Abstract
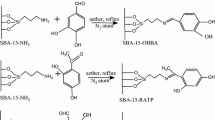
2,3-Dihydroxybenzaldehyde modified silica gel (SGDHB) was prepared and then used for the removal of boron. Adsorption of boron on the SGDHB was examined with respect to the equilibrium adsorption, kinetics and as a function of pH. Boron is strongly retained on the SGDHB between pH 7 and pH 9. The results indicated that the adsorption equilibrium was well described by the Langmuir equation. The SGDHB exhibited an excellent sorption capacity with 3.812 mmol B/g SGDHB under experimental conditions. The material shows reasonably rapid sorption ability, with boron in 25 mL of 0.01 M H3BO3 solution being removed almost completely within 30 min of contact time with 0.12 g of the modified silica gel. The SGDHB was demonstrated to be an efficient sorbent for the removal of boron.









Similar content being viewed by others
References
Akyuz, E., Imamoglu, M., & Altundag, H. (2013). Selective determination of Cr(VI) by FAAS after solid phase extraction on bis(3-aminopropyl)amine-bonded silica gel. Atomic Spectroscopy, 34, 146–153.
Alan, M., Kara, D., & Fisher, A. (2007). Preconcentration of heavy metals and matrix elimination using silica gel chemically modified with 2,3-dihydroxybenzaldehyde. Separation Science and Technology, 42, 879–895.
Alkan, M., & Doğan, M. (2001). Adsorption of copper(II) onto perlite. Journal of Colloid and Interface Science, 243, 280–291.
Allen, S. J., Mckay, G., & Porter, J. F. (2004). Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. Journal of Colloid and Interface Science, 280, 322–333.
Al-Muhtaseb, A. A. H., Ibrahim, K. A., Albadarin, A. B., Ali-Khashman, O., Walker, G. M., & Ahmad, M. N. M. (2011). Remediation of phenol-contaminated water by adsorption using poly(methyl methacrylate) (PMMA). Chemical Engineering Journal, 168, 691–699.
Ayers, R.S.(1994). Water quality for agriculture (Report on FAO Irrigating and Drainage, M-56 ISBN, 92-5-102263-1), Rome.
Badruk, M., Kabay, N., Demircioglu, M., Mordogan, H., & Ipekoglu, U. (1999a). Removal of boron from wastewater of geothermal power plant by selective ion-exchange resins. I. Batch sorption-elution studies. Separation Science and Technology, 34(13), 2553–2569.
Badruk, M., Kabay, N., Demircioglu, M., Mordogan, H., & Ipekoglu, U. (1999b). Removal of boron from wastewater of geothermal power plant by selective ion-exchange resins. II. Column sorption-elution studies. Separation Science and Technology, 34(15), 2981–2995.
Boncukcuoglu, R., Yilmaz, A. E., Kocakerim, M. M., & Copur, M. (2004). An empirical model for kinetics of boron removal from boron containing wastewaters by ion exchange in a batch reactor. Desalination, 160, 159–l 66.
Cagirdi, D., Altundag, H., Imamoglu, M., & Tuzen, M. (2014). Solid-phase extraction of copper(II) in water and food samples using silica gel modified with bis(3-aminopropyl)amine and determination by flame atomic absorption spectrometry. Journal of AOAC International, 97, 1137–1142.
De Moraes, S. V. M., Brasil, J. L., Milcharek, C. D., Martins, L. C., Laranjo, M. T., Gallas, M. R., Benvenutti, E. V., & Lima, E. C. (2005). Use of 1,3-diaminepropane-3-propyl grafted onto a silica gel as a sorbent for flow-injection spectrophotometric determination of copper (II) in digests of biological materials and natural waters. Spectrochimica Acta Part A, 62, 398–406.
Demey, H., Vincent, T., Ruiz, M., Nogueras, M., Sastre, A. M., & Guibal, E. (2014). Boron recovery from seawater with a new low-cost adsorbent material. Chemical Engineering Journal, 254, 463–471.
Fan, J., Qin, Y., Ye, C., Peng, P., & Wu, C. (2008). Preparation of the diphenylcarbazone-functionalized silica gel and its application to on-line selective solid-phase extraction and determination of mercury by flow-injection spectrophotometry. Journal of Hazardous Materials, 150, 343–350.
Foo, K. Y., & Hameed, B. H. (2010). Review, Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal, 156, 2–10.
Franson, M.A.H. (1995). Standard methods for examination of water and waste water, American Publication Health Associations.
Freundlich, H. (1906). Ueber die adsorption in Loesungen. Leipzig: Engelmann.
Helfferich, F. (1962). Ion exchange. New York: McGraw-Hill.
Ho, Y., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34, 451–465.
Howe, P. D. (1998). A review of boron effects in the environment. Biological Trace Element Research, 66, 153–166.
Imamoglu, M., & Gunes, V. (2012). Solid phase extraction of Au and Pd by silica gel functionalized with triethylenetetramine for determination by FAAS. Atomic Spectroscopy, 33, 205–211.
Sasaki, K., Qiu, X., Miyawaki, J., Ideta, K., Takamori, H., Moriyama, S., Hirajima, T. (2013). Contribution of boron-specific resins containing N-methylglucamine groups to immobilization of borate/boric acid in a permeable reactive barrier comprising agglomerated MgO. Desalination, 337, 109–116.
Isaacs-Paez, E. D., Leyva-Ramos, R., Jacobo-Azuara, A., Martinez-Rosales, J. M., & Flores-Cano, J. V. (2014). Adsorption of boron on calcined AlMg layered double hydroxide from aqueous solutions. Mechanism and effect of operating conditions. Chemical Engineering Journal, 245, 248–257.
Kluczka, J., Korolewicz, T., Zołotajkin, M., Simka, W., & Raczek, M. (2013). A new adsorbent for boron removal from aqueous solutions. Environmental Technology, 34, 1369–1376.
Kunin, R., & Preuss, A. F. (1964). Characterization of a boron-specific ion exchange resin. Industrial & Engineering Chemistry Product Research and Development, 3, 304–306.
Lenntech, Water treatment solutions, http://www.lenntech.com/periodic/elements/b.htm, Accessed 9 July 2014.
Limousin, G., Gaudet, J. P., Charlet, L., Szenknect, S., Barthes, V., & Krimissa, M. (2007). Sorption isotherms: a review on physical bases, modeling and measurement. Applied Geochemistry, 22, 249–275.
Liu, P., Su, Z., Wu, X., & Pu, Q. (2002). Application of isodiphenylthiourea immobilized silica gel to flow injection on-line microcolumn preconcentration and separation coupled with flame atomic absorption spectrometry for interference free determination of trace silver, gold, palladium and platinum in geological and metallurgical samples. Journal of Analytical Atomic Spectrometry, 17, 125–130.
Liu, H., Qing, B., Ye, X., Li, Q., Lee, K., & Wu, Z. (2009). Boron adsorption by composite magnetic particles. Chemical Engineering Journal, 151, 235–240.
Martell, A. E., & Smith, R. M. (1989). Critical stability constants (Vol. 6, p. 398). New York: Plenum Press.
McKay, G., & Poots, V. J. P. (1980). Kinetics and diffusion processes in colour removal from effluent using wood as an adsorbent. Journal of Chemical Technology and Biotechnology, 30, 279–292.
McKay, G., Blair, H. S., & Gardner, R. J. (1982). Adsorption of dyes on chitin. I. Equilibrium studies. Journal of Applied Polymer Science, 27, 3043–3057.
Nishihama, S., Sumiyoshi, Y., Ookubo, T., & Yoshizuka, K. (2013). Adsorption of boron using glucamine-based chelate adsorbents. Desalination, 310, 81–86.
Parsaei, M., Goodarzi, M. S., & Nasef, M. M. (2011). Adsorption study for removal of boron using ion exchange resin in Bach system. 2011 2nd International Conference on Environmental Science and Technology, IPCBEE (Vol. 6, pp. 398–402). Singapore: IACSIT Press.
Poslu, K., & Dudeney, A. W. L. (1983). Solvent-extraction of boron with 2-ethyl-1,3-hexanediol and 2-chloro-4-(1,1,3,3-tetramethylbutyl)-6-methylol-phenol in petroleum ether, kerosene and chloroform solutions. Hydrometallurgy, 10, 47–60.
Sanchez-Ramos, S., Medina-Hemandez, M. J., & Sagrado, S. (1998). Flow injection spectrophotometric determination of boron in ceramic materials. Talanta, 45, 835–842.
Schilde, U., & Uhlemann, E. (1992). A simple method for the control of ion-exchange processes with boric acid using specific chelating resins. Reactive Polymers, 18, 155–158.
Senkal, B. F., & Bicak, N. (2003). Polymer supported iminodipropylene glycol functions for removal of boron. Reactive & Functional Polymers, 55, 27–33.
Thakur, N., Kumar, S. A., Shinde, R. N., Pandey, A. K., Kumar, S. D., & Reddy, A. V. R. (2013). Extractive fixed-site polymer sorbent for selective boron removal from natural water. Journal of Hazardous Materials, 260, 1023–1031.
Ting, T. M., Hoshina, H., Seko, N., & Tamada, M. (2013). Removal of boron by boron-selective adsorbent prepared using radiation induced grafting technique. Desalination and Water Treatment, 51, 2602–2608.
Vijayaraghavan, K., Padmesh, T. V. N., Palanivelu, K., & Velan, M. (2006). Biosorption of nickel(II) ions onto Sargassumwightii: application of two-parameter and three parameter isotherm models. Journal of Hazardous Materials, B133, 304–308.
Weber, T. W., & Chakravorti, R. K. (1974). Pore and solid diffusion models for fixed-bed adsorbers. AIChE Journal, 20, 228–238.
Yılmaz, A. E., Boncukcuoglu, R., Yılmaz, M. T., & Kocakerim, M. (2005). Adsorption of boron from boron-containing wastewaters by ion exchange in a continuous reactor. Journal of Hazardous Materials, B117, 221–226.
Yoshimura, K., Miyazaki, Y., Sawada, S., & Waki, H. (1996). 11 B NMR studies on complexation of borate with linear and crosslinked polysaccharides. Journal of the Chemical Society, Faraday Transactions, 92, 651–656.
Yu, S., Xue, H., Fan, Y., & Shi, R. (2013). Synthesis, characterization of salicylic-HCHO polymeric resin and its evaluation as a boron adsorbent. Chemical Engineering Journal, 219, 327–334.
Yurdakoç, M., Seki, Y., Karahan, S., & Yurdakoç, K. (2005). Kinetic and thermodynamic studies of boron removal by Siral 5, Siral 40, and Siral 80. Journal of Colloid and Interface Science, 286, 440–446.
Zelmanov, G., & Semiat, R. (2014). Boron removal from water and its recovery using iron (Fe+3) oxide/hydroxide-based nanoparticles (NanoFe) and NanoFe-impregnated granular activated carbon as adsorbent. Desalination, 333, 107–117.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kara, D. Removal of Boron from Aqueous Solution by 2,3-Dihydroxybenzaldehyde Modified Silica Gel. Water Air Soil Pollut 226, 223 (2015). https://doi.org/10.1007/s11270-015-2461-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2461-z