Abstract
The photolysis of the antimicrobial triclocarban (TCC) in aqueous systems under simulated sunlight irradiation was studied. The effects of several abiotic parameters, including solution pH, initial TCC concentration, presence of natural organic matter, and most common inorganic anions in surface waters, were investigated. The results show that the photolysis of TCC followed pseudo-first-order kinetics. The TCC photolysis rate constant increased with increasing solution pH and decreasing the initial TCC concentration. Compared with the TCC photolysis in pure water, the presence of aqueous bicarbonate, nitrate, humic acids, and its sodium salt decreased the TCC photolysis rate, but fulvic acid increased the TCC photolysis rate. The electron spin resonance and reactive oxygen species scavenging experiments indicated that TCC may undergo two different types of phototransformation reactions: direct photolysis and energy transfer to generate 1O2. The main degradation products were tentatively identified by gas chromatography interfaced with mass spectrometry (GC-MS), and a possible degradation pathway was also proposed.








Similar content being viewed by others
References
Ahn KC, Zhao B, Chen J, Cheredenichenko G, Sanmarti E, Denision MS et al (2008) In vitro biological activities of the antimicrobials triclocarban, its analogues, and triclosan in bioassay screens: receptor-based bioassay screens. Environ Health Pers 116:1203–1210
Audu AA, Heyn AA (1988) Comparative hydrolysis of substituted ureas in a mixed alcoholic-water solution. Water Res 22:1155–1162
Bahnmüller S, Gunten UV, Canonica S (2014) Sunlight-induced transformation of sulfadiazine and sulfamethoxazole in surface waters and wastewater effluents. Water Res 57:183–192
Bautitz IR, Nogueira RFP (2007) Degradation of tetracycline by photo-Fenton process-solar irradiation and matrix effects. J Photochem Photobioa A Chem 187:33–39
Behnajady MA, Modirshahla N, Hamzavi R (2006) Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst. J Hazard Mater 133:226–232
Boyd GR, Reemtsma H, Grimm DA, Mitra S (2003) Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. Sci Total Environ 311:135–149
Calza P, Medana C, Padovano E, Giancotti V, Minero C (2013) Fate of selected pharmaceuticals in river waters. Environ Sci Pollut Res 20:2262–2270
Chen Y, Hu C, Qu J, Yang M (2008) Photodegradation of tetracycline and formation of reactive oxygen species in aqueous tetracycline solution under simulated sunlight irradiation. J Photochem Photobiol A Chem 197:81–87
Chu S, Metcalfe CD (2007) Simultaneous determination of triclocarban and triclosan in municipal biosolids by liquid chromatography–tandem mass spectrometry. J Chromatogr A 1164:212–218
Coogan MA, La Point TW (2008) Snail bioaccumulation of triclocarban, triclosan, and methyltriclosan in a north texas, USA, stream affected by wastewater treatment plant runoff. Environ Toxicol Chem 27:1788–1793
Coogan MA, Edziyie RE, La Point TW, Venable BJ (2007) Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere 67:1911–1918
Dimou AD, Sakkas VA, Albanis TA (2005) Metolachlor photodegradation study in aqueous media under natural and simulated solar irradiation. J Agric Food Chem 53:694–701
Ding SL, Wang XK, Jiang WQ, Meng X, Zhao RS, Wang C et al (2013) Photodegradation of the antimicrobial triclocarban in aqueous systems under ultraviolet radiation. Environ Sci Pollut Res 20:3195–3201
Gangwang LG, Liu H, Zhang N, Wang Y (2012) Photodegradation of salicylic acid in aquatic environment: effect of different forms of nitrogen. Sci Total Environ 435–43:573–577
Gómez-Pacheco CV, Sánchez-Polo M, Rivera-Utrilla J, López-Peñalver JJ (2012) Tetracycline degradation in aqueous phase by ultraviolet radiation. Chem Eng J 187:89–95
Guerard JJ, Miller PL, Trouts TD, Chin YP (2009) The role of fulvic acid composition in the photosensitized degradation of aquatic contaminants. Aquat Sci 71:160–169
Halden RU, Paull DH (2005) Co-occurrence of triclocarban and triclosan in US water resources. Environ Sci Technol 39:1420–1426
Hinther A, Bromba CM, Wulff JE, Helbing CC (2011) Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems. Environ Sci Technol 45:5395–5402
Hoigne J, Bader H (1983) Rate constants of reactions of ozone with organic and inorganic compounds in water.II. Dissociating organic compounds. Water Res 17:185–194
Hu C, Hu X, Guo J, Qu J (2006) Efficient destruction of pathogenic bacteria with NiO/SrBi2O4 under visible light irradiation. Environ Sci Technol 40:5508–5513
Ji Y, Zeng C, Ferronato C, Chovelon JM, Yang X (2012) Nitrate-induced photodegradation of atenolol in aqueous solution: kinetics, toxicity and degradation pathways. Chemosphere 88:644–649
Lam MW, Tantuco K, Mabury SA (2003) PhotoFate: a new approach in accounting for the contribution of indirect photolysis of pesticides and pharmaceuticals in surface waters. Environ Sci Technol 37:899–907
Li Y, Niu J, Wang W (2011a) Photolysis of Enrofloxacin in aqueous systems under simulated sunlight irradiation: kinetics, mechanism and toxicity of photolysis products. Chemosphere 85:892–897
Li Y, Niu JF, Yin LF, Wang WL, Bao YP, Chen J et al (2011b) Photocatalytic degradation kinetics and mechanism of pentachlorophenol based on superoxide radicals. J Environ Sci 23:1911–1918
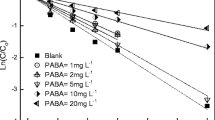
Mao L, Meng C, Zeng C, Ji Y, Yang X, Gao S (2011) The effect of nitrate, bicarbonate and natural organic matter on the degradation of sunscreen agent p-aminobenzoic acid by simulated solar irradiation. Sci Total Environ 409:5376–5381
Mihas O, Kalogerakis N, Psillakis E (2007) Photolysis of 2,4-dinitrotoluene in various water solutions: effect of dissolved species. J Hazard Mater 146:535–539
Minella M, Merlo MP, Maurino V, Minero C, Vione D (2013) Transformation of 2,4,6-trimethylphenol and furfuryl alcohol, photosensitized by Aldrich humic acids subject to different filtration procedures. Chemosphere 90:306–311
Nelieu S, Perreau F, Bonnemoy F, Ollitrault M, Azam D, Lagadic L (2009) Sunlight nitrate-induced photodegradation ofchlorotoluron:evidence of the process in aquatic mesocosms. Environ Sci Technol 43:3148–3154
Niu J, Li Y, Wang W (2013) Light-source-dependent role of nitrate and humic acid in tetracycline photolysis: kinetics and mechanism. Chemosphere 92:1423–1429
Pinna MV, Pusino A (2012) Direct and indirect photolysis of two quinolinecarboxylic herbicides in aqueous systems. Chemosphere 86:655–658
Richard C, Vialaton D, Aguer JP, Andreux F (1997) Transformation of monuron photosensitized by soil extracted humic substances: energy or hydrogen transfer mechanism? J Photochem Photobiol A 111:264–271
Sanches S, Leitão C, Penetra A, Cardoso VV, Ferreira E, Benoliel MJ et al (2011) Direct photolysis of polycyclic aromatic hydrocarbons in drinking water sources. J Hazard Mater 192:1458–1465
Sapkota A, Heidler J, Halden RU (2007) Detection of triclocarban and two co-contaminating chlorocarbanilides in US aquatic environments using isotope dilution liquid chromatography tandem mass spectrometry. Environ Res 103:21–29
Schebb NH, Inceoglu B, Morisseau C, Ahn KC, Gee SJ, Hammock BD (2011) Investigation of human exposure to triclocarban after showering, and preliminary evaluation of its biological effects. Environ Sci Technol 45:3109–3115
Tercero ELA, Neamtu M, Frimmel FH (2007) The effect of nitrate, Fe(III) and bicarbonate on the degradation of bisphenol A by simulated solar UV-irradiation. Water Res 41:4479–4487
Vione D, Khanra S, Das R, Minero C, Maurino V, Brigante M, Mailhot G (2010) Effect of dissolved organic compounds on the photodegradation of the herbicide MCPA in aqueous solution. Water Res 44:6053–6062
Wolters A, Steffens M (2005) Photodegradation of antibiotics on soil surfaces: laboratory studies on sulfadiazine in an ozone-controlled environment. Environ Sci Technol 39:6071–6078
Xu Y, Nguyen TV, Reinhard M, Yew-Hoong Gin K (2011) Photodegradation kinetics of p-tert-octylphenol, 4-tert-octylphenoxy-acetic acid and ibuprofen under simulated solar conditions in surface water. Chemosphere 85:790–796
Zarate FM Jr, Schulwitz SE, Stevens KJ, Venables BJ (2012) Bioconcentration of triclosan, methyl-triclosan, and triclocarban in the plants and sediments of a constructed wetland. Chemosphere 88:323–329
Acknowledgments
The authors are thankful to the financial supports by the National Natural Science Foundation of China (No. 21077069 and 21007035).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Rights and permissions
About this article
Cite this article
Ding, SL., Wang, XK., Jiang, WQ. et al. Influence of pH, inorganic anions, and dissolved organic matter on the photolysis of antimicrobial triclocarban in aqueous systems under simulated sunlight irradiation. Environ Sci Pollut Res 22, 5204–5211 (2015). https://doi.org/10.1007/s11356-014-3686-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3686-x