouzid, M., & Djadi, A. (2015). Revaluation of activated sludge and chicken manure through composting by aerobic process. African Journal of Agricultural Research, 10, 4831–4836. https://doi.org/10.5897/AJAR2015.9950.
Chen, H. S., Huang, Q. Y., Liu, L. N., Cai, P., Liang, W., & Li, M. (2010). Poultry manure compost alleviatesthephytotoxicity of soil cadmium: influence on growth of pakchoi (Brassica chinensis L.). Pedosphere, 20(1), 63–70. https://doi.org/10.1016/S1002-0160%2809%2960283-6.
De Gisi, S., Lofrano, G., Grassi, M., & Notarnicola, M. (2016). Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustainable Materials and Technologies, 9, 10–40. https://doi.org/10.1016/j.susmat.2016.06.002.
Eugene, A. J., Xia, S. S., & Guzman, M. I. (2016). Aqueous photochemistry of glyoxylic acid. The Journal of Physical Chemistry A, 120(21), 3817–3826. https://doi.org/10.1021/acs.jpca.6b00225.
Galende, M. A., Becerril, J. M., Gómez-Sagasti, M. T., Barrutia, O., Garbisu, C., & Hernández, A. (2014). Agro-industrial wastes as effective amendments for ecotoxicity reduction and soil health improvement in aided phytostabilization. Environmental Science and Pollution Research, 21(17), 10036–10044. https://doi.org/10.1007/s11356-014-2752-8.
Haroon, B., Irshad, M., Hafeez, F., Pervez, A., & Faridullah, A. P. (2019). Fractionation of heavy metals in contaminated soil after amendment with composted cow manure and poultry litter. Arabian Journal of Geosciences, 12(6). https://doi.org/10.1007/s12517-019-4395-z.
He, Z., Mao, J., Honeycutt, C. W., Ohno, T., Hunt, J. F., & Cade-Menun, B. J. (2009). Characterization of plant-derived water extractable organic matter by multiple spectroscopic techniques. Biology and Fertility of Soils, 45(6), 609–616. https://doi.org/10.1007/s00374-009-0369-8.
He, X.-S., Xi, B.-D., Jiang, Y.-H., He, L.-S., Li, D., Pan, H.-W., & Bai, S.-G. (2013). Structural transformation study of water-extractable organic matter during the industrial composting of cattle manure. Microchemical Journal, 106, 160–166. https://doi.org/10.1016/j.microc.2012.06.004.
He, Z., Zhang, M., Cao, X., Li, Y., Mao, J., & Waldrip, H. M. (2015). Potential traceable markers of organic matter in organic and conventional dairy manure using ultraviolet–visible and solid-state 13C nuclear magnetic resonance spectroscopy. Organic Agriculture, 5(2), 113–122. https://doi.org/10.1007/s13165-014-0092-0.
He, Z., Pagliari, P. H., & Waldrip, H. M. (2016). Applied and environmental chemistry of animal manure: a review. Pedosphere, 26(6), 779–816. https://doi.org/10.1016/S1002-0160(15)60087-X.
Huang, J., Yu, Z., Gao, H., Yan, X., Chang, J., Wang, C., Hu, J., & Zhang, L. (2017). Chemical structures and characteristics of animal manures and composts during composting and assessment of maturity indices. PLoS ONE, 12(6). https://doi.org/10.1371/journal.pone.0178110.
Jalali, M., & Jalili, A. (2010). Competitive adsorption of trace elements in calcareous soils as affected by sewage sludge, poultry manure, and municipal waste compost. Environmental Earth Sciences, 63(4), 731–739. https://doi.org/10.1007/s12665-010-0742-9.
Jalali, M., & Khanboluki, G. (2007). Leaching of zinc, cadmium, and lead in a sandy soil due to application of poultry litter. Soil & Sediment Contamination, 16(1), 47–60. https://doi.org/10.1080/15320380601077818.
Jalali, M., & Latifi, Z. (2017). Measuring and simulating effect of organic residues on the transport of cadmium, nickel, and zinc in a calcareous soil. Journal of Geochemical Exploration, 184, 372–380. https://doi.org/10.1016/j.gexplo.2017.05.001.
Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements from soil to human. Springer, Verlag, Berlin. https://doi.org/10.1007/978-3-540-32714-1.
Karathanasis, A. D., & Johnson, D. M. C. (2006). Subsurface transport of Cd, Cr, and Mo mediated by biosolid colloids. Science of The Total Environment, 354(2-3), 157–169. https://doi.org/10.1016/j.scitotenv.2005.01.025.
Karathanasis, A. D., Johnson, D. M. C., & Matocha, C. J. (2005). Biosolid colloid-mediated transport of copper, zinc, and lead in waste-amended soils. Journal of Environment Quality, 34(4), 1153. https://doi.org/10.2134/jeq2004.0403.
Katoh, M., Kitahara, W., & Sato, T. (2013). Sorption of lead in animal manure compost: contributions of inorganic and organic fractions. Water, Air, & Soil Pollution, 225(1). https://doi.org/10.1007/s11270-013-1828-2.
Katoh, M., Kitahara, W., Sato, T.(2016). Role of inorganic and organic fractions in animal manure compost in lead immobilization and microbial activity in soil. Applied and Environmental Soil Science, 1–9, https://doi.org/10.1155/2016/7872947
Kucharski, P., Bialecka, B.(2019). Poultry manure as a substrate for agriculture and the chemical industry. International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, 19 (5.2), 611-618, 10.5593/sgem2019/5.2/s20.076
Li, Z., & Shuman, L. M. (1997). Mobility of Zn, Cd and Pb in soils as affected by poultry litter extract—I. leaching in soil columns. Environmental Pollution, 95(2), 219–226. https://doi.org/10.1016/S0269-7491(96)00074-7.
Liu, L., Chen, H., Cai, P., Liang, W., & Huang, Q. (2009). Immobilization and phytotoxicity of Cd in contaminated soil amended with chicken manure compost. Journal of Hazardous Materials, 163(2-3), 563–567. https://doi.org/10.1016/j.jhazmat.2008.07.004.
Martinho, J., Campos, B., Brás, I., & Silva, E. (2015). The role of compost properties in sorption of heavy metals.Environment Protection Engineering, 41, 57-65. https://doi.org/10.5277/epe150205.
Merlin, N., Nogueira, B. A., de Lima, V. A., & Santos, L. M. (2014). Application of fourier transform infrared spectroscopy, chemical and chemometrics analyses to the characterization of agro-industrial waste. Química Nova, 37(10), 1584–1588. https://doi.org/10.5935/0100-4042.20140259.
Michalak, I., Chojnacka, K., & Witek-Krowiak, A. (2013). State of the art for the biosorption process—a review. Applied Biochemistry and Biotechnology, 170(6), 1389–1416. https://doi.org/10.1007/s12010-013-0269-0.
Miller, J. O., & Karathanasis, A. D. (2014). Biosolid colloids as environmental contaminant carriers. The Role of Colloidal Systems in Environmental Protection, 1–18. https://doi.org/10.1016/B978-0-444-63283-8.00001-6.
Moawad, M. M. (2002). Complexation and thermal studies of uric acid with some divalent and trivalent metal ions of biological interest in the solid state. Journal of Coordination Chemistry, 55(1), 61–78. https://doi.org/10.1080/00958970211872.
Mohammed, A.S., Kapri, A., Goel, R.(2011). Heavy metal pollution: source, impact, and remedies. Biomanagement of Metal-Contaminated Soils, 1–28, https://doi.org/10.1007/978-94-007-1914-9_1
Mowrer, J., Kissel, D. E., Cabrera, M., & Hassan, S. M. (2013). Nondegradative extraction and measurement of uric acid from poultry litter. Soil Science Society of America Journal, 77(4), 1413. https://doi.org/10.2136/sssaj2012.0337.
Mowrer, J., Cabrera, M., Rasmussen, T., & Cassity-Duffey, K. (2014). Nitrogen in stored poultry litter: uric acid and xanthine. Journal of Environment Quality, 43(6), 2137. https://doi.org/10.2134/jeq2014.05.0240.
Murphy, V., Hughes, H., & McLoughlin, P. (2007). Cu(II) binding by dried biomass of red, green and brown macroalgae. Water Research, 41(4), 731–740. https://doi.org/10.1016/j.watres.2006.11.032.
Onija, O., Borodi, G., Kacso, I., Pop, M., Dadarlat, D., Bratu, I., & Jumate, N. (2012). Preparation and characterization of urea-oxalic acid solid form. AIP Conference Proceedings, 1425. 35-38. https://doi.org/10.1063/1.3681960.
Otten, D. E., Petersen, P. B., & Saykally, R. J. (2007). Observation of nitrate ions at the air/water interface by UV-second harmonic generation. Chemical Physics Letters, 449(4-6), 261–265. https://doi.org/10.1016/j.cplett.2007.10.081.
Panagos, P., Van Liedekerke, M., Yigini, Y., &Montanarella, L.(2013). Contaminated sites in Europe: review of the current situation based on data collected through a European network. Journal of environmental and public health, 158764, https://doi.org/10.1155/2013/158764
Park, J. H., Lamb, D., Paneerselvam, P., Choppala, G., Bolan, N., & Chung, J.-W. (2011). Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. Journal of Hazardous Materials, 185(2-3), 549–574. https://doi.org/10.1016/j.jhazmat.2010.09.082.
Park, J., Cho, K. H., Ligaray, M., & Choi, M.-J. (2019). Organic matter composition of manure and its potential impact on plant growth. Sustainability, 11(8), 2346. https://doi.org/10.3390/su11082346.
Redington, R., & Liang, C.-K. (1984). Vibrational spectra of glyoxylic acid monomers. Journal of Molecular Spectroscopy, 104, 25–39. https://doi.org/10.1016/0022-2852(84)90242-X.
Rocco, C., Seshadri, B., Adamo, P., Bolan, N. S., Mbene, K., & Naidu, R. (2018). Impact of waste-derived organic and inorganic amendments on the mobility and bioavailability of arsenic and cadmium in alkaline and acid soils. Environmental Science and Pollution Research., 25(26), 25896–25905. https://doi.org/10.1007/s11356-018-2655-1.
Rupiasih, N. N., & Vidyasagar P.B. (2009). Analytical study of humic acid from various sources commonly used as fertilizer: emphasis on heavy metal content. International Journal of Design & Nature and Ecodynamics, 4, 32–46. https://doi.org/10.2495/DNE-V4-N1-32-41.
Sabir, M., Hanafi, M. M., Aziz, T., Ahmad, H. R., Zia-Ur-Rehman, M., Saifullah, G. M., & Hakeem, K. R. (2013). Comparative effect of activated carbon, pressmud and poultry manure on immobilization and concentration of metals in maize (Zea mays) grown on contaminated soil. International Journal of Agriculture and Biology, 15, 559–564.
Saleem, A., Parveen, S., Khan, M.J.(2016). Effect of biochar, farmyard manure and poultry manure on Zn adsorption in calcareous alkaline soil. Sarhad Journal of Agriculture, 32(4): 354-363, 10.17582/journal.sja/2016.32.4.354.363
Santos, A., Bertoli, M. P., Borges, A. C., Gomes, A. C. C. P., Garcia, R. A. B., Jerusa, S., & Trevisan, M. G. (2018). New organomineral complex from humic substances extracted from poultry wastes: synthesis, characterization and controlled release study. Journal of the Brazilian Chemical Society, 29(1), 140–150. https://doi.org/10.21577/0103-5053.20170122.
Sekkoum, K., Cheriti, A., Taleb, S., & Belboukhari, N. (2016). FTIR spectroscopic study of human urinary stones from El Bayadh district (Algeria). Arabian Journal of Chemistry, 9(3), 330–334. https://doi.org/10.1016/j.arabjc.2011.10.010.
Shokalu, O., Adetunji, M. T., Bodunde, J. G., Akintoye, H. A., & Azeez, J. O. (2016). Cadmium adsorption as influenced by poultry manure addition in soils of south - western Nigeria. Archives of Agronomy and Soil Science., 63(8), 1070–1081. https://doi.org/10.1080/03650340.2016.1261118.
Uchimiya, M., Lima, I. M., Thomas Klasson, K., Chang, S., Wartelle, L. H., & Rodgers, J. E. (2010). Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. Journal of Agricultural and Food Chemistry, 58(9), 5538–5544. https://doi.org/10.1021/jf9044217.
Zhang, C., Clark, G. J., Patti, A. F., Bolan, N., Cheng, M., Sale, P. W. G., & Tang, C. (2015). Contrasting effects of organic amendments on phytoextraction of heavy metals in a contaminated sediment. Plant and Soil, 397(1-2), 331–345. https://doi.org/10.1007/s11104-015-2615-1.
Zhou, D.-M., Hao, X.-Z., Wang, Y.-J., Dong, Y.-H., & Cang, L. (2005). Copper and Zn uptake by radish and pakchoi as affected by application of livestock and poultry manures. Chemosphere, 59(2), 167–175. https://doi.org/10.1016/j.chemosphere.2004.11.008.
Zhou, W., Ren, L., & Zhu, L. (2017). Reducement of cadmium adsorption on clay minerals by the presence of dissolved organic matter from animal manure. Environmental Pollution, 223, 247–254. https://doi.org/10.1016/j.envpol.2017.01.019.
Funding
The paper has been prepared in the frames of the project: “Design of a product for substitution of phosphate rocks—DEASPHOR” co-funded by the National Centre for Research and Development (ERA-MIN2/DEASPHOR/2/2019, project value: 1 202 730.20 PLN) under the ERA-MIN2 Joint Call 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kucharski, P., Białecka, B., Śliwińska, A. et al. Evaluation of specific capacity of poultry litter in heavy metal sorption. Water Air Soil Pollut 232, 35 (2021). https://doi.org/10.1007/s11270-021-04984-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-04984-w