Abstract
Lead is a toxic metal, and its characterization in contaminated soils is crucial to the success of a remediation, especially for the soil washing, one of most commonly used technologies. In this study, we propose a convenient approach that combines sedimentary hydro-classification with semi-quantitative powder X-ray diffraction analysis for characterizing the Pb-bearing minerals in soils. The approach was applied to two samples (YYm and YYu-1) collected from a closed Cu–Pb–Zn mine in the Tohoku region of Japan. The samples were taken from adjacent areas but had different appearances (YYm was a gray soil and YYu-1 was a creamy colored soil). The coarser YYm fractions had higher Pb contents than the finer YYm fractions, but the finer YYu-1 fractions (diameters < 32 μm) had higher Pb contents than the coarser YYu-1 fractions. The semi-quantitative powder X-ray diffraction analysis showed that the main Pb-containing minerals in the YYm and YYu-1 samples were galena and plumbojarosite, respectively. Tessier sequential extractions were also performed, and 1 M sodium acetate leached 21% and 65% of the Pb from the YYm and YYu-1 samples, respectively. This suggested that most of the Pb in the YYu-1 sample was ion-exchangeable and was more easily leached compared with that in the YYm sample. The findings indicate that it is important to accurately characterize the Pb-bearing minerals (especially naturally occurring Pb) present in contaminated soils before selecting appropriate remediation techniques and conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lead (Pb) is a naturally occurring toxic metal that is found in the Earth’s crust. Pb has been widely used around the world in batteries, ceramic products, fuel, paint, and various consumer products. Pb pollution problems can be anthropogenic or natural. More than 400,000 contaminated sites and more than 60% of them are polluted with heavy metals in Japan, among which Pb is the most important heavy metal pollutant. Japanese law on countermeasures to soil contamination (No. 53 enacted in 2003) was amended in 2010 to cover naturally occurring heavy metals. The amended law also constrains the “dig and haul” approach and encourages the use of in situ and on-site remediation techniques. However, the development of low cost and environmentally friendly in situ and on-site remediation techniques remains challenging.
Soil contaminated with heavy metals can, in principle, be treated using three types of remediation technique, containment, extraction/removal, and solidification/stabilization (US EPA 1991; Liu et al. 2018). Containment can involve surface capping, encapsulation, or transfer to landfill and is used for soil removed from a contaminated site. Removal can involve soil washing (Isoyama and Wada 2007; Yoo et al. 2017), soil flushing (Manca et al. 2018), electrokinetic (EK) extraction (Acar et al. 1995), and phytoremediation (Alaboudi et al. 2018), but only soil washing has been used in practice, with the other techniques still being developed or having been used only at pilot scales. Solidification and stabilization can involve vitrification and chemical stabilization, but there are limited uses for treated soil (US EPA 1991). Soil washing is the only relatively well-established on-site technique for removing heavy metals from contaminated soils (Liu et al. 2018). EK extraction may be able to be further developed for in situ remediation (López-Vizcaíno et al. 2016; Ji et al. 2019). Soil washing decreases the volume of contaminated soil by separating particles of different sizes but depends on the contaminants being predominantly adsorbed to fine particles (Anderson et al. 1999; Isoyama and Wada 2007; Alaboudi et al. 2020). EK extraction has some advantages over other techniques, including the ability to remove contaminants from soil being less restricted by depth and contaminant concentration (Reddy and Cameselle 2009). EK extraction has been used at many contaminated sites in Europe but has not yet been used at contaminated sites in Japan. It is necessary to understand the bearing minerals of the target contaminant present in naturally polluted soil when designing a remediation process whatever remediation technique is used. The objective of this study is not to compare the advantages and disadvantages among different technologies and/or develop an improved remediation technology but to propose a convenient and practically applicable approach for characterizing metal-bearing minerals in contaminated soils, because such an approach can be a common fundamental basis for effectively selecting and designing a remediation technology. Here, we will take Pb (the most important naturally occurring heavy metal in Japan and other countries having similar geological conditions) as an example and propose an easily implemented approach that combines sedimentary hydro-classification with semi-quantitative powder X-ray diffraction (PXRD) analysis to determine the Pb-bearing minerals and the Pb concentrations in samples of naturally polluted soil.
2 Materials and Methods
2.1 Soil Samples
Two soil samples, labeled YYm and YYu-1, with high Pb contents were collected from a slope surface at a closed Cu–Pb–Zn mine in the Tohoku region of Japan. The sampling locations were adjacent to each other with a distance less than 3 m, but the soil samples had different appearances to the naked eye, YYm was gray, and YYu-1 was creamy-colored (Fig. 1). The soil samples were dried, and coarse particles (diameters > 2 mm) were removed. The samples were then fractionated by dispersing them in water and separating fractions with different settling velocities (Dietrich 1982; Tan et al. 2012; Zhang et al. 2017). The YYm sample was separated into fractions with particle diameters 4–8, 8–16, 16–32, 32–64, 64–100, 100–250, 250–500, and > 500 μm. The YYu-1 sample was separated into fractions with the same particle diameters less than 500 μm and also 500–1000 and 1000–2000 μm. The pellets of soil samples pressed in plastic rings for different fractions are shown in Fig. 1.
2.2 Analytical Methods
The chemical compositions of the sample fractions were determined using a Niton XL 3 t-900S-M portable X-ray fluorescence spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The fractions were also subjected to PXRD analysis using Cu Kα1 radiation (40 kV, 200 mA) using a Rigaku SmartLab X-ray diffractometer with a D/tex Ultra detector (Rigaku, Tokyo, Japan) at the Geological Survey of Japan (Tsukuba, Japan). Each sample fraction was ground with an agate pestle and mortar to give a powder fine enough to avoid problems associated with micro-absorption. Silicon powder was used as an internal standard. A mixture of 0.5 g of a sample fraction and 0.5 g of silicon powder was mounted on a nonreflecting silicon plate. Whole patterns of 2θ values from 3 to 70° were acquired at 10°/min to allow the major constituent minerals being semi-quantitatively analyzed. Narrow patterns from 2θ values between 20 and 40° were acquired at 0.6°/min to allow chlorite, vermiculite, illite, quartz, plumbojarosite, galena, pyrite, sphalerite, and anatase being semi-quantitatively analyzed. The integrated intensities of chlorite (at ~ 6.2°), vermiculite (~ 6.2°), illite (~ 8.5°), quartz (~ 26.7°), plumbojarosite (~ 29.1°), galena (~ 30.7°), pyrite (~ 33.1°), sphalerite (~ 47.5°), and anatase (~ 53.9°) were used to perform the semi-quantitative analyses. The semi-quantitative results for the minerals in the YYm and YYu-1 samples were calculated assuming that the integrated intensity of the silicon standard (at ~ 32.0°) was 50.
The morphologies and chemical compositions of the minerals in the YYm and YYu-1 samples were investigated by SEM-EDX: Scanning electron microscopy (JSM-6610LV) with energy dispersive x-ray spectroscopy (Inca Energy 250X-Max50). It was difficult to polish the YYm and YYu-1 samples, so the samples were attached to double-sided sticky tape and coated with carbon before being analyzed by scanning electron microscopy with energy dispersive x-ray spectroscopy.
The YYm and YYu-1 samples were subjected to the Tessier sequential extraction procedure (Tessier et al. 1979; Filgueiras et al. 2002; Zimmerman and Weindorf 2010), and differences between the Pb extraction rates for different samples were calculated.
3 Results and Discussion
3.1 Concentration of Heavy Metals in Soil Samples
The Pb as well as As, Cu, and Zn contents in the YYm and YYu-1 fractions are shown in Table 1. The YYm sample had higher Pb, As, Cu, and Zn contents compared with the YYu-1 sample. The Pb contents in the YYm and YYu-1 samples were 1172–32,275 and 1296–5839 mg/kg, respectively. The particles of different sizes had different Pb, As, Cu, and Zn contents. Strong positive correlations were found between the Pb, As, Cu, and Zn contents and particle size for the YYm sample (Fig. 2), with coarser particles having higher Pb, As, Cu, and Zn contents. In contrast, the Pb and As contents were higher in the YYu-1 fractions with particle diameters < 32 μm than in the fractions with larger particle diameters (Table 1 and Fig. 2). These results implied that assuming contaminants being predominantly adsorbed to fine particles is not always the case, and the YYm and YYu-1 samples contained different minerals that host As and Pb.
3.2 Mineralogical Compositions
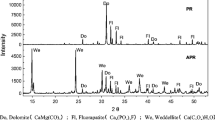
The PXRD patterns and semi-quantitative analysis result for each sample fraction are shown in Fig. 3 (YYm, from 25 to 50°; YYu-1, from 25 to 35°) and Table 2. The YYm samples contained chlorite, galena, illite, pyrite, quartz, and sphalerite, and the YYu-1 samples contained anatase, illite, plumbojarosite, pyrite, quartz, and vermiculite. The YYm samples contained large amounts of sulfide minerals (e.g., sphalerite). In contrast, hardly any sulfide was detected in the YYu-1 samples, but the YYu-1 samples contained sulfate minerals (e.g., plumbojarosite). The Pb and Zn contents of the YYm fractions strongly correlated with the galena and sphalerite contents determined by PXRD (Fig. 4). The Pb and As contents of the YYu-1 fractions positively correlated with the plumbojarosite contents. These results suggested that the main Pb-hosting minerals in the YYm and YYu-1 samples were galena and plumbojarosite, respectively.
The results of scanning electron microscopy with energy dispersive x-ray spectroscopy observations and chemical analyses are shown in Fig. 5 and Table 3, respectively. Galena, pyrite, and sphalerite (sulfide minerals) crystals were found in YYm particles with diameters > 64 μm, and plumbojarosite (a sulfate mineral) was found in the YYu-1 sample with diameters < 32 μm. Arsenic was detected in some of the plumbojarosite crystals. These data agreed with the PXRD data.
3.3 Mechanisms for Different Behaviors between Two Samples
Pb and As can be leached from primary sulfides during weathering and recrystallize as secondary hydrous sulfates, hydroxides, and carbonates in the oxidized zones (as gossan) of sulfide deposits (Jeong and Lee 2003). In particular, jarosite is a common secondary mineral in the oxidized zones of sulfide deposits (Moon et al. 2013). Jarosite contains traces of Pb released from the decomposition of primary sulfides, and other minerals such as beudantite (PbFe3(AsO4)(SO4)(OH)6) and plumbojarosite (PbFe6(SO4)4)(OH)12) which contain large amounts of Pb (Boyle 1993). We concluded that primary sulfides in the area from which the YYm sample was collected decomposed and were recrystallized to be plumbojarosite in the area from which the YYu-1 sample was collected.
The Pb results for the Tessier sequential extractions are shown in Table 4. The 1 M sodium acetate extraction removed 21% and 65% of the Pb from the YYm and YYu-1 samples, respectively, suggesting that most of the Pb in the YYu-1 sample was ion-exchangeable but most of the Pb in the YYm sample was not ion-exchangeable. Plumbojarosite is a Pb analog of jarosite, the K in jarosite being replaced by Pb. The Pb in plumbojarosite in the YYu-1 sample would therefore have been able to be removed by 1 M sodium acetate through ion exchange.
3.4 Applicability of the Approach Proposed in this Study
Various on-site techniques such as “dig and haul,” surface capping, and wet sieving (or grain-size sorting) have been used to remediate heavy metal contamination of soils (Futami 2007). “Dig and haul” and surface capping lead to problems in terms of the changes in land functions. Grain-size sorting simply separates contaminated soil into highly contaminated and less-contaminated fractions. Soil particles with diameters < 75 μm are generally treated as contaminated (Narabe et al. 2007), assuming that fine particles (e.g., clay minerals) strongly adsorb heavy metals (Kumpiene et al. 2017). Our results illustrated that this is not always the case, especially for naturally polluted soils such as the YYm sample. The Tessier sequential extraction procedure allows the speciation of a heavy metal in soil to be divided into exchangeable, carbonate-bound, iron- and manganese-oxide-bound, organic-matter-bound, and residual fractions (Carapeto and Purchase 2000). Only exchangeable heavy metal ions can be removed by washing soil. In EK extraction, application of a direct electric current causes oxidation at the anode, and an acid front is generated (Acar and Alshawabkeh 1993). The decrease in the pH can accelerate heavy metal leaching from contaminated soil and remove most of the heavy metal bound to carbonates. EK extraction can therefore remove both exchangeable heavy metals and carbonate-bound heavy metals. Contaminated soils, especially naturally contaminated soils, are generally affected by complex geological process, so it is essential to characterize the forms of contaminants present in polluted soils.
The YYu-1 fractions with diameters < 32 μm had high Pb contents (Table 1 and Fig. 2), but the YYm fractions with diameters > 64 μm had high Pb contents. Removing particles with diameters < 75 μm by commonly used grain-size sorting technique cannot therefore be used to remove contaminants contained in large diameter particles. If the grain-size sorting, or wet sieving technology, is considered for treating a naturally polluted soil contaminated with heavy metals, it is suggestible to characterizing the particle size-and-mineral-dependent concentrations of targeted heavy metals in advance using the approach proposed in this study that combines sedimentary hydro-classification with semi-quantitative powder X-ray diffraction analysis. In addition, the use of sequential extraction is also helpful for detailed understanding of the speciation and leaching properties of targeted heavy metals from a soil. In other words, characterization of the bearing minerals of heavy metal contaminants present in soils is key to selecting an appropriate remedial technique and/or designing a remediation process.
4 Conclusions
Accurately characterizing the Pb-bearing minerals (especially naturally occurring Pb) present in a polluted soil is essential to successful remediation of the soil. The approach that combines sedimentary hydro-classification with semi-quantitative PXRD analysis (using silicon as a standard) proposed in this study was successfully applied to two samples collected from a naturally polluted area in Japan. The minerals that host Pb in the samples were clearly identified in the fractions with different particle diameters. Contaminants, such as toxic Pb, are not necessarily contained in fine particles as assumed in the grain-size sorting technique. Cautions should be excised especially when treating naturally polluted soils. We are planning further studies to allow the method described here to be used in practice.
References
Acar, Y. B., & Alshawabkeh, A. N. (1993). Principles of electrokinetic remediation. Environmental Science & Technology, 27(13), 2638–2647.
Acar, Y. B., Gale, R. J., Akram, G., Alshawabkeh, N., Marks, R. E., Puppala, S., Bricka, M., & Parker, R. (1995). Electrokinetic remediation: Basics and technology status. Journal of Hazardous Materials, 40, 117–137.
Alaboudi, K. A., Ahmed, B., & Brodie, G. (2018). Phytoremediation of Pb and cd contaminated soils by using sunflower (Helianthus annuus) plant. Annals of Agricultural Sciences, 63, 123–127.
Alaboudi, K. A., Ahmed, B., & Brodie, G. (2020). Soil washing technology for removing heavy metals from a contaminated soil: A case study. Polish Journal of Environmental Studies, 29(1), 1–8.
Anderson, R., Rasor, E., & Ryn, F. V. (1999). Particle size separation via soil washing to obtain volume reduction. Journal of Hazardous Materials, 66(1), 89–98.
Boyle, D. R. (1993). Oxidation of massive sulfide deposits in the Bathurst mining camp, New Brunswick: Natural analogues for acid drainage in temperate climates. In N. A. Charles & D. W. Blowes (Eds.), Environmental geochemistry of sulfide oxidation (pp. 535–550). American Chemical Society, Washington DC: ACS Symposium Series.
Carapeto, C., & Purchase, D. (2000). Use of sequential extraction procedures for the analysis of cadmium and lead in sediment samples from a constructed wetland. Bulletin of Environmental Contamination and Toxicology, 64, 51–58.
Dietrich, W. R. (1982). Settling velocity of natural particles. Water Resources Research, 18(6), 1615–1626.
Filgueiras, A. V., Lavilla, I., & Bendicho, C. (2002). Chemical sequential extraction for metal partitioning in environmental solid samples. Journal of Environmental Monitoring, 4, 823–857.
Futami, T. (2007). Practical approach to remediating heavy metal-contaminated soil by grain sorting, gravity separation, and grain-surface attrition. Journal of Geography (Chigaku Zasshi), 116(6), 932–940 (In Japanese).
Isoyama, M., & Wada, S. I. (2007). Remediation of Pb-contaminated soils by washing with hydrochloric acid and subsequent immobilization with calcite and allophanic soil. Journal of Hazardous Materials, 143, 636–642.
Jeong, G. Y., & Lee, B. Y. (2003). Secondary mineralogy and microtextures of weathered sulfides and manganoan carbonates in mine waste-rock dumps, with implications for heavy-metal fixation. American Mineralogist, 88, 1933–1942.
Ji, H., Huang, W. Q., Xing, Z. X., Zuo, J. Q., Wang, Z. A., & Yang, K. (2019). Experimental study on removing heavy metals from the municipal solid waste incineration fly ash with the modified electrokinetic remediation device. Scientific Reports, 9, 8271.
Kumpiene, J., Nordmark, D., Carabante, I., Suziedelyte-Visockiene, J., & Aksamitauskas, V. C. (2017). Remediation of soil contaminated with organic and inorganic wood impregnation chemicals by soil washing. Chemosphere, 184, 13–19.
Liu, L., Li, W., Song, W., & Guo, M. (2018). Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Science of the Total Environment, 633, 206–219.
López-Vizcaíno, R., Navarro, V., León, M., Risco, C., Rodrigo, M. A., Sáez, C., & Cañizares, P. (2016). Scale-up on electrokinetic remediation: Engineering and technological parameters. Journal of Hazardous Materials, 315, 135–143.
Manca, P. P., Caredda, P., & Orru, G. (2018). The applicability of soil flushing technology in a metallurgical plant. International Journal of Coal Science & Technology, 5(1), 70–77.
Moon, Y., Zhang, Y. S., Sonn, Y. K., Hyun, B. K., Song, Y., & Moon, H. S. (2013). Mineralogical characterization related to physico-chemical conditions in the pyrite-rich tailings in Guryong mine, Korea. Soil Science and Plant Nutrition, 59, 509–521.
Narabe, Y., Kawakami, S., & Kawabe, L. (2007). Improvements of particle sorting and reduction of contamination for soil washing. Proceedings of 13th symposium on groundwater and soil contamination and their countermeasures, 567–563 (in Japanese).
Reddy, K., & Cameselle, C. (2009). Electrochemical remediation Technologies for Polluted Soils. John Wiley & Sons, Hoboken, NJ: Sediments and Groundwater.
Tan, X. L., Zhang, G. P., Yin, H., Reed, A. H., & Furukawa, Y. (2012). Characterization of particle size and settling velocity of cohesive sediments affected by a neutral exopolymer. International Journal of Sediment Research, 27, 473–485.
Tessier, A., Campbell, P. G. C., & Bisson, B. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51, 844–885.
United States Environmental Protection Agency (US EPA). (1991). Treatment of lead-contaminated soils. United States Environmental Protection Agency, Washington, DC, EPA540/2-91/009.
Yoo, J. C., Park, S. M., Yoon, G. S., Tsang, D. C. W., & Baek, K. (2017). Effects of lead mineralogy on soil washing enhanced by ferric salts as extracting and oxidizing agents. Chemosphere, 185, 501–508.
Zhang, N., Zhu, W., He, H. T., Lv, Y. Y., & Wang, S. W. (2017). Experimental study on settling velocity of soil particles in dredged slurry. Marine Georesources & Geotechnology, 35(6), 747–757.
Zimmerman, A. J., & Weindorf, D. C. (2010). Heavy metal and trace metal analysis in soil by sequential extraction: A review of procedures. International Journal of Analytical Chemistry, 2010, Article ID 387803, 1–7.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoshino, M., Zhang, M., Suzuki, M. et al. Characterization of Pb-Bearing Minerals in Polluted Soils from Closed Mine Sites. Water Air Soil Pollut 231, 176 (2020). https://doi.org/10.1007/s11270-020-04548-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04548-4