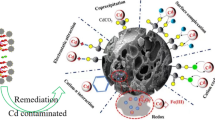
Abstract
In this study, a magnetic montmorillonite composite (MMC) was prepared by a coprecipitation method, evaluated as sorbent for Cd(II) ions and characterized by IR spectroscopy, SEM, EDX, VSM, and zeta potential measurement. The adsorption potential of MMC was tested in both batch and continuous modes at different initial pH levels, sorbent dosages, contact times, ionic strengths, flow rates, and bed depths. The adsorption performance of MMC was found maximum at an original solution pH of 5.9 with 0.18 g sorbent for Cd(II). Fast adsorption equilibrium was established within 10 min. Kinetic and isotherm studies showed that the adsorption data followed the pseudo-second-order kinetic model and Langmuir isotherm. The maximum monolayer adsorption capacity of MMC was 0.325 mmol g−1. Cd(II) adsorption was mainly controlled by ion exchange, electrostatic interaction, and surface complexation mechanisms. Desorption and reusability studies revealed that MMC can be a promising alternative for the effective adsorption of Cd(II) in aqueous solutions of environmental remediation.





Similar content being viewed by others
References
Akar, T., Sayin, F., Turkyilmaz, S., & Tunali Akar, S. (2017). The feasibility of Thamnidium elegans cells for color removal from real wastewater. Process Safety and Environmental Protection, 105, 316–325. https://doi.org/10.1016/j.psep.2016.11.017.
Aksu, Z., & Çağatay, Ş. Ş. (2006). Investigation of biosorption of Gemazol Turquise Blue-G reactive dye by dried Rhizopus arrhizus in batch and continuous systems. Separation and Purification Technology, 48(1), 24–35. https://doi.org/10.1016/j.seppur.2005.07.017.
Alese, M. O., Agbaje, M. A., & Alese, O. O. (2018). Cadmium induced damage in Wistar rats, ameliorative potentials of progesterone. Journal of Trace Elements in Medicine and Biology, 50, 276–282. https://doi.org/10.1016/j.jtemb.2018.07.014.
Argun, M. E., Dursun, S., Ozdemir, C., & Karatas, M. (2007). Heavy metal adsorption by modified oak sawdust: thermodynamics and kinetics. Journal of Hazardous Materials, 141(1), 77–85. https://doi.org/10.1016/j.jhazmat.2006.06.095.
Bulgariu, D., & Bulgariu, L. (2016). Potential use of alkaline treated algae waste biomass as sustainable biosorbent for clean recovery of cadmium (II) from aqueous media: batch and column studies. Journal of Cleaner Production, 112, 4525–4533. https://doi.org/10.1016/j.jclepro.2015.05.124.
Chang, J., Ma, J., Ma, Q., Zhang, D., Qiao, N., Hu, M., & Ma, H. (2016). Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Applied Clay Science, 119, 132–140. https://doi.org/10.1016/j.clay.2015.06.038.
Chen, J., Yan, L.-G., Yu, H.-Q., Li, S., Qin, L.-L., Liu, G.-Q., Li, Y.-F., & Du, B. (2016). Efficient removal of phosphate by facile prepared magnetic diatomite and illite clay from aqueous solution. Chemical Engineering Journal, 287, 162–172. https://doi.org/10.1016/j.cej.2015.11.028.
Chowdhury, S., Mishra, R., Saha, P., & Kushwaha, P. (2011). Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination, 265(1), 159–168. https://doi.org/10.1016/j.desal.2010.07.047.
Cottet, L., Almeida, C. A. P., Naidek, N., Viante, M. F., Lopes, M. C., & Debacher, N. A. (2014). Adsorption characteristics of montmorillonite clay modified with iron oxide with respect to methylene blue in aqueous media. Applied Clay Science, 95, 25–31. https://doi.org/10.1016/j.clay.2014.03.023.
Dubinin, M. M. (1947). The equation of the characteristic curve of activated charcoal. Doklady Akademii Nauk SSSR, 55, 327–329.
Ezzeddine, Z., Batonneau-Gener, I., Pouilloux, Y., Hamad, H., Saad, Z., & Kazpard, V. (2015). Divalent heavy metals adsorption onto different types of EDTA-modified mesoporous materials: effectiveness and complexation rate. Microporous and Mesoporous Materials, 212, 125–136. https://doi.org/10.1016/j.micromeso.2015.03.013.
Feng, Y., Gong, J.-L., Zeng, G.-M., Niu, Q.-Y., Zhang, H.-Y., Niu, C.-G., Deng, J.-H., & Yan, M. (2010). Adsorption of Cd (II) and Zn (II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chemical Engineering Journal, 162(2), 487–494. https://doi.org/10.1016/j.cej.2010.05.049.
Freundlich, H. (1906). Über die absorption in lösungen. Über Die Adsorption in Lösungen, 385–470.
Futalan, C. M., Kan, C.-C., Dalida, M. L., Pascua, C., & Wan, M. W. (2011). Fixed-bed column studies on the removal of copper using chitosan immobilized on bentonite. Carbohydrate Polymers, 83, 697–704. https://doi.org/10.1016/j.carbpol.2010.08.043.
Ge, F., Li, M.-M., Ye, H., & Zhao, B.-X. (2012). Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. Journal of Hazardous Materials, 211-212, 366–372. https://doi.org/10.1016/j.jhazmat.2011.12.013.
Gong, J.-L., Wang, B., Zeng, G.-M., Yang, C.-P., Niu, C.-G., Niu, Q.-Y., Zhou, W.-J., & Liang, Y. (2009). Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. Journal of Hazardous Materials, 164(2), 1517–1522. https://doi.org/10.1016/j.jhazmat.2008.09.072.
Gournis, D., Papachristodoulou, C., Maccallini, E., Rudolf, P., Karakassides, M. A., Karamanis, D. T., Sage, M.-H., Palstra, T. T. M., Colomer, J.-F., Papavasileiou, K. D., Melissas, V. S., & Gangas, N. H. (2010). A two-dimensional magnetic hybrid material based on intercalation of a cationic Prussian blue analog in montmorillonite nanoclay. Journal of Colloid and Interface Science, 348(2), 393–401. https://doi.org/10.1016/j.jcis.2010.04.068.
Hall, K. R., Eagleton, L. C., Acrivos, A., & Vermeulen, T. (1966). Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Industrial and Engineering Chemistry Fundamentals, 5(2), 212–223. https://doi.org/10.1021/i160018a011.
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34(5), 451–465. https://doi.org/10.1016/S0032-9592(98)00112-5.
Ivanets, A. I., Srivastava, V., Roshchina, M. Y., Sillanpää, M., Prozorovich, V. G., & Pankov, V. V. (2018). Magnesium ferrite nanoparticles as a magnetic sorbent for the removal of Mn2+, Co2+, Ni2+ and Cu2+ from aqueous solution. Ceramics International, 44(8), 9097–9104. https://doi.org/10.1016/j.ceramint.2018.02.117.
Jia, L., Yao, X., Ma, J., & Long, C. (2017). Adsorption kinetics of water vapor on hypercrosslinked polymeric adsorbent and its comparison with carbonaceous adsorbents. Microporous and Mesoporous Materials, 241, 178–184. https://doi.org/10.1016/j.micromeso.2016.12.028.
Jorfi, S., Shooshtarian, M. R., & Pourfadakari, S. (2019). Decontamination of cadmium from aqueous solutions using zeolite decorated by Fe3O4 nanoparticles: adsorption modeling and thermodynamic studies. International Journal of Environmental Science and Technology. https://doi.org/10.1007/s13762-019-02350-2.
Kalantari, K., Ahmad, M. B., Fard Masoumi, H. R., Shameli, K., Basri, M., & Khandanlou, R. (2015). Rapid and high capacity adsorption of heavy metals by Fe3O4/montmorillonite nanocomposite using response surface methodology: preparation, characterization, optimization, equilibrium isotherms, and adsorption kinetics study. Journal of the Taiwan Institute of Chemical Engineers, 49, 192–198. https://doi.org/10.1016/j.jtice.2014.10.025.
Karunanayake, A. G., Todd, O. A., Crowley, M., Ricchetti, L., Pittman, C. U., Anderson, R., Mohan, D., & Mlsna, T. (2018). Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. Chemical Engineering Journal, 331, 480–491. https://doi.org/10.1016/j.cej.2017.08.124.
Kausar, A., Iqbal, M., Javed, A., Aftab, K., Nazli, Z.-I.-H., Bhatti, H. N., & Nouren, S. (2018). Dyes adsorption using clay and modified clay: a review. Journal of Molecular Liquids, 256, 395–407. https://doi.org/10.1016/j.molliq.2018.02.034.
Khairy, M., El-Safty, S. A., & Shenashen, M. A. (2014). Environmental remediation and monitoring of cadmium. TrAC Trends in Analytical Chemistry, 62, 56–68. https://doi.org/10.1016/j.trac.2014.06.013.
Khan, M. A., Otero, M., Kazi, M., Alqadami, A. A., Wabaidur, S. M., Siddiqui, M. R., Alothman, Z. A., & Sumbul, S. (2019). Unary and binary adsorption studies of lead and malachite green onto a nanomagnetic copper ferrite/drumstick pod biomass composite. Journal of Hazardous Materials, 365, 759–770. https://doi.org/10.1016/j.jhazmat.2018.11.072.
Khezami, L., Alwqyan, T. S., Bououdina, M., Al-Najar, B., Shaikh, M. N., Modwi, A., & Taha, K. K. (2019). Dependence of phase distribution and magnetic properties of milled and annealed ZnO·Fe2O3 nanostructures as efficient adsorbents of heavy metals. Journal of Materials Science: Materials in Electronics, 30(10), 9683–9694. https://doi.org/10.1007/s10854-019-01303-2.
Kim, W.-K., Shim, T., Kim, Y.-S., Hyun, S., Ryu, C., Park, Y.-K., & Jung, J. (2013). Characterization of cadmium removal from aqueous solution by biochar produced from a giant Miscanthus at different pyrolytic temperatures. Bioresource Technology, 138, 266–270. https://doi.org/10.1016/j.biortech.2013.03.186.
Krishna Murthy, T. P., Gowrishankar, B. S., Chandra Prabha, M. N., Kruthi, M., & Hari Krishna, R. (2019). Studies on batch adsorptive removal of malachite green from synthetic wastewater using acid treated coffee husk: equilibrium, kinetics and thermodynamic studies. Microchemical Journal, 146, 192–201. https://doi.org/10.1016/j.microc.2018.12.067.
Lagergren, S. (1898). Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar, 24, 1–39.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 40(9), 1361–1403. https://doi.org/10.1021/ja02242a004.
Li, C., Yang, X., Xu, Y., Li, L., & Wang, Y. (2018a). Cadmium detoxification induced by salt stress improves cadmium tolerance of multi-stress-tolerant Pichia kudriavzevii. Environmental Pollution, 242, 845–854. https://doi.org/10.1016/j.envpol.2018.07.058.
Li, J., Zheng, B., He, Y., Zhou, Y., Chen, X., Ruan, S., Yang, Y., Dai, C., & Tang, L. (2018b). Antimony contamination, consequences and removal techniques: a review. Ecotoxicology and Environmental Safety, 156, 125–134. https://doi.org/10.1016/j.ecoenv.2018.03.024.
Lima, É. C., Adebayo, M. A., & Machado, F. M. (2015). In C. P. Bergmann & F. M. Machado (Eds.), Carbon nanomaterials as adsorbents for environmental and biological applications (pp. 33–69). Cham: Springer International Publishing.
Lin, J., Su, B., Sun, M., Chen, B., & Chen, Z. (2018). Biosynthesized iron oxide nanoparticles used for optimized removal of cadmium with response surface methodology. Science of the Total Environment, 627, 314–321. https://doi.org/10.1016/j.scitotenv.2018.01.170.
Liu, H., Chen, W., Liu, C., Liu, Y., & Dong, C. (2014). Magnetic mesoporous clay adsorbent: preparation, characterization and adsorption capacity for atrazine. Microporous and Mesoporous Materials, 194, 72–78. https://doi.org/10.1016/j.micromeso.2014.03.038.
Liu, C., Cai, W., & Liu, L. (2018a). Hydrothermal carbonization synthesis of Al-pillared montmorillonite@carbon composites as high performing toluene adsorbents. Applied Clay Science, 162, 113–120. https://doi.org/10.1016/j.clay.2018.06.005.
Liu, Z., Li, X., Zhan, P., Hu, F., & Ye, X. (2018b). Removal of cadmium and copper from water by a magnetic adsorbent of PFM: adsorption performance and micro-structural morphology. Separation and Purification Technology, 206, 199–207. https://doi.org/10.1016/j.seppur.2018.06.007.
Mahramanlioglu, M., Kizilcikli, I., & Bicer, I. O. (2002). Adsorption of fluoride from aqueous solution by acid treated spent bleaching earth. Journal of Fluorine Chemistry, 115(1), 41–47. https://doi.org/10.1016/S0022-1139(02)00003-9.
Masoudi, R., Moghimi, H., Azin, E., & Taheri, R. A. (2018). Adsorption of cadmium from aqueous solutions by novel Fe3O4- newly isolated Actinomucor sp. bio-nanoadsorbent: functional group study. Artificial Cells, Nanomedicine, and Biotechnology, 46(sup3), S1092–S1101. https://doi.org/10.1080/21691401.2018.1533841.
McKay, G., Blair, H. S., & Gardner, J. R. (1982). Adsorption of dyes on chitin I. Equilibrium studies. Journal of Applied Polymer Science, 27(8), 3043–3057. https://doi.org/10.1002/app.1982.070270827.
Mohan, D., Kumar, H., Sarswat, A., Alexandre-Franco, M., & Pittman, C. U. (2014). Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chemical Engineering Journal, 236, 513–528. https://doi.org/10.1016/j.cej.2013.09.057.
Mohubedu, R. P., Diagboya, P. N. E., Abasi, C. Y., Dikio, E. D., & Mtunzi, F. (2019). Magnetic valorization of biomass and biochar of a typical plant nuisance for toxic metals contaminated water treatment. Journal of Cleaner Production, 209, 1016–1024. https://doi.org/10.1016/j.jclepro.2018.10.215.
Naseem, K., Begum, R., Wu, W., Usman, M., Irfan, A., Al-Sehemi, A. G., & Farooqi, Z. H. (2019). Adsorptive removal of heavy metal ions using polystyrene-poly(N-isopropylmethacrylamide-acrylic acid) core/shell gel particles: adsorption isotherms and kinetic study. Journal of Molecular Liquids, 277, 522–531. https://doi.org/10.1016/j.molliq.2018.12.054.
Oliveira, L. C. A., Rios, R. V. R. A., Fabris, J. D., Sapag, K., Garg, V. K., & Lago, R. M. (2003). Clay–iron oxide magnetic composites for the adsorption of contaminants in water. Applied Clay Science, 22(4), 169–177. https://doi.org/10.1016/S0169-1317(02)00156-4.
Oliveira, L. C. A., Petkowicz, D. I., Smaniotto, A., & Pergher, S. B. C. (2004). Magnetic zeolites: a new adsorbent for removal of metallic contaminants from water. Water Research, 38(17), 3699–3704. https://doi.org/10.1016/j.watres.2004.06.008.
Özcan, A. S., Erdem, B., & Özcan, A. (2005). Adsorption of Acid Blue 193 from aqueous solutions onto BTMA-bentonite. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 266(1), 73–81. https://doi.org/10.1016/j.colsurfa.2005.06.001.
Peng, S.-H., Wang, R., Yang, L.-Z., He, L., He, X., & Liu, X. (2018). Biosorption of copper, zinc, cadmium and chromium ions from aqueous solution by natural foxtail millet shell. Ecotoxicology and Environmental Safety, 165, 61–69. https://doi.org/10.1016/j.ecoenv.2018.08.084.
Pokhrel, D., & Viraraghavan, T. (2008). Organic arsenic removal from an aqueous solution by iron oxide-coated fungal biomass: an analysis of factors influencing adsorption. Chemical Engineering Journal, 140(1–3), 165–172. https://doi.org/10.1016/j.cej.2007.09.038.
Purkayastha, D., Mishra, U., & Biswas, S. (2014). A comprehensive review on Cd (II) removal from aqueous solution. Journal of Water Process Engineering, 2, 105–128. https://doi.org/10.1016/j.jwpe.2014.05.009.
Pyrzynska, K. (2019). Removal of cadmium from wastewaters with low-cost adsorbents. Journal of Environmental Chemical Engineering, 7(1), 102795. https://doi.org/10.1016/j.jece.2018.11.040.
Razmara, Z., Saheli, S., Eigner, V., & Dusek, M. (2019). Synthesis, crystal structure and magnetic properties of a new tri-nuclear iron (II, III) complex, a precursor for the preparation of superparamagnetic Fe3O4 nanoparticles applicable in the removal of Cd2+. Applied Organometallic Chemistry, 33(5), e4880. https://doi.org/10.1002/aoc.4880.
Saha, P., & Chowdhury, S. (2011). In T. Mizutani (Ed.), Thermodynamics. Croatia: InTech.
Smith, J. M. (1970). Chemical engineering kinetics. USA: McGraw-Hill Inc..
Tian, Y., Ji, C., Zhao, M., Xu, M., Zhang, Y., & Wang, R. (2010). Preparation and characterization of baker’s yeast modified by nano-Fe3O4: application of biosorption of methyl violet in aqueous solution. Chemical Engineering Journal, 165(2), 474–481. https://doi.org/10.1016/j.cej.2010.09.037.
Tinas H., Caliskan E., Ozbek N., Akman, S. (2016). Preparation of Fe3O4 @ montmorillonite composite as an effective sorbent for the removal of lead and cadmium from wastewater samples. Turkish Journal of Chemistry, 40, 974-978. https://doi.org/10.3906/kim-1605-79.
Trinh, T. T. P. N. X., Quang, D. T., Tu, T. H., Dat, N. M., Linh, V. N. P., Van Cuong, L., Nghia, L. T. T., Loan, T. T., Hang, P. T., Phuong, N. T. L., Phong, M. T., Nam, H. M., & Hieu, N. H. (2019). Fabrication, characterization, and adsorption capacity for cadmium ions of graphene aerogels. Synthetic Metals, 247, 116–123. https://doi.org/10.1016/j.synthmet.2018.11.020.
Tu, Y.-J., You, C.-F., & Chang, C.-K. (2012). Kinetics and thermodynamics of adsorption for Cd on green manufactured nano-particles. Journal of Hazardous Materials, 235-236, 116–122. https://doi.org/10.1016/j.jhazmat.2012.07.030.
Vijayaraghavan, K., Jegan, J., Palanivelu, K., & Velan, M. (2004). Removal of nickel (II) ions from aqueous solution using crab shell particles in a packed bed up-flow column. Journal of Hazardous Materials, B113, 223–230. https://doi.org/10.1016/j.jhazmat.2004.06.014.
Weber, W. J., & Morris, J. C. (1963). Kinetics of adsorption on carbon from solution. Journal of the Sanitary Engineering Division, 89(2), 31–60.
Wu, J., Lu, J., Zhang, C., Zhang, Z., & Min, X. (2019). Adsorptive removal of tetracyclines and fluoroquinolones using yak dung biochar. Bulletin of Environmental Contamination and Toxicology, 102(3), 407–412. https://doi.org/10.1007/s00128-018-2516-0.
Yadanaparthi, S. K. R., Graybill, D., & von Wandruszka, R. (2009). Adsorbents for the removal of arsenic, cadmium, and lead from contaminated waters. Journal of Hazardous Materials, 171(1), 1–15. https://doi.org/10.1016/j.jhazmat.2009.05.103.
Zhao, D., Li, J., Lv, L., Zhang, M., Liu, Z., & An, S. (2019). Effect of cadmium contamination on the eutrophic secondary pollution of aquatic macrophytes by litter decomposition. Journal of Environmental Management, 231, 1100–1105. https://doi.org/10.1016/j.jenvman.2018.11.027.
Acknowledgments
This work was partially supported by the Commission of Scientific Research Projects of Eskisehir Osmangazi University (ESOGU) with the project number 2015-786. The authors gratefully acknowledge the financial support of ESOGU.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tunali Akar, S., Sayin, F., Ozdemir, I. et al. A Natural Montmorillonite-Based Magsorbent as an Effective Scavenger for Cadmium Contamination. Water Air Soil Pollut 231, 409 (2020). https://doi.org/10.1007/s11270-020-04743-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04743-3