Abstract
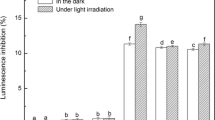
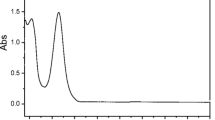
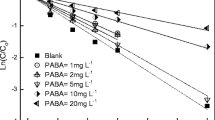
Photolysis is a major transformation pathway for triclosan, an antibacterial agent frequently detected in aquatic environment. Though many studies have been conducted on the influence of dissolved organic matter on the photolysis of triclosan, there are still controversies and the mechanism involved is still not clear. In the present study, influence of humic acid on the photolysis of triclosan in molecular form and anionic form was investigated. Reactive substances involved were identified and photolysis pathways were proposed. The addition of humic acid significantly enhanced the photolysis of triclosan in molecular form and inhibited that of triclosan in anionic form. •OH and intra-humic acid 1O2 played the dorminant role in the enhanced photolysis of triclosan. Different photolysis pathways of triclosan in different forms in the presence of humic acid were proposed, and dioxin products were not found during the indirect photolysis. Here, we show that humic acid has the opposing effects on the photolysis of triclosan in different forms. These findings will help us better understand the photolysis process of triclosan in natural waters.



Similar content being viewed by others
References
Anger, C. T., Sueper, C., Blumentritt, D. J., McNeill, K., Engstrom, D. R., & Arnold, W. A. (2013). Quantification of triclosan, chlorinated triclosan derivatives, and their dioxin photoproducts in lacustrine sediment cores. Environmental Science and Technology, 47, 1833–1843.
Aranami, K., & Readman, J. W. (2007). Photolytic degradation of triclosan in freshwater and seawater. Chemosphere, 66, 1052–1056.
Bedoux, G., Roig, B., Thomas, O., Dupont, V., & Le Bot, B. (2012). Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environmental Science and Pollution Research International, 19, 1044–1065.
Bester, K. (2005). Fate of triclosan and triclosan-methyl in sewage treatment plants and surface waters. Archives of Environment Contamination and Toxicology, 49, 9–17.
Bianco, A., Fabbri, D., Minell, M., Brigante, M., Mailhot, G., Maurino, V., Minero, C., & Vione, D. (2015). New insights into the environmental photochemistry of 5-chloro-2-(2,4-dichlorophenoxy) phenol (triclosan): reconsidering the importance of indirect photoreactions. Water Research, 72, 271–280.
Buth, J. M., Grandbois, M., Vikesland, P. J., McNeill, K., & Arnold, W. A. (2009). Aquatic photochemistry of chlorinated triclosan derivatives: potential source of polychlorodibenzo-p-dioxins. Environmental Toxicology and Chemistry, 28, 2555–2563.
Chen, L., Tang, X., Shen, C., Chen, C., & Chen, Y. (2012). Photosensitized degradation of 2,4′,5-trichlorobiphenyl (PCB 31) by dissolved organic matter. Journal of Hazardous Materials, 201–202, 1–6.
Dhillon, G. S., Kaur, S., Sarma, S. J., Brar, S. K., Cledon, M., Verma, M., & Surampalli, R. Y. (2015). Triclosan: current status, occurrence, environmental risks and bioaccumulation potential. International Journal of Environmental Research and Public Health, 12, 5657–5684.
Halladja, S., ter Halle, A., Aguer, J. P., Boulkamh, A., & Richard, C. (2007). Inhibition of humic substances mediated photooxygenation of furfuryl alcohol by 2,4,6-trimethylphenol: evidence for reactivity of the phenol with humic triplet excited states. Environmental Science and Technology, 41, 6066–6073.
Hassett, J. P. (2006). Dissolved natural organic matter as a microreactor. Science, 311, 1723–1724.
Latch, D. E., Packer, J. L., Stender, B. L., Vanoverbeke, J., Arnold, W. A., & McNeill, K. (2005). Aqueous photochemistry of triclosan: formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo- p-dioxin, and oligomerization products. Environmental Toxicology and Chemistry, 24, 517–525.
Leech, D. M., Snyder, M. T., & Wetzel, R. G. (2009). Natural organic matter and sunlight accelerate the degradation of 17β-estradiol in water. Science of the Total Environment, 407, 2087–2092.
Mezcua, M., Gomez, M. J., Ferrer, I., Aguera, A., Hernando, M. D., & Fernandez-Alba, A. R. (2004). Evidence of 2,7/2,8-dibenzodichloro-p-dioxin as a photodegradation product of triclosan in water and wastewater samples. Analytica Chimica Acta, 524, 241–247.
Singer, H., Müller, S., Tixier, C., & Pillonel, L. (2002). Triclosan: occurrence and fate of a widely used biocide in the aquatic environment: field measurements in wastewater treatment plants, surface waters, and lake sediments. Environmental Science and Technology, 36, 4998–5004.
Tixier, C., Singer, H. P., Canonica, S., & Müller, S. R. (2002). Phototransfomation of triclosan in surface waters: a relevant elimination process for this widely used biocide-laboratory studies, field measurements, and modeling. Environmental Science and Technology, 36, 3482–3489.
Wong-Wah-Chung, P., Rafqah, S., Voyard, G., & Sarakha, M. (2007). Photochemical behaviour of triclosan in aqueous solutions: kinetic and analytical studies. Journal of Photochemistry and Photobiology A, 191, 201–208.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 41301545) and the Natural Science Foundation of Jiangsu Province (No. BK20130961). The authors also want to thank Dr. Peter Edwards for language editing for this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Wang, Z., Wang, Z. et al. Influence of Humic Acid on the Photolysis of Triclosan in Different Dissociation Forms. Water Air Soil Pollut 227, 318 (2016). https://doi.org/10.1007/s11270-016-3024-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3024-7