Abstract
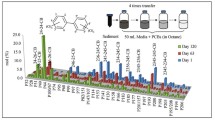
This study aims to evaluate extensively the inhibition of six PCB (polychlorinated biphenyls) congeners in batch reactors under fermentative-methanogenic condition. The reactors with anaerobic sludge were fed with mineral medium, co-substrates (ethanol and sodium formate), and five PCB concentrations. The maximum methane production (MMP) in the reactor without PCB (RC), with 0.5 (R0.5), 1.5 (R1.5), 3.0 (R3.0), 4.5 (R4.5), and 6.0 mg/L (R6.0) of PCB, was 654.83, 193.08, 111.65, 104.60, 96.67, and 79.50 μmolCH4/gTVS, respectively. The methane inhibition for the reactors R0.5, R1.5, R3.0, R4.5, and R6.0 were 70, 83, 84, 85, and 88 %, respectively. The concentration that causes 50 % of inhibition (IC50) for PCB was 0.03 mg/L. The inhibition results present two different profiles according to the concentration range. The concentration range of 0.5 to 3.0 mg/L of PCB inhibited the acetoclastic microorganisms and the concentration of 4.5 to 6.0 mg/L inhibited both methanogenic and acidogenic population. The acidogenic populations were less sensitive to the PCB than the methanogenic. Lower methane production and organic matter removal were verified in all reactors with PCB compared to RC, without PCB. The microbial community highlighted lower diversity index for reactors with higher PCB concentration. In the reactors with PCB, the populations of bacteria domain were more susceptible to composition changes than the archaea domain. The inhibitory effect of PCB is concentration-dependent and affected differently the populations of organisms in the reactor. Moreover, the range of 4.5 to 6.0 mg/L of PCB severely inhibited the anaerobic community.



Similar content being viewed by others
References
Abramowicz, D. A. (1990). Aerobic and anaerobic biodegradation of PCBs: a review. Crit Rev Biotechnol, 10, 241–251. doi:10.3109/07388559009038210.
Abreu, A. A., Alves, J. I., Pereira, M. A., et al. (2010). Engineered heat treated methanogenic granules: a promising biotechnological approach for extreme thermophilic biohydrogen production. Bioresour Technol, 101, 9577–9586. doi:10.1016/j.biortech.2010.07.070.
Ahmed, M. N., Sinha, S. N., Vemula, S. R., et al. (2016). Accumulation of polychlorinated biphenyls in fish and assessment of dietary exposure: a study in Hyderabad City, India. Environ Monit Assess, 188, 94. doi:10.1007/s10661-015-5068-3.
Angelidaki, I., Alves, M. M., Bolzonella, D., et al. (2009). Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays. Water Sci Technol, 59, 927–934.
APHA American Public Health Association. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington, DC.
ATSDR Agency for Toxic Substances and Disease Registry. (2000). Toxicological profile for polychlorinated biphenyls (PCBs). Atlanta: U.S. Department of Health and Human Services, Public Health Service.
Balasubramani, A., Howell, N. L., & Rifai, H. S. (2014). Polychlorinated biphenyls (PCBs) in industrial and municipal effluents: concentrations, congener profiles, and partitioning onto particulates and organic carbon. Sci Total Environ, 473, 702–713. doi:10.1016/j.scitotenv.2013.12.105.
Boone, D. R., Chynoweth, D. P., Mah, R. A., et al. (1993). Ecology and microbiology of biogasification. Biomass and Bioenergy, 5, 191–202.
Borja, J., Taleon, D. M., Auresenia, J., & Gallardo, S. (2005). Polychlorinated biphenyls and their biodegradation. Process Biochem, 40, 1999–2013. doi:10.1016/j.procbio.2004.08.006.
Buzzini, A. P., Sakamoto, I. K., Varesche, M. B., & Pires, E. C. (2006). Evaluation of the microbial diversity in an UASB reactor treating wastewater from an unbleached pulp plant. Process Biochem, 41, 168–176. doi:10.1016/j.procbio.2005.06.009.
Cámara, B., Herrera, C., González, M., et al. (2004). From PCBs to highly toxic metabolites by the biphenyl pathway. Environ Microbiol, 6, 842–850. doi:10.1111/j.1462-2920.2004.00630.x.
Carriger, J. F., Castro, J., & Rand, G. M. (2015). Screening historical water quality monitoring data for chemicals of potential ecological concern: hazard assessment for selected inflow and outflow monitoring stations at the water conservation areas, South Florida. Water, Air, Soil Pollut, 227, 27. doi:10.1007/s11270-015-2710-1.
Casserly, C., & Erijman, L. (2003). Molecular monitoring of microbial diversity in an UASB reactor. Int Biodeterior Biodegradation, 52, 7–12. doi:10.1016/S0964-8305(02)00094-X.
Chan, Y. J., Chong, M. F., Law, C. L., & Hassell, D. G. (2009). A review on anaerobic–aerobic treatment of industrial and municipal wastewater. Chem Eng J, 155, 1–18. doi:10.1016/j.cej.2009.06.041.
Chen, K.-F., Chang, Y.-C., & Huang, S.-C. (2012). Biodegradation potential of MTBE and BTEX under aerobic, nitrate reducing, and methanogenic conditions at a gasoline-contaminated site. Desalin Water Treat, 48, 278–284.
Ferraz Júnior, A. D. N., Wenzel, J., Etchebehere, C., & Zaiat, M. (2014). Effect of organic loading rate on hydrogen production from sugarcane vinasse in thermophilic acidogenic packed bed reactors. Int J Hydrogen Energy, 39, 16852–16862. doi:10.1016/j.ijhydene.2014.08.017.
García-Morales, J. L., Nebot, E., Romero, L. I., & Sales, D. (2001). Comparison between acidogenic and methanogenic inhibition caused by linear alkylbenzene-sulfonate (LAS). Chem Biochem Eng Q, 15, 13–19.
Gomes, B. C., Adorno, M. A. T., Okada, D. Y., et al. (2014). Analysis of a microbial community associated with polychlorinated biphenyl degradation in anaerobic batch reactors. Biodegradation, 25, 797–810. doi:10.1007/s10532-014-9700-7.
Gonzalez-Mille, D. J., Espinosa-Reyes, G., Rivero-Pérez, N. E., et al. (2013a). Vertical distributions of organochlorine pesticides and polychlorinated biphenyls in an agricultural soil core from the Guanzhong Basin, China. Water, Air, Soil Pollut, 224, 1781–1795. doi:10.1007/s10661-014-4159-x.
Gonzalez-Mille, D. J., Espinosa-Reyes, G., Rivero-Pérez, N. E., et al. (2013b). Distribution and ecological risk of polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) in surface sediments from the Bizerte lagoon, Tunisia. Water, Air, Soil Pollut, 224, 1781–1795. doi:10.1007/s11356-013-1709-7.
Griffiths, R. I., Whiteley, A. S., Donnell, A. G. O., & Bailey, M. J. (2000). Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl Environ Microbiol, 66, 5488–5491.
Grimm, F. A., Hu, D., Kania-Korwel, I., et al. (2015). Metabolism and metabolites of polychlorinated biphenyls. Crit Rev Toxicol, 45, 245–272. doi:10.3109/10408444.2014.999365.
Hardman, D. J., McEldowney, S., & Waite, S. (1993). Pollution: ecology and biotreatment. Longman Scientific & Technical.
Harrad, S., Abdallah, M. A.-E., & Oluseyi, T. (2016). Polybrominated diphenyl ethers and polychlorinated biphenyls in dust from cars, homes, and offices in Lagos, Nigeria. Chemosphere, 146, 346–353. doi:10.1016/j.chemosphere.2015.12.045.
Kania-Korwel, I., & Lehmler, H.-J. (2016). Toxicokinetics of chiral polychlorinated biphenyls across different species—a review. Environ Sci Pollut Res Int, 23, 2058–2080. doi:10.1007/s11356-015-4383-0.
Kaya, D., Imamoglu, I., & Sanin, F. D. (2013b). Impact of PCB-118 and transformer oil toxicity on anaerobic digestion of sludge: anaerobic toxicity assay results. Chemosphere, 92, 821–827. doi:10.1016/j.chemosphere.2013.04.032.
Kaya, D., Imamoglu, I., & Sanin, F. D. (2013a). Anaerobic mesophilic digestion of waste activated sludge in the presence of 2,3′,4,4′,5-pentachlorobiphenyl. Int Biodeterior Biodegradation, 83, 41–47. doi:10.1016/j.ibiod.2013.04.003.
Kim I. S., Young J. C., Tabak H. H. (1994). Kinetics of acetogenesis and methanogenesis in anaerobic reactions under toxic conditions. Water Environ Res 66:119–132. doi: http://dx.doi.org/10.2175/WER.66.2.5.
Krocker, E. J., Schulte, D. D., Sparling, A. B., & Lapp, H. M. (1979). Anaerobic treatment process stability. Water Pollut Control Fed, 51, 718–727.
Kudo, Y., Nakajima, T., Miyaki, T., & Oyaizu, H. (1997). Methanogen flora of paddy soils in Japan. FEMS Microbiol Ecol, 23, 39–48. doi:10.1111/j.1574-6941.1997.tb00354.x.
Lawrence, J., & Tosine, H. M. (1976). Adsorption of polychlorinated biphenyls from aqueous solutions and sewage. Environ Sci Technol, 10, 381–383. doi:10.1021/es60115a007.
Moreda, J. M., Arranz, A., Fdez De Betoño, S., et al. (1998). Chromatographic determination of aliphatic hydrocarbons and polyaromatic hydrocarbons (PAHs) in a sewage sludge. Sci Total Environ, 220, 33–43. doi:10.1016/S0048-9697(98)00238-1.
Motteran, F., Braga, J. K., Sakamoto, I. K., & Varesche, M. B. A. (2014). Methanogenic potential of an anaerobic sludge in the presence of anionic and nonionic surfactants. Int Biodeterior Biodegradation, 96, 198–204. doi:10.1016/j.ibiod.2014.10.001.
Muyzer, G., Waal, E. C. D. E., & Uitierlinden, A. G. (1993). Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol, 59, 695–700.
Nakhla, G., Kochany, J., & Lugowski, A. (2002). Evaluation of PCBs biodegradability in sludges by various microbial cultures. Environ Prog, 21, 85–93. doi:10.1002/ep.670210210.
Nielsen, H., Brunak, S., & von Heijne, G. (1999). Machine learning approaches for the prediction of signal peptides and other protein sorting signals. Protein Eng Des Sel, 12, 3–9. doi:10.1093/protein/12.1.3.
Ochoa-Herrera, V., Banihani, Q., León, G., et al. (2009). Toxicity of fluoride to microorganisms in biological wastewater treatment systems. Water Res, 43, 3177–3186. doi:10.1016/j.watres.2009.04.032.
Patel, G. B., Agnew, B. J., & Dicaire, C. J. (1991). Inhibition of pure cultures of methanogens by benzene ring compounds. Appl Envir Microbiol, 57, 2969–2974.
Patureau, D., & Trably, E. (2006). Impact of anaerobic and aerobic processes on polychlorobiphenyl removal in contaminated sewage sludge. Biodegradation, 17, 9–17. doi:10.1007/s10532-005-1920-4.
Penteado, E. D., Lazaro, C. Z., Sakamoto, I. K., & Zaiat, M. (2013). Influence of seed sludge and pretreatment method on hydrogen production in packed-bed anaerobic reactors. Int J Hydrogen Energy, 38, 6137–6145. doi:10.1016/j.ijhydene.2013.01.067.
Piringer, G., & Bhattacharya, S. (1999). Toxicity and fate of pentachlorophenol in anaerobic acidogenic systems. Water Res, 33, 2674–2682. doi:10.1016/S0043-1354(98)00496-5.
Saia, F. T., Damianovic, M. H. R. Z., Cattony, E. B. M., et al. (2007). Anaerobic biodegradation of pentachlorophenol in a fixed-film reactor inoculated with polluted sediment from Santos-São Vicente Estuary, Brazil. Appl Microbiol Biotechnol, 75, 665–672. doi:10.1007/s00253-007-0841-z.
Sierra-Alvarez, R., & Lettinga, G. (1991). The effect of aromatic structure on the inhibition of acetoclastic methanogenesis in granular sludge. Appl Microbiol Biotechnol. doi:10.1007/BF00180586.
Speece, R. E. (1996). Anaerobic biotechnology for industrial wastewaters (1st ed.). Nashville: Archae Press.
Stevens, J., Green, N. J., & Jones, K. C. (2001). Survey of PCDD/Fs and non-ortho PCBs in UK sewage sludges. Chemosphere, 44, 1455–1462. doi:10.1016/S0045-6535(00)00474-4.
Tartakovsky, B., Hawari, J., & Guiot, S. R. (2000). Enhanced dechlorination of Aroclor 1242 in an anaerobic continuous bioreactor. Water Res, 34, 85–92.
Tiedje, J. M., Quensen, J. F., III, Chee-Sanford, J., et al. (1993). Microbial reductive dechlorination of PCBs. Biodegradation, 4, 231–240.
Uberoi, V., & Bhattacharya, S. K. (1997). Effects of chlorophenols and nitrophenols on the kinetics of propionate degradation in sulfate-reducing anaerobic systems. Environ Sci Technol, 31, 1607–1614. doi:10.1021/es960223i.
UNEP United Nations Environment Programme. (2012). Terms of reference of the Polychlorinated Biphenyls Elimination Network—(PEN).
van Bodegom, P. (2007). Microbial maintenance: a critical review on its quantification. Microb Ecol, 53, 513–523. doi:10.1007/s00248-006-9049-5.
Wang, Y., Hu, J., Lin, W., et al. (2016). Health risk assessment of migrant workers’ exposure to polychlorinated biphenyls in air and dust in an e-waste recycling area in China: Indication for a new wealth gap in environmental rights. Environ Int, 87, 33–41. doi:10.1016/j.envint.2015.11.009.
Warren, E., Bekins, B. A., Godsy, E. M., & Smith, V. K. (2003). Inhibition of acetoclastic methanogenesis in crude oil- and creosote-contaminated groundwater. Bioremediat J, 7, 139–149.
Xu, K., Liu, H., Li, X., et al. (2010). Typical methanogenic inhibitors can considerably alter bacterial populations and affect the interaction between fatty acid degraders and homoacetogens. Appl Microbiol Biotechnol, 87, 2267–2279.
Zwietering, M. H., Jongenburger, I., Rombouts, F. M., & Van, K. (1990). Modeling of the bacterial growth curve modeling of the bacterial growth curve. Appl Environ Microbiol, 56, 1875–1881.
Acknowledgments
Financial support for this study was provided by a grant from CNPq (Conselho Nacional de Pesquisa), project number 141878/2012-6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
de Lima e Silva, M.R., Motteran, F., Sakamoto, I.K. et al. Anaerobic Toxicity Assay of Polychlorinated Biphenyl: Focus on Fermentative-Methanogenic Community. Water Air Soil Pollut 227, 316 (2016). https://doi.org/10.1007/s11270-016-3016-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3016-7