Abstract
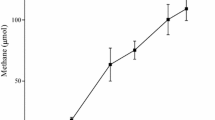
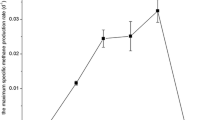
The effects of two typical methanogenic inhibitors [2-bromoethanesulfonate (BES) and chloroform (CHCl3)] on the bacterial populations were investigated using molecular ecological techniques. Terminal restriction fragment length polymorphism analyses (T-RFLP) in combination with clone library showed that both the toxicants not only inhibited methanogenic activity but also considerably altered the bacterial community structure. Species of low % G + C Gram-positive bacteria (Clostridiales), high % G + C Actinomycetes, and uncultured Chloroflexi showed relatively greater tolerance of CHCl3, whereas the BES T-RFLP patterns were characterized by prevalence of Geobacter hydrogenophilus and homoacetogenic Moorella sp. In addition, due to indirect thermodynamic inhibition caused by high hydrogen partial pressures, the growth of obligately syntrophic acetogenic Syntrophomonas and Syntrophobacter was also affected by selective inhibition of methanogenesis. Interestingly, by comparing the fermentative intermediates detected in BES- and CHCl3-treated experiments, it was furthermore found that when methanogenesis is specifically inhibited, the syntrophic interaction between hydrogen-producing fatty acid degraders and hydrogen-utilizating homoacetogens seemed to be strengthened.






Similar content being viewed by others
References
Amann R (1995) In situ identification of microorganisms by whole cell hybridization with rRNA-targeted nucleic acid probes. In: Akkermans A, van Elsas J, de Bruin F (eds) Molecular microbial ecology manual, 1st edn. Kluwer, London, pp 1–15
Babel S, Fukushi K, Sitanrassamee B (2004) Effect of acid speciation on solid waste liquefaction in an anaerobic acid digester. Water Res 38:2417–2423
Bauchop T (1967) Inhibition of rumen methanogenesis by methane analogues. J Bacteriol 94:171–175
Bedard DL (2008) A case study for microbial biodegradation: anaerobic bacterial reductive dechlorination of polychlorinated biphenyls—from sediment to defined medium. Annu Rev Microbiol 62:253–270
Buckel W (1999) Anaerobic energy metabolism. In: Lengeler JW, Drews G, Schlegel HG (eds) Biology of the prokaryotes. Wiley-Blackwell, Stuttgart, pp 278–326
Chidthaisong A, Conrad R (2000) Specificity of chloroform, 2-bromoethanesulfonate and fluoroacetate to inhibit methanogenesis and other anaerobic processes in anoxic rice field soil. Soil Biol Biochem 32:977–988
Chiu PC, Lee M (2001) 2-Bromoethanesulfonate affects bacteria in a trichloroethene-dechlorinating culture. Appl Environ Microbiol 67:2371–2374
Chouari R, Paslier DL, Daegelen P, Ginestet P, Weissenbach J, Sghir A (2005) Novel predominant archaeal and bacterial groups revealed by molecular analysis of an anaerobic sludge digester. Environ Microbiol 7:1104–1115
Conrad R, Klose M (2000) Selective inhibition of reactions involved in methanogenesis and fatty acid production on rice roots. FEMS Microbiol Ecol 34:27–34
Cord-Ruwisch R, Lovley DR, Schink B (1998) Growth of Geobacter sulfurreducens with acetate in syntrophic cooperation with hydrogen-oxidizing anaerobic partners. Appl Environ Microbiol 64:2232–2236
Davenport RJ, Curtis TP, Goodfellow M, Stainsby FM, Bingley M (2000) Quantitative use of fluorescent in situ hybridization to examine relationships between mycolic acid-containing actinomycetes and foaming in activated sludge plants. Appl Environ Microbiol 66:1158–1166
de Bok FAM, Plugge CM, Stams AJM (2004) Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res 38:1368–1375
Drake HL, Horn MA, Wüst PK (2009) Intermediary ecosystem metabolism as a main driver of methanogenesis in acidic wetland soil. Environ Microbiol Rep 1:307–318
Galagan J, Nusbaum C, Roy A, Endrizzi M, Macdonald P, FitzHugh W, Calvo S, Engels R, Smirnov S, Atnoor D (2002) The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res 12:532–542
Hansen KH, Ahring BK, Raskin L (1999) Quantification of syntrophic fatty acid–oxidizing bacteria in a mesophilic biogas reactor by oligonucleotide probe hybridization. Appl Environ Microbiol 65:4767–4774
Harmsen HJM, Kengen HMP, Akkermans ADL, Stams AJM, Devos WM (1996) Detection and localization of syntrophic propionate-oxidizing bacteria in granular sludge by in situ hybridization using 16S rRNA-based oligonucleotide probes. Appl Environ Microbiol 62:1656–1663
Hu B, Chen S (2007) Pretreatment of methanogenic granules for immobilized hydrogen fermentation. Int J Hydrogen Energy 32:3266–3273
Iannotti E, Kafkewitz D, Wolin M, Bryant M (1973) Glucose fermentation products in Ruminococcus albus grown in continuous culture with Vibrio succinogenes: changes caused by interspecies transfer of H2. J Bacteriol 114:1231–1240
Jackson BE, McInerney MJ (2002) Anaerobic microbial metabolism can proceed close to thermodynamic limits. Nature 415:454–456
Kemp PF, Aller JY (2004) Estimating prokaryotic diversity: when are 16S rDNA libraries large enough? Limnol Oceanogr Methods 2:114–125
Kim JR, Min B, Logan BE (2005) Evaluation of procedures to acclimate a microbial fuel cell for electricity production. Appl Microbiol Biotechnol 68:23–30
Löffler FE, Ritalahti KM, Tiedje JM (1997) Dechlorination of chloroethenes is inhibited by 2-bromoethanesulfonate in the absence of methanogens. Appl Environ Microbiol 63:4982–4985
Makras L, Vuyst LD (2006) The in vitro inhibition of Gram-negative pathogenic bacteria by bifidobacteria is caused by the production of organic acids. Int Dairy J 16:1049–1057
McInerney MJ, Bryant MP (1981) Basic principles of bioconversions in anaerobic digestion and methanogenesis. In: Sofer SS, Zaborsky OR (eds) Biomass conversion processes for energy and fuels. Plenum, New York, pp 277–296
Narihiro T, Sekiguchi Y (2007) Microbial communities in anaerobic digestion processes for waste and wastewater treatment: a microbiological update. Curr Opin Biotechnol 18:273–278
Noll M, Matthies D, Frenzel P, Frenzel M, Liesack W (2005) Succession of bacterial community structure and diversity in a paddy soil oxygen gradient. Environ Microbiol 7:382–395
Sauer M, Porro D, Mattanovich D, Branduardi P (2008) Microbial production of organic acids: expanding the markets. Trends Biotechnol 26:100–108
Scholten JCM, Conrad R (2000) Energetics of syntrophic propionate oxidation in defined batch and chemostat cocultures. Appl Environ Microbiol 66:2934–2942
Siriwongrungson V, Zeng RJ, Angelidaki I (2007) Homoacetogenesis as the alternative pathway for H2 sink during thermophilic anaerobic degradation of butyrate under suppressed methanogenesis. Water Res 41:4204–4210
Stams AJ, Plugge CM (2009) Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat Rev Microbiol 7:568–577
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Microbiol Mol Biol Rev 41:100–180
Tiquia SM (2005) Microbial community dynamics in manure composts based on 16S and 18S rDNA T-RFLP profiles. Environ Technol 26:1101–1114
Ungerfeld EM, Rust SR, Boone DR, Liu Y (2004) Effects of several inhibitors on pure cultures of ruminal methanogens. J Appl Microbiol 97:520–526
Valdez-Vazqueza I, Poggi-Varaldo HM (2009) Hydrogen production by fermentative consortia. Renew Sustain Energy Rev 13:1000–1013
Wüst PK, Horn MA, Drake HL (2009) Trophic links between fermenters and methanogens in a moderately acidic fen soil. Environ Microbiol 11:1395–1409
Wilms R, Köpke B, Sass H, Chang TS, Cypionka H, Engelen B (2006) Deep biosphere-related bacteria within the subsurface of tidal flat sediments. Environ Microbiol 8:709–719
Xu K, Liu H, Chen J (2010) Effect of classic methanogenic inhibitors on the quantity and diversity of archaeal community and the reductive homoacetogenic activity during the process of anaerobic sludge digestion. Bioresour Technol 101:2600–2607
Zelles L, Palojarvi A, Kandeler E, von Lützow M, Winter K, Bai QY (1997) Changes in soil microbial properties and phospholipid fatty acid fractions after chloroform fumigation. Soil Biol Biochem 29:1325–1336
Acknowledgments
We thank the anonymous reviewers for the valuable comments and suggestions that improved the manuscript. This study was financially supported by the Major State Basic Research Development Program (973 Program) of China (No. 2007CB714036), the Tai-Lake Water Specific Program of Jiangsu Province (No. BS2007098), the Key Technologies R&D Program (Social Development) of Jiangsu Province (No. BE2008627), and State Key Lab of Urban Water Resource and Environment (HIT; No. QAK200807).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 39 kb)
Rights and permissions
About this article
Cite this article
Xu, K., Liu, H., Li, X. et al. Typical methanogenic inhibitors can considerably alter bacterial populations and affect the interaction between fatty acid degraders and homoacetogens. Appl Microbiol Biotechnol 87, 2267–2279 (2010). https://doi.org/10.1007/s00253-010-2708-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2708-y