Abstract
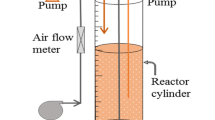
Black liquor is generated from the pretreatment process of biomass-based bioethanol production and due its environmental impact, should be treated effectively before discharged to the water body. Chemical treatment using coagulation-flocculation method was commonly used for wastewater treatment. In the case of black liquor, chemical treatment is often insufficient and further treatment was needed to degrade lignin in order to reduce its black coloration. This present study investigated the two-step treatment to decolorize black liquor using chemical coagulation-flocculation and biological treatment using white-rot fungus Trametes versicolor INACC F200. The biological treatment was optimized by applying a response surface methodology (RSM) of the utilization of CuSO4 concentration, Tween 80 concentration, and agitation. Furthermore, lignin degradation was also confirmed using FTIR and LC-MS. Initial chemical treatment using ferrous sulfate and polyacrylamide as coagulant-flocculant with a ratio of 3:3, resulted in black liquor decolorization at 80.9% and reduced the COD up to 90.77%. A full quadratic stepwise model was utilized with CuSO4 inducer, Tween 80 mediator, and agitation speed as the independent variables. Optimum decolorization of 96.188% was predicted when using 2 mM CuSO4, 2% Tween 80, and an agitation speed of 150 rpm. The highest enzyme activity during the decolorization process was lignin peroxidase (LiP). FT-IR and LC-MS profile showed that lignin-associated bond was eliminated and the molecular weight of lignin was decreased after the treatment. This study concludes the effective decolorization and delignification of black liquor by the two-step chemical and biological treatment.








Similar content being viewed by others
References
Adnan, L. A., Mohd Yusoff, A. R., Hadibarata, T., & Khudhair, A. B. (2014). Biodegradation of bis-azo dye Reactive Black 5 by white-rot fungus Trametes gibbosa sp. WRF 3 and its metabolite characterization. Water, Air, & Soil Pollution, 225, 2119.
Amriani, F., Sari, A. A., Abimanyu, H., & Tachibana, S. (2017). Evaluation of lignin-based black liquor decolorization by Trametes versicolor U 80. AIP Conference Proceedings, 1803, 020001.
Ansari, K. B., & Gaikar, V. G. (2014). Green hydrotropic extraction technology for delignification of sugarcane bagasse by using alkybenzene sulfonates as hydrotropes. Chemical Engineering Science, 115, 157–166.
Archibald, F. S., Bourbonnais, R., Jurasek, L., Paice, M. G., & Reid, I. D. (1997). Kraft pulp bleaching and delignification by Trametes versicolor. Journal of Biotechnology, 53, 215–236.
Balan, K., Sathishkumar, P., & Palvannan, T. (2012). Decolorization of malachite green by laccase: Optimization by response surface methodology. Journal of the Taiwan Institute of Chemical Engineers, 43, 776–782.
Belsare, D. K., & Prasad, D. Y. (1988). Decolorization of effluent from the bagasse-based pulp mills by white-rot fungus, Schizophyllum commune. Applied Microbiology and Biotechnology, 28, 301–304.
Bonugli-Santos, R. C., Vieira, G. A., Collins, C., Fernandes, T. C., Marin-Morales, M. A., Murray, P., & Sette, L. D. (2016). Enhanced textile dye decolorization by marine-derived basidiomycete Peniophora sp. CBMAI 1063 using integrated statistical design. Environmental Science and Pollution Research, 23, 8659–8668.
Boyle, C. D., Kropp, B. R., & Reid, I. D. (1992). Solubilization and mineralization of lignin by white rot fungi. Applied and Environmental Microbiology, 58, 3217–3224.
Bratby, J. (2016). Coagulation and flocculation in water and wastewater treatment. London: IWA Publishing.
Bugg, T. D., Ahmad, M., Hardiman, E. M., & Rahmanpour, R. (2011). Pathways for degradation of lignin in bacteria and fungi. Natural Product Reports, 28, 1883–1896.
Chandra, R., & Abhishek, A. (2011). Bacterial decolorization of black liquor in axenic and mixed condition and characterization of metabolites. Biodegradation, 22, 603–611.
Da Re, V., & Papinutti, L. (2011). Black liquor decolorization by selected white-rot fungi. Applied Biochemistry and Biotechnology, 165, 406–415.
Daâssi, D., Frikha, F., Zouari-Mechichi, H., Belbahri, L., Woodward, S., & Mechichi, T. (2012). Application of response surface methodology to optimize decolourization of dyes by the laccase-mediator system. Journal of Environmental Management, 108, 84–91.
Font, X., Caminal, G., Gabarrell, X., Romero, S., & Vicent, M. T. (2003). Black liquor detoxification by laccase of Trametes versicolor pellets. Journal of Chemical Technology & Biotechnology, 78, 548–554.
Garg, A., Mishra, I. M., & Chand, S. (2010). Effectiveness of coagulation and acid precipitation processes for the pre-treatment of diluted black liquor. Journal of Hazardous Materials, 180, 158–164.
Guillén-Jiménez, F. d. M., Cristiani-Urbina, E., Cancino-Díaz, J. C., Flores-Moreno, J. L., & Barragán-Huerta, B. E. (2012). Lindane biodegradation by the Fusarium verticillioides AT-100 strain, isolated from Agave tequilana leaves: Kinetic study and identification of metabolites. International Biodeterioration & Biodegradation, 74, 36–47.
Hadibarata, T., & Kristanti, R. A. (2013). Biodegradation and metabolite transformation of pyrene by basidiomycetes fungal isolate Armillaria sp. F022. Bioprocess and Biosystems Engineering, 36, 461–468.
Hadibarata, T., Tachibana, S., & Askari, M. (2011). Identification of metabolites from phenanthrene oxidation by phenoloxidases and dioxygenases of Polyporus sp. S133. Journal of Microbiology and Biotechnology, 21, 299–304.
Hadibarata, T., Abdullah, F., Yusoff, A. R. M., Ismail, R., Azman, S., & Adnan, N. (2012a). Correlation study between land use, water quality, and heavy metals (Cd, Pb, and Zn) content in water and green lipped mussels Perna viridis (Linnaeus.) at the Johor Strait. Water, Air, & Soil Pollution, 223, 3125–3136.
Hadibarata, T., Khudhair, A. B., & Salim, M. R. (2012b). Breakdown products in the metabolic pathway of anthracene degradation by a ligninolytic fungus Polyporus sp. S133. Water, Air, & Soil Pollution, 223, 2201–2208.
Helmy, S. M., El Rafie, S., & Ghaly, M. Y. (2003). Bioremediation post-photo-oxidation and coagulation for black liquor effleunt treatment. Desalination, 158, 331–339.
Higuchi, T. (1989). Mechanisms of lignin degradation by lignin peroxidase and laccase of white-rot fungi. Plant Cell Wall Polymers, American Chemical Society, pp. 482–502.
Irfan, M., Butt, T., Imtiaz, N., Abbas, N., Khan, R. A., & Shafique, A. (2017). The removal of COD, TSS and colour of black liquor by coagulation–flocculation process at optimized pH, settling and dosing rate. Arabian Journal of Chemistry, 10, S2307–S2318.
Jamal, F., Qidwai, T., Pandey, P. K., Singh, R., & Singh, S. (2011). Azo and anthraquinone dye decolorization in relation to its molecular structure using soluble Trichosanthes dioica peroxidase supplemented with redox mediator. Catalysis Communications, 12, 1218–1223.
Jayasinghe, C., Imtiaj, A., Lee, G. W., Im, K. H., Hur, H., Lee, M. W., Yang, H.-S., & Lee, T.-S. (2008). Degradation of three aromatic dyes by white rot fungi and the production of ligninolytic enzymes. Mycobiology, 36, 114–120.
Justino, C. I., Duarte, K., Loureiro, F., Pereira, R., Antunes, S. C., Marques, S. M., Gonçalves, F., Rocha-Santos, T. A. P., & Freitas, A. C. (2009). Toxicity and organic content characterization of olive oil mill wastewater undergoing a sequential treatment with fungi and photo-Fenton oxidation. Journal of Hazardous Materials, 172, 1560–1572.
Kersten, P., & Cullen, D. (2007). Extracellular oxidative systems of the lignin-degrading Basidiomycete Phanerochaete chrysosporium. Fungal Genetics and Biology, 44, 77–87.
Kim, W., Gamo, Y., Sani, Y., Wusiman, M. Y., Ogawa, S., Karita, S., & Goto, M. (2006). Effect of tween 80 on hydrolytic activity and substrate accessibility of carbohydrolase I (CBH I) from Trichoderma viride. Asian Australian Journal of Animal Science, 19, 684–689.
Kirk, T. K., Schultz, E., Connors, W. J., Lorenz, L. F., & Zeikus, J. G. (1978). Influence of culture parameters on lignin metabolism by Phanerochaete chrysosporium. Archives of Microbiology, 117, 277–285.
Lara, M. A., Rodriguez-Malaver, A. J., Rojas, O. J., Holmquist, O., González, A. M., Bullón, J., Peñaloza, N., & Araujo, E. (2003). Black liquor lignin biodegradation by Trametes elegans. International Biodeterioration & Biodegradation, 52, 167–173.
Leonowicz, A., Matuszewska, A., Luterek, J., Ziegenhagen, D., Wojtaś-Wasilewska, M., Cho, N.-S., Hofrichter, M., & Rogalski, J. (1999). Biodegradation of lignin by white rot fungi. Fungal Genetics and Biology, 27, 175–185.
Liu, Y., Hu, T., Wu, Z., Zeng, G., Huang, D., Shen, Y., He, X., Lai, M., & He, Y. (2014). Study on biodegradation process of lignin by FTIR and DSC. Environmental Science and Pollution Research, 21, 14004–14013.
Montgomery, D. C. (2012). Design and analysis of experiments, 8th Edition, John Wiley & Sons, Incorporated.
Murugesan, K., Dhamija, A., Nam, I.-H., Kim, Y.-M., & Chang, Y.-S. (2007). Decolourization of reactive black 5 by laccase: Optimization by response surface methodology. Dyes and Pigments, 75, 176–184.
Orrego, R., Pandelides, Z., Guchardi, J., & Holdway, D. (2011). Effects of pulp and paper mill effluent extracts on liver anaerobic and aerobic metabolic enzymes in rainbow trout. Ecotoxicology and Environmental Safety, 74, 761–768.
Paice, M. G., Reid, I. D., Bourbonnais, R., Archibald, F. S., & Jurasek, L. (1993). Manganese peroxidase, produced by Trametes versicolor during pulp bleaching, demethylates and delignifies kraft pulp. Applied and Environmental Microbiology, 59, 260–265.
Perestelo, F., Falcón, M. A., Pérez, M. L., Corominas Roig, E., & de la Fuente Martin, G. (1989). Bioalteration of kraft pine lignin by Bacillus megaterium isolated from compost piles. Journal of Fermentation and Bioengineering, 68, 151–153.
Rahmat, N. A., Ali, A. A., Salmiati, Hussain, N., Muhamad, M. S., Kristanti, R. A., & Hadibarata, T. (2016). Removal of remazol brilliant blue R from aqueous solution by adsorption using pineapple leaf powder and lime peel powder. Water, Air, & Soil Pollution, 227, 105.
Rajwar, D., & Rai, J. (2015). Kraft black liquor decolorization by fungi isolated from contaminated pulp and paper mill sludge. International Journal of Recent Scientific Research, 6, 7770–7775.
Sahadevan, L. D. M., Misra, C. S., & Thankamani, V. (2016). Characterization of lignin-degrading enzymes (LDEs) from a dimorphic novel fungus and identification of products of enzymatic breakdown of lignin. 3 Biotech, 6, 56.
Salvachúa, D., Katahira, R., Cleveland, N. S., Khanna, P., Resch, M. G., Black, B. A., Purvine, S. O., Zink, E. M., Prieto, A., Martínez, M. J., Martínez, A. T., Simmons, B. A., Gladden, J. M., & Beckham, G. T. (2016). Lignin depolymerization by fungal secretomes and a microbial sink. Green Chemistry, 18, 6046–6062.
Sari, A. A., Tachibana, S., & Itoh, K. (2012). Determination of co-metabolism for 1,1,1-trichloro-2,2-bis(4-chlorophenyl) ethane (DDT) degradation with enzymes from Trametes versicolor U97. Journal of Bioscience and Bioengineering, 114, 176–181.
Sari, A. A., Kurniawan, H. H., Nurdin, M., & Abimanyu, H. (2015). Decolorization of black liquor wastewater generated from bioethanol process by using oil palm empty fruit bunches. Energy Procedia, 68, 254–262.
Si, J., Cui, B.-K., & Dai, Y.-C. (2013). Decolorization of chemically different dyes by white-rot fungi in submerged cultures. Annals of Microbiology, 63, 1099–1108.
Su, Y., Yu, X., Sun, Y., Wang, G., Chen, H., & Chen, G. (2018). Evaluation of screened lignin-degrading fungi for the biological pretreatment of corn stover. Scientific Reports, 8, 5385.
Verma, A. K., Dash, R. R., & Bhunia, P. (2012). A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. Journal of Environmental Management, 93, 154–168.
Vrsanska, M., Voberkova, S., Langer, V., Palovcikova, D., Moulick, A., Adam, V., & Kopel, P. (2016). Induction of laccase, lignin peroxidase and manganese peroxidase activities in white-rot Fungi using copper complexes. Molecules, 21, 1553.
Wu, J., Xiao, Y.-Z., & Yu, H.-Q. (2005). Degradation of lignin in pulp mill wastewaters by white-rot fungi on biofilm. Bioresource Technology, 96, 1357–1363.
Yamanaka, R., Soares, C. F., Matheus, D. R., & Machado, K. M. G. (2008). Lignolytic enzymes produced by Trametes villosa ccb176 under different culture conditions. Brazilian Journal of Microbiology, 39, 78–84.
Zhang, H., Nie, S., Qin, C., Zhang, K., & Wang, S. (2018). Effect of hot chlorine dioxide delignification on AOX in bagasse pulp wastewater. Cellulose, 25, 2037–2049.
Acknowledgments
This research was supported by INSINAS – Ministry of Research, Technology and Higher Education, Indonesia, for the 2018 Fiscal Year. The part of this research was presented in International Conference on Engineering and Natural Science – Summer Session 2018 that funded by overseas conference grant (Bantuan Seminar Luar Negeri / BSLN) - Ministry of Research, Technology and Higher Education, Indonesia for the 2018 Fiscal Year.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sari, A.A., Hadibarata, T., Hanifah, U. et al. Bioethanol Mill Wastewater Purification by Combination of Coagulation-Flocculation and Microbial Treatment of Trametes versicolor INACC F200. Water Air Soil Pollut 230, 224 (2019). https://doi.org/10.1007/s11270-019-4270-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4270-2