Abstract
The impact of cerium oxide nanoparticles, bulk cerium oxide and ionic cerium nitrate on the plant development as well as the uptake and further translocation of Cu, Mn, Zn and Fe by sugar pea (Pisum sativum L.) was investigated. Plants were cultivated in the laboratory pot experiments using the modified Hoagland solutions supplemented with cerium compounds at the 200 mg L−1 Ce level. Analysis of variance proved that cerium oxide nanoparticles significantly decreased Cu, Mn, Zn and Fe concentrations in roots and above ground parts of the pea plants. The latter ions are presumably transported via symplastic pathways and may compete with nanoparticles for similar carriers. The lowest impact on the plant growth and the metal uptake was observed under the bulk CeO2 treatment. On the contrary, strongest interactions were observed for supplementation with ionic cerium nitrate. The highly beneficial effect of cerium oxide nanoparticles on the plant growth was not supported by this study. The latter conclusion is of particular relevance when environmental impact of cerium compounds on the waste management, municipal urban low emissions and food production is to be concerned.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rapid development of nanoscience and nanotechnology prompted wide application of nanoparticles (NPs) in technology, medicine and agriculture. They are employed in a number of products including electronic devices, advanced fuels, textiles, paintings and coatings, personal care products, pharmaceuticals and agrochemicals (Tsazuki 2009; Hua et al. 2012; De Almeida et al. 2014; Dasgupta et al. 2015; Vance et al. 2015; Rai et al. 2018; Rajeshkumar and Naik 2018; Consumer Product Inventory 2018). These widespread applications make nanoparticles increasingly abundant in the environment. Unfortunately, this amazing progress is not free from risks related to toxicity and environmental fate of nanomaterials (NMs) (Lead and Smith 2009; Peralta-Videa et al. 2011; Kamali et al. 2019). In particular, the realistic assessment of their impact on the environment cannot be made without proper estimation of their production worldwide. Unfortunately, available data are usually based on estimates and forecasts only (Aitken et al. 2006; Hendren et al. 2011; Holden et al. 2014; Keller and Lazareva 2014). Furthermore, nanomaterials are released to environment as objects of diverse structure and toxicity, starting from the rather simply isolated particles and ending up at complex entities with nanoparticles embedded into matrix elements.
Nowadays, cerium dioxide nanoparticles are among the most widely used in technology (Ma et al. 2015) with forecasts indicating their continuously increasing importance for medicine and energy production (Das et al. 2013; Montini et al. 2016). According to the highly respected survey by Piccinno et al. (2012) the global annual production of cerium oxide nanomaterials has approached 1000 tonnes in 2012. However, the European Commission estimates are around 10,000 tonnes (European Commission, Commission Staff Working Paper 2012). So far, the cerium-based nanomaterials have found numerous applications as efficient electrolyte additives for solid oxide fuel cells (Monteiro-Riviere and Orsière 2007), abrasives for chemical-mechanical planarization of computer chips (Zantye et al. 2004; Kim et al. 2011), cosmetics additives (Zholobak et al. 2014), nanosensors (Maduraiveeran et al. 2018; Olgun et al. 2018) and in advanced water treatment technologies (Recillas et al. 2010). However, the highest impact on environment will presumably follow from the increasing usage of cerium dioxide as a diesel fuel additive. It significantly improves the overall efficiency of engines and reduces emission of the harmful carbon monoxide by its oxidation to carbon dioxide. The latter was confirmed in tests on coaches exploited in a normal traffic which unequivocally proved that cerium oxide lowered ultrafine particles emissions from the diesel exhaust (Park 2007; Lead and Smith 2009). Therefore, the cerium levels in soils near roads would also increase as a result of ambient particle deposition (Gottschalk and Nowack 2011; Cao et al. 2017).
Nanomaterials containing cerium dioxide are among the contaminants of emerging concern (Sauvé and Desrosiers 2014; Noguera-Oviedo and Aga 2016). Like many hazardous materials, ceria nanoparticles are often not embedded in a surrounding matrix and may be prone to migrations. They readily interact with all components of the environment. Their occurrence is widespread but influence on living organisms still needs to be evaluated. The relevant literature data are quite contradictory with emphasis put either on positive or negative influence of CeO2 NPs on human health and plant development (Achari and Kowshik 2018; Dimkpa 2014; Pošćić et al. 2017; Hussain et al. 2019). The continuously growing production, widespread use and resistance to abundant waste treatment technologies addresses the issue of surplus nanomaterial storage and waste management.
NPs approach plants through variety of mechanisms which are strongly dependent on their size, morphology, charge, settings and agglomeration (Pérez-de-Luque 2017; Yang et al. 2017; Zhang et al. 2019). All those factors affect the plant response and need to be carefully evaluated. Unfortunately, environmental impact is often biased by the methodology of a particular experiment (Paterson et al. 2011). Especially, Kabir et al. (2018) in their recent review on environmental impact of nanomaterials clearly indicate that the toxic effects of NMs should be assessed by more objective means. The latter should fully ensure high comparability and transferability of results. This issue presents a real challenge as numerous experimental conditions are combined with diverse plant species and types of NPs (Majumdar et al. 2014). Therefore, divergent methodologies can be substantially biased. This problem has been clearly illustrated by Montes et al. (2017) in the review on the phytotoxicity of diverse NPs as administered to Arabidopsis thaliana. The authors approached several difficulties related to incomparability of results published by different investigators. In the conclusion, they suggested that model plants should be subjected to tests with NPs of standardized concentrations and sizes. This approach is of particular relevance when risk assessment is to be concerned (Layet et al. 2017; Núñez and de la Rosa-Alvarez 2018).
Usually, two major methodologies are being applied for evaluation of the NPs impact on plants. The more popular one relies on the long-term growth in soils supplemented with representative concentrations of NPs. Nonetheless, the advantages of soilless-hydroponic cultivation has also been recognized (Deng et al. 2014). The latter is well suited for studying the NPs’ outcome on plants with distinct advantages over the traditional soil systems. It facilitates prompt separation of root tissues with a special emphasis put on fine root hairs and precise administration of nutrients. In particular, plants grown in controlled homogeneous liquid solution are more uniform and give statistically significant, reproducible results (Nguyen et al. 2016).
Green peas are among the most extensively used legumes worldwide. The latter are valuable source of proteins, essential amino acids, vitamins and minerals. Since ancient Greek times, they have been extensively employed in traditional nutrition strategies (Nikolopoulou et al. 2007). The global green pea production is steadily growing over the years and recently approached 20 million tonnes (Food and Agriculture Organization of the United Nations, FAOSTAT 2018). They are often grown on heavily polluted soils and therefore understanding of the mechanism and ways by which metals enter the plant body is of general interest. Pea is an important nonmodel plant in a postgenomic era of applied system biology (Kulaeva et al. 2017). It has been an object of experimental work well before Mendel’s genetic discoveries (Smýkal 2014). Pea genetic diversity is a challenge for genome studies. Nevertheless, based on the analysis of the transcriptome resources, the pea can now be treated as a biochemical model plant with near-complete transcript coverage regarding different aerial tissues (Franssen et al. 2011).
In this paper, the effect of cerium oxide nanoparticles on physiological changes and uptake of copper, zinc, manganese and iron by sugar pea plants cultivated in the model hydroponic pot experiments in modified Hoagland solutions is reported. The latter four elements are critical for plant growth and photosynthesis (Pakrasi et al. 2004; Hänsch and Mende 2009). Plant response induced by the uptake in the accumulation of cerium oxide NPs was recently reported for Brassica napus L., Helianthus annuus L., Raphanus sativus L., Phaselous vulgaris and Oryza sativa L. by Rossi et al. (2017a), Tassi et al. (2017), Zhang et al. (2017), Salehi et al. (2018) and Ramírez-Olvera et al. (2018), respectively. However, to our knowledge, this is the first experimental work on the heavy metal uptake by pea originated by emerging nanopollutants. This work follows our ongoing investigations on metal migration strategies in plants initiated by chemical stressors of the environmental, waste management and agricultural concerns (Adamczyk-Szabela et al. 2015; Adamczyk-Szabela et al. 2017; Skiba et al. 2017; Skiba and Wolf 2017).
2 Materials and Methods
2.1 Cerium Compounds
CeO2 (99.995%) in a bulk, cubic form (as confirmed by the powder X-ray diffraction technique) and Ce (NO3)3·6H2O (99.999%) were purchased from Sigma Aldrich and used without purification. Commercially available cerium oxide nanoparticles (Byk, Additives & Instruments) were obtained from the manufacturer as a liquid dispersion stabilized by ammonium citrate. Transmission electron microscopy was used to evaluate the structure, shape and size of cerium oxide nanoparticles. Ten microliters of sample was placed on 200-mesh copper grids with carbon surface, washed in deionized water and dried at room temperature. Images were collected with the JEOL-1010 instrument (Su 2017), the average particle size was 25.8 ± 13.9 nm (Fig. 1).
The abbreviations Ce-b, Ce-n and Ce-i for bulk form of CeO2, the nanoparticles of CeO2 and Ce (NO3)3, respectively, are used in the following text.
2.2 Experimental Setup
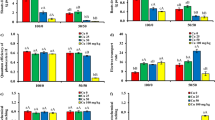
Iłówiecki sugar pea (Pisum sativum L.) quality class A seeds from “PNOS” Co. Ltd., Ożarów Mazowiecki were used in the study. Four series, each contained six pots with ten plants, were applied. Seeds were surface sterilized with 70% ethanol for 10 min and rinsed extensively with distilled water. They were placed on a moistened filter paper in Petri dishes to germinate in darkness for 3 days at 22 °C. At this point, their average stage of growth was 09 according to the BBCH scale (Meier 2018). Seedlings were further hydroponically grown for 4 days at 21 °C on aerated Hoagland solution containing KNO3 (0.51 g L−1), Ca (NO3)2·4H2O (1.18 g L−1), MgSO4·7H2O (0.49 g L−1), KH2PO4 (0.14 g L−1), H3BO3 (0.6 mg L−1), MnCl2·4H2O (0.4 mg L−1), ZnSO4·7H2O (0.05 mg L−1), CuSO4·5H2O (0.05 mg L−1), FeEDTA (10.28 mg L−1) and Na2MoO4·2H2O (0.02 mg L−1) at pH 5.9; the medium was used without further sterilization. The light intensity of 170 μE/m2s, 16/8 h day/night photoperiod was applied and the growth medium was changed twice every 48 h. Subsequently, three series were administered with 750 mL of the medium per pot and supplemented with bulk cerium oxide, cerium oxide nanoparticles and Ce (NO3)3 at the Ce concentration of 200 mg L−1, respectively. This concentration affects plants physiology but it is not lethal for pea. In fact, majority of studies on toxicity of CeO2 NPs to terrestrial plants use concentrations in the range 1–1000 mg/L−1 (Holden et al. 2014; Rossi et al. 2017b), while the phytotoxicity test as recommended by the Environmental Protection Agency, USA (Zhang et al. 2019) approach the 2000 mg L−1 level. The fourth series was a reference treated with original Hoagland solution. Liquid media were changed every 24 h. Plants were harvested after 12 days of cerium administration when (on average) they reached growth stage 15 at the BBCH scale. Shoots and roots were separated. The latter were carefully rinsed with deionized water and later gently dried with a filter paper. The lengths of roots and stems were carefully measured (Fig. 2). Weights of the fresh and dry (incubation at 55 °C to the constant weight) shoots and roots were determined (Figs. 3 and 4).
2.3 Determination of Metals in Pea Roots and Shoots
Plant samples (0.6 g shoots and 0.3 g roots) were digested in the mixture of concentrated HNO3 and HCl (6:1, v/v) using the Anton Paar Multiwave 3000 closed system instrument. The content of Cu, Zn, Fe and Mn was determined by the flame atomic absorption spectrometry (FAAS) with the GBC 932 plus spectrometer. Results are summarized in Table 1. The reliability of the analytical procedures was checked using certified references materials: INCT-MPH-2 (Dybczyński et al. 2004) and IAEA-V-10 (Reference sheet IAEA-V-10, 2000). Detailed numerical data are given in the Supplementary material (Table S1).
2.4 Statistical Analysis
A one-way analysis of variance (ANOVA) as implemented in OriginPro 2016 was used to test the influence of cerium compounds on heavy metal accumulation in sugar pea cultivated in Hoagland solution. The 0.95 probability level was applied. Detailed numerical data are given in the Supplementary material (Table S2).
2.5 Tolerance Indices, Transfer Coefficients, Bioaccumulation and Translocation Factors
The tolerance index (TI) was calculated as the ratio of an average root length for plants cultivated in cerium-induced stress conditions related to the respective root size in a raw reference treatment (Buendía-González et al. 2010) (Fig. S1). Metal uptake was evaluated by transfer coefficients (TCs) and bioaccumulation factors (BAFs). They are defined as ratios of particular element concentration in roots and shoots, respectively, related to its content in the growing medium (Galal and Shehata 2015; Chen et al. 2016; Rezania et al. 2016). Metal distribution inside the plant body was assessed by translocation factor (TF) which is the ratio of element concentration in above ground parts of the plant to that in roots (Shi and Cai 2009; Ha et al. 2011; Testiati et al. 2013; Xiao et al. 2015). Those factors give a better estimate of metal migration than the raw metal contents and should be evaluated altogether. This formalism was successfully applied by us for the pesticide-induced heavy metal uptake and further accumulation in wheat (Skiba et al. 2017; Skiba and Wolf 2017). TCs, TFs and BAFs computed for four series of cultures are presented in Fig. S2.
3 Results and Discussion
Copper, manganese, zinc and iron contents in roots and shoots of sugar pea plant cultivated in Hoagland solution supplemented with cerium compound of divergent constitutions are summarized in Table 1. As expected, plants grown in untreated control solution accumulate metals mostly in roots. Administration of cerium derivatives prompted decrease of almost all metal contents. The major exception was observed for zinc which concentration distinctively increased in roots treated with the Ce-b and Ce-i. However, increase of the zinc content in shoots was firmly observed for plants treated with the Ce-b only. In view of the well-recognized low solubility of cubic CeO2 (Dahle and Arai 2015), this result is quite unexpected indeed. We speculate that zinc accumulation in roots is a thermodynamically driven process initiated by the release of small quantities of hydrated CeO2 particles from the solid phase of Ce-b. In solution, they exist as a hydrated micellar structure which may be prone to ionic zinc adsorption. These entities may work as zinc molecular carries. They can enter the plant root tissue through the well-recognized mechanisms like capillary forces, pores on cell walls, intercellular plasmodesmata or highly regulated symplastic route (Zhang et al. 2011). Decrease of zinc concentration in roots treated with Ce-n indicates that the transfer ability of CeO2 NPs acting as “Trojan horse” carriers show limited significance in the studied system (Du et al. 2018; Naasz et al. 2018; Rossi et al. 2018). Similar effects were reported by Ebbs et al. (2016) who observed that carrot displayed significantly less accumulation of Zn, Cu and Ce from respective ENPs than from the ionic forms of particular element.
Once in the plant body, zinc ions are further stabilized by several processes of which those involving phytochelatins are generally acknowledged (Prasad and Hagemeyer 1999; Rauser 1999; Viehweger 2014). However, other mechanisms such as complexation with organic acids have been also identified (Fontes et al. 2014). Iron and manganese are important elements essential for the proper plant growth. Their mutual interactions are widely recognized indeed (Chatzistathis 2014). According to Kabata-Pendias (2011) and Dias et al. (2009) the Fe/Mn concentration ratio should be in the range 1.5 to 2.5. In this work, the largest alterations were observed for plants treated with Ce-i (16.6 and 7.0 for roots and shoots, respectively), while values lower than 3.0 were for determined for other treatments (Table S3).
The root and shoot lengths accompanied by corresponding fresh and dry weights are summarized in Figs. 2, 3 and 4, respectively. All cerium supplementations decreased the root lengths and partially affected green parts of the plant (Figs. 2 and S1). The most pronounced effect was observed for Ce-i which practically suppressed the root growth. However, the green parts of the plant responded in a more diverse way. The Ce-b did not influence the shoot lengths, while Ce-n increased them by 5%. The ionic cerium inhibited the plant growth essentially. The latter was also reflected by the characteristic pattern of plant weights with the lowest being observed for the Ce-i treatment.
The heavy metal uptake of Pisum sativum L. plants was evaluated with the transfer coefficients augmented with bioaccumulation and translocation factors as summarized in Fig. S2. TCs for plants cultivated in the reference, untreated Hoagland solution are in the order Zn>Mn>Cu>Fe. Supplementation of the growth media with the Ce-b did not affect this arrangement, while the administration with Ce-n interchanged that order for copper and manganese, only. Application of the Ce-i led to the order Zn>Cu>Fe>Mn. A visible reduction of all TCs as induced by the Ce-n treatment indicated that in the Hoagland medium environment the solution-root transfer was significantly restricted. The bioconcentration of metals in shoots was evaluated by BAFs. Surprisingly, they were ordered in the same way over all treatments including control (Zn>Cu>Mn>Fe). The largest BAF reduction was observed for Mn upon the Ce-i administration.
Migration of metals in the plant body may be easily examined with the translocation factor. The average TFs are shown in Fig. S2. All values except that of manganese subjected to Ce-i (1.28) were lower than unity. Translocation factors for the reference treatment were in the order Cu>Zn>Mn ≈ Fe. The Ce-b and Ce-n treatments interchanged the manganese position in that order, while the Ce-i administration changed the sequence as follows: Mn>Cu>Fe>Zn.
The influence of cerium compounds on heavy metal accumulation by plants was evaluated by one-way ANOVA at the 0.95 probability level (Fig. 5). Detailed numerical data are given in the Supplementary material (Table S2). The null hypothesis was whether cerium compounds influence the metal transfer from Hoagland solution and their further accumulation in plant. Calculations clearly showed that all cerium compounds affected heavy metal transfer. However, only Ce-n application resulted in a decrease of all metal concentrations in the plant.
The influence of cerium compounds on copper, manganese, zinc and iron accumulation in roots (a) and shoots (b) of pea cultivated in Hoagland solution as evaluated by one-way ANOVA at the 0.95 probability level. Grey colour shows combination for which the average metal concentration in a plant tissue increases after the cerium compound treatment. Black colour represents a decrease of respective metal concentration while white indicates no change
The lowest impact on the plant growth and water content as well as copper, manganese and iron uptake was observed under the Ce-b treatment. This supplement facilitated only zinc transport and accumulation. The fully soluble ionic Ce-i was the most toxic to the plant leading to the highest biomass and in parallel, also to the water content reduction (Table S4). Similar to the Ce-b treatment, the increase of zinc content in roots was observed. The Ce-n prompted significant decrease of concentrations for all investigated metals either in roots or shoots. Interestingly, this treatment affected the root size and increased the stem lengths above the control level. The metal uptake by roots may proceed via nonselective apoplastic or selective symplastic mechanisms (Hanć et al. 2016; Edelstein and Ben-Hur 2018). The latter is as an energy-consuming transmembrane pathway strongly dependent on metal transporter proteins (Dalir et al. 2017). On the contrary, the apoplastic bypass, sometimes referred as a passive pathway, is correlated with transpiration (Qiu et al. 2012). In this study, we firmly observed a decrease of copper, manganese, zinc and iron concentrations in roots upon cerium oxide nanoparticle treatment. We speculate that the latter ions are transported via symplastic pathways and compete with Ce-n for similar carriers. Those mechanisms depend on the carrier concentration which is strictly related to the rate of a particular protein synthesis. According to Ma et al. (2016), cerium oxide NPs tend to alter regulation of genes which are responsible either for encoding metal ion transporters or activity of a distinct enzyme. In particular, low accumulation of Fe can be related to downregulation of IRT1 and IRT2 iron–regulating genes induced by the Ce-n toxicity. Similar mechanisms developed by plants to avoid the harmful effects of nanoparticles and involving genes of the IRT family for Cd, Cu, Zn, Co and Mn were also reported (Taylor et al. 2014).
4 Conclusions
Our results unequivocally show that cerium compounds at any molecular, ionic or nanosize levels alter Cu, Mn, Zn and Fe uptake and their further migration in Pisum sativum L. The latter metals have been found in photosynthetic electron transport complexes (Yruela 2013) and may be used as rough photosynthesis indicators (Sheoran and Singh 1996). The strongest influence was found for plants cultivated in Hoagland solutions supplemented with Ce-i and Ce-n. This conclusion is of particular importance when plants are cultivated on soils subjected to steadily growing and unrestricted cerium dioxide emissions as originated from popular diesel fuel additives. Complex interactions which restrain heavy metal uptake in either normal or stress conditions are important indicators of the metals migration mechanisms. They are of special relevance when environmental impact of cerium compounds on the waste management, municipal urban low emissions and food production is to be concerned.
References
Achari, G. A., & Kowshik, M. (2018). Recent developments on nanotechnology in agriculture: plant mineral nutrition, health, and interactions with soil microflora. Journal of Agricultural and Food Chemistry, 66, 8647–8661. https://doi.org/10.1021/acs.jafc.8b00691.
Adamczyk-Szabela, D., Markiewicz, J., & Wolf, W. M. (2015). Heavy metal uptake by herbs. IV. Influence of soil pH on the content of heavy metals in Valeriana officinalis L. Water, Air & Soil Pollution, 226(4), 106–114. https://doi.org/10.1007/s11270-015-2360-3.
Adamczyk-Szabela, D., Romanowska-Duda, Z., Lisowska, K., & Wolf, W. M. (2017). Heavy metal uptake by herbs. V. Metal accumulation and physiological effects induced by Thiuram in Ocimum basilicum L. Water, Air & Soil Pollution, 228(9), 334–348. https://doi.org/10.1007/s11270-017-3508-0.
Aitken, R. J., Chaudhry, M. Q., Boxall, A. B. A., & Hull, M. (2006). Manufacture and use of nanomaterials: current status in the UK and global trends. Occupational Medicine, 56, 300–306. https://doi.org/10.1093/occmed/kql051.
Buendía-González, L., Orozco-Villafuerte, J., Cruz-Sosa, F., Barrera-Díaz, C. E., & Vernon-Carter, E. J. (2010). Prosopis laevigata a potential chromium (VI) and cadmium (II) hyperaccumulator desert plant. Bioresource Technology, 101, 5862–5867. https://doi.org/10.1016/j.biortech.2010.03.027.
Cao, Z., Stowers, C., Rossi, L., Zhang, W., Lombardini, L., & Ma, X. (2017). Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (Glycine max (L.) Merr.). Environmental Science: Nano, 4, 1086–1094. https://doi.org/10.1039/C7EN00015D.
Chatzistathis, T. (2014). Plant responses to iron, manganese, and zinc deficiency stress. In P. Ahmad & S. Rasool (Eds.), Emerging technologies and management of crop stress tolerance: volume 1 - biological techniques (pp. 293–311). New York: Elsevier.
Chen, H., Yuan, X., Li, T., Hu, S., Ji, J., & Wang, C. (2016). Characteristics of heavy metal transfer and their influencing factor in different soil-crop systems of the industrialization region, China. Ecotoxicology and Environmental Safety, 126, 193–201. https://doi.org/10.1016/j.ecoenv.2015.12.042.
Consumer Product Inventory 2018. An inventory of nanotechnology-based consumer products introduced on the market. http://www.nanotechproject.org/cpi/. Accessed 31 May 2019.
Dahle, J. T., & Arai, Y. (2015). Environmental geochemistry of cerium: applications and toxicology of cerium oxide nanoparticles. International Journal of Environmental Research and Public Health, 12, 1252–1278. https://doi.org/10.3390/ijerph120201253.
Dalir, N., Khoshgoftarmanesh, A. H., Massah, A., & Shariatmadari, H. (2017). Plasma membrane ATPase and H+ transport activities of microsomal membranes from wheat roots under Ni deficiency conditions as affected by exogenous histidine. Environmental and Experimental Botany, 135, 56–62. https://doi.org/10.1016/j.envexpbot.2016.12.009.
Das, S., Dowding, J. M., Klump, K. E., McGinnis, J. F., Self, W., & Seal, S. (2013). Cerium oxide nanoparticles: applications and prospects in nanomedicine. Nanomedicine-UK, 8(9), 1483–1508. https://doi.org/10.2217/nnm.13.133.
Dasgupta, N., Ranjan, S., Mundekkad, D., & Ramalingman, C. (2015). Nanotechnology in agro-food: from field to plate. Food Research International, 69, 381–400. https://doi.org/10.1016/j.foodres.2015.01.005.
De Almeida, M. P., Pereira, E., Baptista, P., Gomes, I., Figueiredo, S., Soares, L., et al. (2014). Gold nanoparticles as (bio) chemical sensors. In M. Valcárcel & A. I. López-Lorente (Eds.), Comprehensive analytical chemistry (pp. 529–567). Elsevier: Amsterdam. https://doi.org/10.1016/B978-0-444-63285-2.00013-4.
Deng, Y.-Q., White, J. C., & Xing, B.-S. (2014). Interactions between engineered nanomaterials and agricultural crops: Implications for food safety. Journal of Zhejiang University-Science A, 15(8), 552–572.
Dias, A. S., Lidon, F. C., & Ramalho, J. C. (2009). IV. Heat stress in Triticum: kinetics of Fe and Mn accumulation. Brazilian Journal of Plant Physiology, 21(2), 153–164. https://doi.org/10.1590/S1677-04202009000200008.
Dimkpa, C. O. (2014). Can nanotechnology deliver the promised benefits without negatively impacting soil microbial life? Journal of Basic Microbiology, 54, 889–904. https://doi.org/10.1002/jobm.201400298.
Du, W., Xu, Y., Yin, Y., Ji, R., & Guo, H. (2018). Risk assessment of engineered nanoparticles and other contaminants in terrestrial plants. Current Opinion in Environmental Science & Health, 6, 21–28. https://doi.org/10.1016/j.coesh.2018.07.010.
Dybczyński, R., Danko, B., Kulisa, K., Maleszewska, E., Polkowska-Motrenko, H., Samczyński, Z., et al. (2004). Preparation and preliminary certification of two new Polish CRMs for inorganic trace analysis. Journal of Radioanalytical and Nuclear Chemistry, 259(3), 409–413.
Ebbs, S. D., Bradfield, S. J., Kumar, P., White, J. C., Musante, C., & Ma, X. (2016). Accumulation of zinc, copper, or cerium in carrot (Daucus carota) exposed to metal oxide nanoparticles and metal ions. Environmental Science: Nano., 3, 114–126. https://doi.org/10.1039/c5en00161g.
Edelstein, M., & Ben-Hur, M. (2018). Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops. Scientia Horticulturae-Amsterdam, 234, 431–444. https://doi.org/10.1016/j.scienta.2017.12.039.
European Commission, Commission Staff Working Paper: Types and uses of nanomaterials, including safety aspects, https://ec.europa.eu/nanotechnology/index_en.html, 2012 https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52012SC0288&from=EN. Accessed 31 May 2019.
Fontes, R. L. F., Pereira, J. M. N., & Neves, J. C. L. (2014). Uptake and translocation of Cd and Zn in two lettuce cultivars. Anais da Academia Brasileira Ciencias, 86(2), 907–922. https://doi.org/10.1590/0001-37652014117912.
Food and Agriculture Organization of the United Nations, FAOSTAT. Food and Agricultural Commodities Production. http://www.fao.org/faostat/en/#data/QC/visualize/, 2018. Accessed 31 May 2019.
Franssen, S. U., Shrestha, R. P., Bräutigam, A., Bornberg-Bauer, E., & Weber, A. P. M. (2011). Comprehensive transcriptome analysis of the highly complex Pisum sativum genome using next generation sequencing. BMC Genomics, 12, 227. https://doi.org/10.1186/1471-2164-12-227.
Galal, T. M., & Shehata, H. S. (2015). Bioaccumulation and translocation of heavy metals by Plantago major L. grown in contaminated soils under the effect of traffic pollution. Ecological Indicators, 48, 244–251. https://doi.org/10.1016/j.ecolind.2014.08.013.
Gottschalk, F., & Nowack, B. (2011). The release of engineered nanomaterials to the environment. Journal of Environmental Monitoring, 13, 1145–1155. https://doi.org/10.1039/c0em00547a.
Ha, N. T. H., Sakakibara, M., Sano, S., & Nhuan, M. T. (2011). Uptake of metals and metalloids by plants growing in a lead–zinc mine area, Northern Vietnam. Journal of Hazardous Materials, 186, 1384–1391. https://doi.org/10.1016/j.jhazmat.2010.12.020.
Hanć, A., Małecka, A., Kutrowska, A., Bagniewska-Zadworna, A., Tomaszewska, B., & Barałkiewicz, D. (2016). Direct analysis of elemental biodistribution in pea seedlings by LA-ICP-MS, EDX and confocal microscopy: imaging and quantification. Microchemical Journal, 128, 305–311.
Hänsch, R., & Mende, R. R. (2009). Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, cl). Current Opinion in Plant Biology, 12, 259–266. https://doi.org/10.1016/j.pbi.2009.05.006.
Hendren, C. O., Mesnard, X., Dröge, J., & Wiesner, M. R. (2011). Estimating production data for five engineered nanomaterials as a basis for exposure assessment. Environmental Science and Technology, 45, 2562–2569. https://doi.org/10.1021/es103300g.
Holden, P. A., Klaessig, F., Turco, R. F., Priester, J. H., Rico, C. M., Avila-Arias, H., et al. (2014). Evaluation of exposure concentration used in assessing manufactured nanomaterial environmental hazards: are they relevant? Environmental Science and Technology, 48, 10541–10551. https://doi.org/10.1021/es502440s.
Hua, M., Zhang, S., Pan, B., Zhang, W., Lv, L., & Zhang, Q. (2012). Heavy metal removal from water/wastewater by nanosized metal oxides: a review. Journal of Hazardous Materials, 211-212, 317–331. https://doi.org/10.1016/j.jhazmat.2011.10.016.
Hussain, I., Singh, A., Singh, N. B., Singh, A., & Singh, P. (2019). Plant-nanoceria interaction: Toxicity, accumulation, translocation and biotransformation. South African Journal of Botany, 121, 239–247.
Kabata-Pendias, A. (2011). Trace elements in soils and plants. Boca Raton: CRC Press Taylor & Francis.
Kabir, E., Kumar, V., Kim, K.-H., Yip, A. C. K., & Sohn, J. R. (2018). Environmental impacts of nanomaterials. Journal of Environmental Management, 225, 261–271. https://doi.org/10.1016/j.jenvman.2018.07.087.
Kamali, M., Persson, K. M., Costa, M. E., & Capela, I. (2019). Sustainability criteria for assessing nanotechnology applicability in industrial wastewater treatment: current status and future outlook. Environment International, 125, 261–276. https://doi.org/10.1016/j.envint.2019.01.055.
Keller, A. A., & Lazareva, A. (2014). Predicted releases of engineered nanomaterials: from global to regional to local. Environmental Science & Technology Letters, 1, 65–70. https://doi.org/10.1021/ez400106t.
Kim, J-Y., Kim, U-S., Byeon, M-S., Kang, W-K., Hwang, K-T. Cho, W-S. (2011). Recovery of cerium from glass polishing slurry. Journal of Rare Earths, 29 (11), 1075–1078. doi:https://doi.org/10.1016/S1002-0721(10)60601-1.
Kulaeva, O.A., Afonin, A.M., Zhernakov, A.I., Tikhnonich, I. A., Zhukov, V.A. (2017). Transcriptomic studies in non-model plants: case of Pisum sativum L. and Medicago lupina L. In F. Marchi, P. Cirillo, E.C. Mateo (Eds.) Applications of RNA-Seq and omics strategies. From microorganisms to human health. (pp412–582). IntechOpen. doi.org/10.5772/intechopen.69057. Accessed 31 May 2019.
Layet, C., Auffan, M., Santaella, C., Chevassus-Rosset, C., Montes, M., Ortet, P., et al. (2017). Evidence that soil properties and organic coating drive the phytoavailability of cerium oxide nanoparticles. Environmental Science & Technology, 51, 9756–9764. https://doi.org/10.1021/acs.est.7b02397.
Lead, J. R., & Smith, E. (2009). Environmental and human health impact of nanotechnology. Chichester: Wiley.
Ma, C., White, J. C., Dhankher, O. P., & Xing, B. (2015). Metal-based Nanotoxicity and detoxification pathways in higher plants. Environmental Science & Technology, 49, 7109–7122. https://doi.org/10.1021/acs.est.5b00685.
Ma, C., Liu, H., Guo, H., Musante, C., Coskun, S. H., Nelson, B. C., et al. (2016). Defense mechanisms and nutrient displacement in Arabidopsis thaliana upon exposure to CeO2 and In2O3 nanoparticles. Environmental Science: Nano, 3, 1369–1379. https://doi.org/10.1039/C6EN00189K.
Maduraiveeran, G., Sasidharan, M., & Ganesan, V. (2018). Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosensors and Bioelectronics, 103, 113–129. https://doi.org/10.1016/j.bios.2017.12.031.
Majumdar, S., Peralta-Videa, J. R., Bandyopadhyay, S., Castillo-Michel, H., Hernandez-Vizcas, J.-A., Sahi, S., et al. (2014). Exposure of cerium oxide nanoparticles to kidney bean shows disturbance in the plant defense mechanisms. Journal of Hazardous Materials, 278, 279–287. https://doi.org/10.1016/j.jhazmat.2014.06.009.
Meier, U. (2018). Growth stages of mono- and dicotyledonous plants: BBCH monograph. Quedlinburg, Julius Kühn-Institut (JKI): Open Agrar RepositoriumU.
Monteiro-Riviere, N. A., & Orsière, T. (2007). Toxicological impacts of nanomaterials. In M. R. Wiesner & J.-Y. BotteroJ-Y (Eds.), Environmental nanotechnology: applications and impacts of nanomaterials (pp. 395–445). London: McGraw-Hill Education – Europe.
Montes, A., Bisson, M.A., Gardella Jr. J.A., Aga, D.S. (2017). Uptake and translocation of engineered nanomaterials: Critical responses observed in terrestrial plants and the model plant Arabidopsis thaliana. Science of the Total Environment, 607–608, 1497–1516. doi: https://doi.org/10.1016/j.scitotenv.2017.06.190.
Montini, T., Melchionna, M., Monai, M., & Fornasiero, P. (2016). Fundamentals and catalytic applications of CeO2-based materials. Chemical Reviews, 116, 5987–6041. https://doi.org/10.1021/acs.chemrev.5b00603.
Naasz, S., Altenburger, R., & Kühnel, D. (2018). Environmental mixtures of nanomaterials and chemicals: the Trojan-horse phenomenon and its relevance for ecotoxicity. Science of the Total Environment, 635, 1170–1181. https://doi.org/10.1016/j.scitotenv.2018.
Nguyen, N. T., McInturf, S. A., & Mendoza-Cózatl, D. G. (2016). Hydroponics: a versatile systems to study nutrient allocation and plant responses to nutrient availability and exposure to toxic elements. Journal of Visualized Experiments, 113, e54317. https://doi.org/10.3791/54317.
Nikolopoulou, D., Grigorakis, K., Stasini, M., Alexis, M. N., & Iliadis, K. (2007). Differences in chemical composition of field pea (Pisum sativum) cultivars: effects of cultivation area and year. Food Chemistry, 103, 847–852. https://doi.org/10.1016/j.foodchem.2006.09.035.
Noguera-Oviedo, K., & Aga, D. S. (2016). Lessons learned from more than two decades of research on emerging contaminants in the environment. Journal of Hazardous Materials, 316, 242–251. https://doi.org/10.1016/j.jhazmat.2016.04.058.
Núñez, E. V., & de la Rosa-Alvarez, G. (2018). Environmental behavior of engineered nanomaterials in terrestrial ecosystems: uptake, transformation and trophic transfer. Current Opinion in Environmental Science and Health, 6, 42–46.
Olgun, F. A. O., Üzer, A., Ozturk, B. D., & Apak, R. (2018). A novel cerium oxide nanoparticles – based colorimetric sensor using tetramethyl benzidine reagent for antioxidant activity assay. Talanta, 182, 55–61. https://doi.org/10.1016/j.talanta.2018.01.047.
Pakrasi, H., Ogawa, T., & Bhattacharrya-Pakrasi, M. (2004). Transport of metals: a key process in oxygenic photosynthesis. In E. M. Aro & B. Andersson (Eds.), Regulation of photosynthesis (pp. 253–262). Kluwer: New York.
Park, B. (2007). Current and future applications of nanotechnology. In R. E. Hester & R. M. Harrison (Eds.), Nanotechnology consequences for human health and environment (pp. 1–19). Cambridge: RSC.
Paterson, G., Macken, A., & Thomas, K. V. (2011). The need for standardized methods and environmental monitoring programs for anthropogenic nanoparticles. Analitical Methods, 3, 1461–1467. https://doi.org/10.1039/c1ay05157a.
Peralta-Videa, J. R., Zhao, L., Lopez-Moreno, M. L., de la Rosa, G., Hong, J., & Gardea-Torresdey, J. L. (2011). Nanomaterials and the environment: a review for the biennium 2008–2010. Journal of Hazardous Materials, 186, 1–15. https://doi.org/10.1016/j.jhazmat.2010.11.020.
Pérez-de-Luque, A. (2017). Interaction of nanomaterials with plants: what do we need for real applications in agriculture? Frontiers in Environmental Science, 5, 12. doi.org/10.3389/fenvs.2017.00012.
Piccinno, F., Gottschalk, F., Seeger, S., & Nowack, B. (2012). Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. Journal of Nanoparticles Research, 14, 1109. https://doi.org/10.1007/s11051-012-1109-9.
Pošćić, F., Schat, H., & Marchiol, L. (2017). Cerium negatively impacts the nutritional status in rapeseed. Science of the Total Environment, 593–594, 735–744. https://doi.org/10.1016/j.scitotenv.2017.03.215.
Prasad, M. N. V., & Hagemeyer, J. (Eds.). (1999). Heavy metal stress in plants. From molecules to ecosystems. Verlag Berlin Heidelberg: Springer.
Qiu, R. L., Tang, Y. T., Zeng, X. W., Thangavel, P., Tang, L., Gan, Y. Y., et al. (2012). Mechanisms of cd Hyperaccumulation and detoxification in heavy metal Hyperaccumulators: how plants cope with Cd. In U. Lüttge, W. Beyschlag, B. Büdel, & D. Francis (Eds.), Progress in botany 73 (pp. 127–159). Springer: Heidelberg.
Rai, P. K., Kumar, V., Lee, S. S., Raza, N., Kim, K.-H., Ok, Y. S., et al. (2018). Nanoparticle-plant interaction: implications in energy, environment, and agriculture. Environment International, 119, 1–19. https://doi.org/10.1016/j.envint.2018.06.012.
Rajeshkumar, S., & Naik, P. (2018). Synthesis and biomedical applications of cerium oxide nanoparticles – a review. Biotechnology Reports (Amsterdam), 17, 1–5. https://doi.org/10.1016/j.btre.2017.11.008.
Ramírez-Olvera, S. M., Trejo-Téllez, L. I., García-Morales, S., Pérez-Sato, J. A., & Gómez-Merino, F. C. (2018). Cerium enhances germination and shoot growth, and alters mineral nutrient concentration in rice. PLoS One, 13(3), e0194691. https://doi.org/10.1371/journal.pone.0194691.
Rauser, W. E. (1999). Structure and function of metal chelators produced by plants. Cell Biochemistry and Biophysics, 31(1), 19–48. https://doi.org/10.1007/BF02738153.
Recillas, S., Colón, J., Casals, E., González, E., Puntes, V., Sánchez, A., et al. (2010). Chromium VI adsorption on cerium oxide nanoparticles and morphology changes during the process. Journal of Hazardous Materials, 184, 425–431. https://doi.org/10.1016/j.jhazmat.2010.08.052.
Reference Material IAEA-V-10. (2000). Trace Elements in Hay (powder). International Atomic Energy Agency, Vienna.
Rezania, S., Taib, S. M., Din, M. F. M., Dahalan, F. A., & Kamyab, H. (2016). Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. Journal of Hazardous Materials, 318, 587–599. https://doi.org/10.1016/j.jhazmat.2016.07.053.
Rossi, L., Zhang, W., & Ma, X. (2017a). Cerium oxide nanoparticles alter the salt stress tolerance of Brassica napus L. by modifying the formation of root apoplastic barriers. Environmental Pollution, 229, 132–138. https://doi.org/10.1016/j.envpol.2017.05.083.
Rossi, L., Zhang, W., Schwab, A. P., & Ma, X. (2017b). Uptake, accumulation, and in planta distribution of coexisting cerium oxide nanoparticles and cadmium in Glycina max (L.) Merr. Environmental Science and Technology, 51, 12815–12824. https://doi.org/10.1021/acs.est.7b03363.
Rossi, L., Sharifan, H., Zhang, W., Schwab, A. P., & Ma, X. (2018). Mutual effects and in planta accumulation of coexisting cerium oxide nanoparticles and cadmium in hydroponically grown soybean (Glycine max (L.) Merr.). Environmental Science: Nano, 5, 150–157. https://doi.org/10.1039/c7en00931c.
Salehi, H., Chehregani, A., Lucini, L., Majd, A., & Gholami, M. (2018). Morphological, proteomic and metabolomic insight into the effect of cerium dioxide nanoparticles to Phaselous vulgaris L. under soil or foliar application. Science of the Total Environment, 616-617, 1540–1551. https://doi.org/10.1016/j.scitotenv.2017.10.159.
Sauvé, S., & Desrosiers, M. (2014). A review of what is an emerging contaminant. Chemistry Central Journal, 8, 15. https://doi.org/10.1186/1752-153X-8-15.
Sheoran, I. S., & Singh, R. (1996). Effect of heavy metals on photosynthesis in higher plants. In Y. P. Abrol, P. Mohanty, & Govindjee (Eds.), Photosynthesis: photoreactions to plant productivity (pp. 451–468). Dordrecht: Springer.
Shi, G., & Cai, Q. (2009). Cadmium tolerance and accumulation in eight potential energy crops. Biotechnology Advances, 27, 555–561. https://doi.org/10.1016/j.biotechadv.2009.04.006.
Skiba, E., & Wolf, W. M. (2017). Commercial phenoxyacetic herbicides control heavy metal uptake by wheat in a divergent way than pure active substances alone. Environmental Sciences Europe, 29, 26. https://doi.org/10.1186/s12302-017-0124-y.
Skiba, E., Kobyłecka, J., & Wolf, W. M. (2017). Influence of 2,4-D and MCPA herbicides on uptake and translocation of heavy metals in wheat (Triticum aestivum L.). Environmental Pollution, 220(B), 882–890. https://doi.org/10.1016/j.envpol.2016.10.072.
Smýkal, P. (2014). Pea (Pisum sativum L.) in biology prior and after Mendel’s discovery. Czech Journal of Genetic and Plant Breeding, 50(2), 52–64.
Su, D. (2017). Advanced electron microscopy characterization of nanomaterials for catalysis. Green Energy & Environment, 2, 70–83. https://doi.org/10.1016/j.gee.2017.02.001.
Tassi, E., Giorgetti, L., Morelli, E., Peralta-Videa, J.-R., Gardea-Torresdey, J. L., & Barbafieri, M. (2017). Physiological and biochemical responses of sunflower (Helianthus annuus L.) exposed to nano-CeO2 and excess boron: modulation of boron phytotoxicity. Plant Physiology and Biochemistry, 110, 50–58. https://doi.org/10.1016/j.plaphy.2016.09.013.
Taylor, A. F., Rylott, E. L., Anderson, C. W. N., & Bruce, N. C. (2014). Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold. PLoS One, 9(4), e93793. https://doi.org/10.1371/journal.pone.0093793.
Testiati, E., Parinet, J., Massiani, C., Laffont-Schwob, I., Rabier, J., Pfeifer, H. R., et al. (2013). Trace metal and metalloid contamination levels in soils and two native plant species of a former industrial site: evaluation of the phytostabilization potential. Journal of Hazardous Materials, 248-249, 131–141. https://doi.org/10.1016/j.jhazmat.2012.12.039.
Tsazuki, T. (2009). Commercial scale production of inorganic nanoparticles. International Journal of Nanotechnology, 6, 567–578. https://doi.org/10.1504/IJNT.2009.024647.
Vance, M. E., Kuiken, T., Vejerano, E. P., McGinnis, S. P., Hochella Jr., M. F., Rejeski, D., et al. (2015). Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein Journal of Nanotechnology, 6, 1769–1780. https://doi.org/10.3762/bjnano.6.181.
Viehweger, K. (2014). How plants cope with heavy metals. Botanical Studies, 55, 35. https://doi.org/10.1186/1999-3110-55-35.
Xiao, R., Bai, J., Lu, Q., Zhao, Q., Gao, Z., Wen, X., et al. (2015). Fractionation, transfer and ecological risks of heavy metals in riparian and ditch wetlands across a 100-year chronsequence of reclamation in estuary of China. Science of the Total Environment, 517, 66–75. https://doi.org/10.1016/j.scitotenv.2015.02.052.
Yang, J., Cao, W., & Rui, Y. (2017). Interactions between nanoparticles and plants: phytotoxicity and defense mechanisms. Journal of Plant Interactions, 12(1), 158–169. https://doi.org/10.1080/17429145.2017.1310944.
Yruela, I. (2013). Transition metals in plant photosynthesis. Metallomics, 5, 1090–1109. https://doi.org/10.1039/C3MT00086A.
Zantye, P. B., Kumar, A., & Sikder, P. B. (2004). Chemical mechanical planarization for microelectronics applications. Materials Science and Enginering: R Reports, 45, 89–220. https://doi.org/10.1016/j.mser.2004.06.002.
Zhang, Z., He, X., Zhang, H., Ma, Y., Zhang, P., Ding, Y., et al. (2011). Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics, 3, 816–822. https://doi.org/10.1039/c1mt00049g.
Zhang, W., Dan, Y., Shi, H., & Ma, X. (2017). Elucidating the mechanisms for plant uptake and in-planta speciation of cerium in radish (Raphanus sativus L.) treated with cerium oxide nanoparticles. Journal of Environmental Chemical Engineering, 5, 572–577. https://doi.org/10.1016/j.jece.2016.12.036.
Zhang, P., Ma, Y., Xie, C., Guo, Z., He, X., Valsami-Jones, E., et al. (2019). Plant species-dependent transformation and translocation of ceria nanoparticles. Environmental Science: Nano, 6, 60–67. https://doi.org/10.1039/C8EN01089G.
Zholobak, N. M., Shcherbakov, A. B., Bogorad-Kobelska, A. S., Ivanova, O. S., Baranchikov, A. Y., Spivak, N. Y., et al. (2014). Panthenol-stabilized cerium dioxide nanoparticles for cosmeceutic formulations against ROS-induced and UV-induced damage. Journal of Photochemistry and Photobiology: B - Biology, 130, 102–108. https://doi.org/10.1016/j.jphotobiol.2013.10.015.
Acknowledgements
MSc Sylwia Michlewska is kindly acknowledged for the TEM CeO2 NPs characterization. The European University Foundation is acknowledged for advising on the legal and social dimension of this study.
Funding
This work received support from the Regional Fund for Environmental Protection and Water Management in Łódź, Poland (grant numbers: 804/BNID/2016 and 58/BN/D2018); additional funding from the Institute of General and Ecological Chemistry of Lodz University of Technology is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 48 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Skiba, E., Wolf, W.M. Cerium Oxide Nanoparticles Affect Heavy Metals Uptake by Pea in a Divergent Way than Their Ionic and Bulk Counterparts. Water Air Soil Pollut 230, 248 (2019). https://doi.org/10.1007/s11270-019-4296-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4296-5