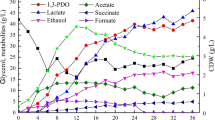
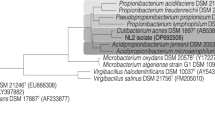
Abstract
Bacteria that harbor the mer operon in their genome are able to enzymatically reduce mercury (II) to the volatile form of mercury Hg (0). Detoxification of contaminated waste by using these bacteria may be an alternative to conventional methods for mercury removal. Residual glycerol from the biodiesel industry can be used as a carbon source to accelerate the process. This work shows for the first time the feasibility of using residual glycerol as a carbon source for Hg removal by bacteria prospected from contaminated environments. Eight bacterial isolates were able to remove mercury and degrade glycerol in mineral medium and residual glycerol. Mercury removal was monitored by atomic absorption spectroscopy and glycerol degradation by high performance liquid chromatography. The best results of mercury removal and glycerol degradation were obtained using isolates of Serratia marcescens M25C (85 and 100 %), Klebsiella pneumoniae PLB (90 and 100 %), Klebsiella oxytoca (90 and 100 %), and Arthrobacter sp. U3 (80 and 75 %), with addition of 0.5 g L−1 yeast extract. The Arthrobacter sp. U3 isolate is common in soils and has proven to be a promising candidate for environment applications due to its low pathogenicity and higher Hg removal and glycerol degradation rates.



Similar content being viewed by others
References
Bafana, A., Krishnamurthi, K., Patil, M., & Chakrabarti, T. (2010). Heavy metal resistance in Arthrobacter ramosus strain G2 isolated from mercuric salt-contaminated soil. Journal of Hazardous Materials, 177, 481–486.
Ball, M. M., Carrero, P., Castro, D., & Yarzabal, L. A. (2007). Mercury resistance in bacterial strains isolated from tailing ponds in a gold mining area near El Callao (Bolivar State, Venezuela). Current Microbiology, 54, 149–154.
Barkay, T., & Wagner-Döbler, I. (2005). Microbial transformations of mercury: potentials, challenges, and achievements in controlling mercury toxicity in the environment. Advances in Applied Microbiology, 57, 1–52.
Barkay, T., Miller, S. M., & Summers, A. (2003). Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiology Reviews, 27, 355–384.
Bernasconi, et al. (2004). Arthrobacter woluwensis subacute infective endocarditis: case report and review of the literature. Clinical Infectious Diseases, 38, 27–31.
Biebl, H., Menzel, K., Zeng, A. P., & Deckwer, W. D. (1999). Microbial production of 1,3-propanediol. Applied Microbiology and Biotechnology, 52, 289–297.
Bodík, I., Blastáková, A., Sedlácek, S., & Hutnan, M. (2009). Biodiesel waste as source of organic carbon for municipal WWTP denitrification. Bioresource Technology, 100, 2452–2456.
Boening, D. W. (2000). Ecological effects, transport, and fate of mercury: a general review. Chemosphere, 40, 1335–1351.
Cabral, L., Giovanella, P., Gianello, C., Bento, F. M., Andreazza, R., & Camargo, F. A. O. (2013). Isolation and characterization of bacteria from mercury contaminated sites in Rio Grande do Sul, Brazil, and assessment of methylmercury removal capability of a Pseudomonas putida V1 strain. Biodegradation, 24, 319–331.
Cabral, L., et al. (2014). Impact of selected anions and metals on the growth and in vitro removal of methylmercury by Pseudomonas putida V1. International Biodeterioration & Biodegradation, 91, 29–36.
Camargo, F. A. O., Bento, F. M., Okeke, B. C., & Frankenberger, W. T. (2004). Hexavalent chromium reduction by an actinomycete, Arthrobacter acrytallopoietes ES 32. Biological Trace Element Research, 97, 183–194.
Camargo, F. A. O., Bento, F. M., & Jacques, R. J. S. (2007). Uso de microrganismos para remediação de metais. In C. A. Ceretta, L. S. Silva, & J. M. Reichert (Eds.), Tópicos em ciência do solo (pp. 468–496). Minas Gerais: Sociedade Brasileira de Ciência do Solo.
Chatziefthimou, A., Crespo-Medina, M., Wang, Y., et al. (2007). The isolation and initial characterization of mercury resistant chemolithotrophic thermophilic bacteria from mercury rich geothermal springs. Extremophiles, 11, 469–479.
Chien, M., Nakahata, R., Ono, T., Miysuke, K., & Endo, G. (2012). Mercury removal and recovery by immobilized Bacillus megaterium MB1. Frontiers of Chemical Science and Engineering, 6, 192–197.
Choi, W. J., Hartono, M. R., Chan, W. H., & Yeo, S. S. (2011). Ethanol production from biodiesel-derived crude glycerol by newly isolated Kluyvera cryocrescen. Applied Microbiology and Biotechnology, 89, 1255–1264.
Daniel, R., Stuertz, K., & Gottschalk, G. (1995). Biochemical and molecular characterization of the oxidative branch of glycerol utilization by Citrobacter freundii. Journal of Bacteriology, 177, 4392–4401.
Dasari, M. A., Kiatsimkul, P. P., Sutterlin, W. R., & Suppes, G. J. (2005). Low-pressure hydrogenolysis of glycerol to propylene glycol. Applied Catalysis A: General, 281, 225–231.
Dash, H. R., & Das, S. (2012). Bioremediation of mercury and importance of bacterial mer genes. International Biodeterioration & Biodegradation, 75, 207–213.
Dash, H. R., Mangwani, N., & Das, S. (2013). Characterization and potential application in mercury bioremediation of highly mercury-resistant marine bacterium Bacillus thuringiensis PW-05. Environmental Science and Pollution Research International, 21, 2642–2653.
Dharmadi, Y., Murarka, A., & Gonzalez, R. (2006). Anaerobic fermentation of glycerol by Escherichia coli: a new platform for metabolic engineering. Biotechnology and Bioengineering, 94, 821–829.
Driscoll, C. T., Mason, R. P., Chan, H. M., Jacob, D. J., & Pirrone, N. (2013). Mercury as a global pollutant: sources, pathways, and effects. Environmental Science & Technology, 47, 4967–4983.
Durnin, G., Clomburg, J., Yeates, Z., Alvarez, P. J. J., Zygourakis, K., Campbell, P., & Gonzalez, R. (2009). Understanding and harnessing the microaerobic metabolism of glycerol in Escherichia coli. Biotechnology and Bioengineering, 103, 148–161.
Erbe, J. L., Taylor, K. B., & Hall, L. M. (1995). Metalloregulation of the cyanobacterial smt locus: identification of SmtB binding sites and direct interaction with metals. Nucleic Acids Research, 23, 2472–2478.
Escapa, A., Manuel, M. F., Morant, A., Gomez, X., Guiot, S. R., & Tartakovsky, B. (2009). Hydrogen production from glycerol in a membraneless microbial electrolysis cell. Energy & Fuels, 23, 4612–4618.
Farrell, R. E., Germida, J. J., & Huang, P. M. (1993). Effects of chemical speciation in growth media on the toxicity of mercury (II). Applied and Environmental Microbiology, 59, 1507–1514.
Felske, A. D. M., Fehr, W., Pauling, B. V., Von Canstein, H., & Wagner-Döbler, I. (2003). Functional profiling of mercuric reductase (merA) genes in biofilm communities of a technical scale biocatalyzer. BMC Microbiology, 3, 22.
Forage, R. G., & Foster, A. M. (1982). Glycerol fermentation in Klebsiella pneumoniae: functions of the coenzyme B12-dependent glycerol and diol dehydratases. Journal of Bacteriology, 149, 413–419.
Fortunato, R., Crespo, J. G., & Reis, M. A. M. (2005). Biodegradation of thiomersal containing effluents by a mercury resistant Pseudomonas putida strain. Water Research, 39, 3511–3522.
Ghosh, S., Sadhukhan, P. C., Ghosh, D. K., Chaudhuri, J., & Mandal, A. (1996). Studies on the effect of mercury and organomercurial on the growth and nitrogen fixation by mercury-resistant Azotobacter strains. Journal of Applied Microbiology, 80, 319–326.
Giovanella, P., Bento, F., Cabral, L., Gianello, C., & Camargo, F. A. O. (2011). Isolamento e seleção de micro-organismos resistentes e capazes de volatilizar mercúrio. Quim Nova, 34, 231–236.
Guerrero, J., Tayà, C., Guisasola, A., & Baeza, J. A. (2012). Glycerol as a sole carbon source for enhanced biological phosphorus removal. Water Research, 46, 2983–2991.
Hobman, J. L. (2007). MerR family transcription activators: similar designs different specifities. Molecular Microbiology, 65, 1275–1278.
Homann, T., Tag, C., Biebl, H., Deckwer, W. D., & Schink, B. (1990). Fermentation of glycerol to 1,3-propanediol by Klebsiella and Citrobacter strains. Applied Microbiology and Biotechnology, 33, 121–126.
Hozzein, W. N., Ali, M. I. A., & Rabie, W. (2008). A new preferential medium for enumeration and isolation of desert actinomycetes. World Journal of Microbiology and Biotechnology, 24, 1547–1552.
Johnson, D. T., & Taconi, K. A. (2007). The glycerin glut: options for the value-added conversion of crude glycerol resulting from biodiesel production. Environmental Progress, 26, 338–348.
Jones, D., & Keddie, R. M. (2006). The genus Arthrobacter. Prokaryotes, 3, 945–960.
Kannan, S. K., & Krishnamoorthy, R. (2006). Isolation of mercury resistant bacteria and influence of abiotic factors on bioavailability of mercury: a case study in Pulicat lake north of Chennai, South East Índia. Science of the Total Environment, 367, 341–353.
Keramati, P., Hoodaji, M., & Tahmourespour, A. (2011). Multi-metal resistance study of bacteria highly resistant to mercury isolated from dental clinic effluent. African Journal of Microbiology Research, 5, 831–837.
Li, J., Zhang, L., Wu, Y., Liu, Y., Zhou, P., Wen, S., et al. (2009). A national survey of polychlorinated dioxins, furans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (DL-PCBs) in human milk in China. Chemosphere, 75, 1236–1242.
Liebert, C. A., Wireman, J., Smith, T., & Summers, A. O. (1997). Phylogeny of mercury resistance (mer) operons of gram-negative bacteria isolated from the fecal flora of primates. Applied and Environmental Microbiology, 63, 1066–1076.
Luers, F., Seyfried, M., Daniel, R., & Gottschalk, G. (1997). Glycerol conversion to 1,3-propanediol by Clostridium pasteurianum: cloning and expression of the gene encoding 1,3-propanediol dehydrogenase. FEMS Microbiology Letters, 154, 337–345.
Ma, Z., Jacobsen, F. E., & Giedroc, D. P. (2009). Coordination chemistry of bacterial metal transport and sensing. Chemistry Review, 109, 4644–4681.
Macis, L., Daniel, R., & Gottschalk, G. (1998). Properties and sequence of the coenzyme B12-dependent glycerol dehydratase of Clostridium pasteurianum. FEMS Microbiology Letters, 164, 21–28.
Maxson, P. (2005). Dynamics of mercury pollution on regional and global scales. In N. Pirrone & K. R. Mahaffey (Eds.), Atmospheric processes and human exposures around the world (pp. 25–50). New York: Springer.
Menzel, K., Zeng, A. P., & Deckwer, W. D. (1997). Enzymatic evidence for an involvement of pyruvate dehydrogenase in the anaerobic glycerol metabolism of Klebsiella pneumoniae. Journal of Biotechnology, 56, 135–142.
Mortazavi, S., Rezaee, A., Khavanin, A., Varmazyar, S., & Jafarzadeh, M. (2005). Removal of mercuric chloride by a mercury resistant Pseudomonas putida strain. Journal of Biological Sciences, 5, 269–273.
Mu, Y., Teng, H., Zhang, D. J., Wang, W., & Xiu, Z. L. (2006). Microbial production of 1, 3-propanediol by Klebsiella pneumoniae using crude glycerol from biodiesel preparations. Biotechnological Letters, 28, 1755–1759.
Murarka, A., Dharmadi, Y., Yazdani, S. S., & Gonzalex, R. (2008). Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Applied and Environmental Microbiology, 74, 1124–1135.
Naja, G. M., & Volesky, B. (2010). Treatment of metal-bearing effluents: removal and recovery. In L. K. Wang, J. P. Chen, Y. T. Hung, & N. K. Shammas (Eds.), Handbook on heavy metals in the environment (pp. 247–291). Boca Raton: CRC Press.
Nakamura, K., Iwahara, M., & Furukama, K. (2001). Screening of organomercurial-volatilizing bacteria in the mercury-polluted sediments and seawater of Minamata Bay in Japan. Clean Product and Process, 3, 104–107.
Nakas, J. P., Schaedle, M., Parkinson, C. M., Coonley, C. E., & Tanenbaum, S. W. (1983). System development of linked-fermentation production of solvents from algal biomass. Applied and Environmental Microbiology, 46, 1017–1023.
Narita, M., Chiba, K., Nishizawa, H., et al. (2003). Diversity of mercury resistance determinants among Bacillus strains isolated from sediment of Minamata Bay. FEMS Microbiology Letters, 223, 73–82.
Németh, A., Kupcsulik, B., & Sevella, B. (2003). 1,3-Propanediol oxidoreductase production with Klebsiella pneumoniae DSM2026. World Journal of Microbiology and Biotechnology, 19, 659–663.
Ní Chadhain, S. M., Schaefer, J. K., Crane, S., Zylstra, G. J., & Barkay, T. (2006). Analysis of mercuric reductase (merA) gene diversity in an anaerobic mercury-contaminated sediment enrichment. Environmental Microbiology, 8, 1746–1752.
Nies, D. H. (1999). Microbial heavy-metal resistance. Applied Microbiology and Biotechnology, 51, 730–750.
Nikel, P. I., Ramirez, M. C., Pettinari, M. J., Méndez, B. S., & Galvagno, M. A. (2010). Ethanol synthesis from glycerol by Escherichia coli redox mutants expressing adhE from Leuconostoc mesenteroides. Journal of Applied Microbiology, 109, 492–504.
Oregaard, G., & Sørensen, S. J. (2007). High diversity of bacterial mercuric reductase genes from surface and sub-surface floodplain soil (Oak Ridge, USA). The ISME Journal, 1, 453–467.
Osborn, A. M., Bruce, K. D., Strike, P., & Ritchie, D. A. (1997). Distribution, diversity and evolution of the bacterial mercury resistance (mer) operon. FEMS Microbiology Review, 19, 239–262.
Pepi, M., et al. (2010). Mercury-resistant bacterial strains Pseudomonas and Psychrobacter spp. isolated from sediments of Orbetello Lagoon (Italy) and their possible use in bioremediation processes. International Biodeterioration & Biodegradation, 65, 85–91.
Pirrone, N., Cinnirella, S., Feng, X., et al. (2010). Global mercury emissions to the atmosphere from natural and anthropogenic sources. Atmos. Chemical Physics, 10, 5951–5964.
Ramamoorthy, S., & Kushner, D. J. (1975). Binding of mercury and other heavy metal ions by microbial growth media. Microbiology Ecology, 2, 162–176.
Rodriguez, M. A. A., Gozzi, A., Abreu Filho, B. A., Zanin, G. M., & Moraes, F. F. (2012). Potential of Klebsiella oxytoca for 1,3-propanediol production from glycerol under excess substrate conditions. African Journal of Biotechnology, 11, 12675–12681.
Rossi, D. M., Costa, J. B., Souza, E. A., Peralba, M. C. R., & Ayub, M. A. Z. (2012). Bioconversion of residual glycerol from biodiesel synthesis into 1,3-propanediol and ethanol by isolated bacteria from environmental consortia. Renewable Energy, 39, 223–227.
Sadhukhan, S., Ghosh, J., Chaudhuri, D. K., & Ghosh, A. (1997). Mandal mercury and organomercurial resistance in bacteria isolated from freshwater fish of wetland fisheries around Calcutta. Environmental Pollution, 97, 71–78.
Sajjaphan, K., Shapir, N., Wackett, L. P., Palmer, M., Blackmon, B., Tomkins, J., & Sadowsky, J. (2004). Arthrobacter aurescens TC1 atrazine catabolism genes trzN, atzB, and atzC are linked on a 160-kilobase region and are functional in Escherichia coli. Applied and Environmental Microbiology, 70, 4402–4407.
Sambrook, J., & Russel, D. W. (2001). Molecular cloning: a laboratory manual (3rd ed.). New York: Cold Spring Arbor.
Sarma, S. J., Brar, S. K., Sydney, E. B., Bihan, Y. L., Buelna, G., & Soccol, C. R. (2012). Microbial hydrogen production by bioconversion of crude glycerol: a review. International Journal of Hydrogen Energy, 37, 6473–6490.
Seifert, C., Bowien, S., Gottschalk, G., & Daniel, R. (2001). Identification and expression of the genes and purification and characterization of the genes products involved in reactivation of coenzyme B12-dependent glycerol dehydratase of Citrobacter freundii. European Journal of Biochemistry, 268, 2369–2378.
Silva, G. P., Mack, M., & Contiero, J. (2009). Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnology Advances, 27, 30–39.
Silver, S., & Hobman, J. L. (2007). Mercury microbiology: resistance systems, environmental aspects, methylation, and human health. Microbiology Monographs, 6, 357–370.
Silver, S., & Phung, L. T. (1996). Bacterial heavy metal resistance: new surprises. Annual Review of Microbiology, 50, 753–789.
Silver, S., & Walderhaug, M. (1992). Gene regulation of plasmid- and chromosomal determined inorganic ion transport in bacteria. Microbiological Reviews, 56, 195–228.
Sone, Y., Mochizuki, Y., Koizawa, K., Nakamura, R., Pan-Hou, H., Itoh, T., & Kiyono, M. (2013). Mercurial-resistance determinants in Pseudomonas strain K-62 plasmid pMR68. AMB Express, 3, 41.
Summers, A. O. (1992). Untwist and shout: a heavy metal responsive transcriptional regulator. Journal of Bacteriology, 174, 3097–3101.
Tao, J., Wang, X., Shen, Y., & Wei, D. (2005). Strategy for the improvement of prodigiosin production by a Serratia marcescens mutant through fed-batch fermentation. World Journal of Microbiology and Biotechnology, 21, 969–972.
Thompson, J. C., & He, B. B. (2006). Characterization of crude glycerol from biodiesel production from multiple feedstocks. Applied Engineering in Agriculture, 22, 261–265.
Tong, I. T., Liao, H. H., & Cameron, D. C. (1991). 1,3-propanediol production by Escherichia coli expressing genes from the Klebsiella pneumoniae dha regulon. Applied and Environmental Microbiology, 57, 3541–3546.
Torà, J. A., Baeza, J. A., Carrera, J., & Oleszkiewicz, J. A. (2011). Denitritation of a high-strength nitrite wastewater in a sequencing batch reactor using different organic carbon sources. Chemical Engineering Journal, 172, 994–998.
UNEP. http://www.chem.unep.ch/mercury/report/GMA-report-TOC.htm. Accessed 23 Sep 2013.
Vetriani, C., et al. (2005). Mercury adaptation among bacteria from a deep-sea hydrothermal vent. Applied and Environmental Microbiology, 71, 220–226.
Wagner-Döbler, I. (2003). Pilot plant for bioremediation of mercury-containing industrial wastewater. Applied Microbiology and Biotechnology, 62, 124–133.
Waldron, K. J., & Robinson, N. J. (2009). How do bacterial cells ensure that metalloproteins get the correct metal? Nature Reviews. Microbiology, 7, 25–35.
Yazdani, S. S., & Gonzales, R. (2007). Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Current Opinion in Biotechnology, 18, 213–219.
Yuan, Q., Sparling, R., Lagasse, P., Lee, Y. M., Taniguchi, D., & Oleszkiewicz, J. A. (2010). Enhancing biological phosphorus removal with glycerol. Water Science and Technology, 61, 1837–1843.
Zeng, A. P., & Sabra, W. (2011). Microbial production of diols as platform chemicals: recent progress. Current Opinion in Biotechnology, 22, 749–757.
Zeroual, Y., Moutaouakkil, A., & Blaghen, M. (2001). Volatilization of mercury by immobilized bacteria (Klebsiella pneumoniae) in different support by using fluidized bed bioreactor. Current Microbiology, 43, 322–327.
Zhang, R., Wang, Y., & Gu, J. D. (2006). Identification of environmental plasmid-bearing Vibrio species isolated from polluted and pristine marine reserves of Hong Kong and resistance to antibiotics and mercury. Antonie van Leeuwenhoek, 89, 307–315.
Zhang, W., Chen, L., & Liu, D. (2012). Characterization of a marine-isolated mercury resistant Pseudomonas putida strain SP1 and its potential application in marine mercury reduction. Applied Microbiology and Biotechnology, 93, 1305–1314.
Zhu, M. M., Lawman, P. D., & Cameron, D. C. (2002). Improving 1.3-propanediol production from glycerol in a metabolically engineered Escherichia coli by reducing accumulation of sn-glycerol-3-phosphate. Biotechnology Progress, 18, 694–699.
Acknowledgments
The authors wish to thank the Brazilian Bureau for Science and Technology (CNPq) and CAPES for the financial support for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giovanella, P., Costa, A.P., Schäffer, N. et al. Detoxification of Mercury by Bacteria Using Crude Glycerol from Biodiesel as a Carbon Source. Water Air Soil Pollut 226, 224 (2015). https://doi.org/10.1007/s11270-015-2480-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2480-9