Abstract
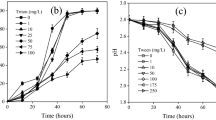
Surfactant can reduce the interfacial tension in liquid–gas system and may probably improve the rate and/or extent of dissolution. This study was conducted to evaluate the effect of three different surfactants (viz., sodium dodecyl sulfate (SDS), Triton X-100, and cetyltrimethylammonium chloride (CTAC)) on CO2 biomineralization by two ureolytic microorganism—Sporosarcina pasteurii and Bacillus megaterium. In S. pasteurii-mediated biomineralization, headspace CO2 content (2.5 mM) was decreased by 40, 52, and 68 % in the presence of SDS, Triton X-100 or CTAC, respectively within the first 8 h of incubation. CO2 removal with B. megaterium in the presence of Triton X-100 (64 %) and CTAC (56 %) was better in comparison to control without surfactant (48 %). However, appreciable CO2 depletion was not observed with SDS, which was just 4 %. On other hand, headspace CO2 loss in the presence of CTAC with B. megaterium did not get biomineralized, as no calcium carbonate was detected. Crystalline phase and morphology of CaCO3 precipitate also varied between ionic and nonionic surfactants. The result suggests that the effect of surfactant on CO2 capture and biomineralization can be largely different, depending on the surfactant and concerned microbial species involved.





Similar content being viewed by others
References
APHA. (2005). Standard Methods for the Examination of Water & Wastewater. American Public Health Association (APHA).
Bachu, S., & Adams, J. J. (2003). Sequestration of CO2 in geological media in response to climate change: capacity of deep saline aquifers to sequester CO2 in solution. Energy Conversion and Management, 44(20), 3151–3175.
Barabesi, C., Galizzi, A., Mastromei, G., Rossi, M., Tamburini, E., & Perito, B. (2007). Bacillus subtilis gene cluster involved in calcium carbonate biomineralization. Journal of Bacteriology, 189(1), 228–35.
Bartnik, F. G. (1992). Interaction of anionic surfactants with proteins, enzymes, and membranes. In C. Gloxhuber & K. Klunstler (Eds.), Anionic surfactants: biochemistry, toxicology, dermatology (2nd ed., pp. 1–42). New York: CRC Press.
Boot-Handford, M. E., Abanades, J. C., Anthony, E. J., Blunt, M. J., Brandani, S., Mac Dowell, N., et al. (2014). Carbon capture and storage update. Energy & Environmental Science, 7(1), 130.
Bramwell, D.-A. P., & Laha, S. (2000). Effects of surfactant addition on the biomineralization and microbial toxicity of phenanthrene. Biodegradation, 11(4), 263–277.
Chen, G., Strevett, K. A., & Vanegas, B. A. (2001). Naphthalene, phenanthrene and surfactant biodegradation. Biodegradation, 12(6), 433–442.
Chibowski, E., Szczes, A., & Holysz, L. (2005). Influence of sodium dodecyl sulfate and static magnetic field on the properties of freshly precipitated calcium carbonate. Langmuir The ACS Journal of Surfaces and Colloids, 21(18), 8114–22.
Chou, C.-W., Seagren, E. A., Aydilek, A. H., & Lai, M. (2011). Biocalcification of sand through ureolysis. Journal of Geotechnical and Geoenvironmental Engineering, 137(12), 1179–1189.
Colomer, A., Pinazo, A., García, M. T., Mitjans, M., Vinardell, M. P., Infante, M. R., et al. (2012). pH-sensitive surfactants from lysine: assessment of their cytotoxicity and environmental behavior. Langmuir The ACS Journal of Surfaces and Colloids, 28(14), 5900–12.
Cserháti, T., Forgács, E., & Oros, G. (2002). Biological activity and environmental impact of anionic surfactants. Environment International, 28(5), 337–348.
Doney, S. C., Fabry, V. J., Feely, R. A., & Kleypas, J. A. (2009). Ocean acidification: the other CO2 problem. Annual Review of Marine Science, 1, 169–92.
Doong, R., & Lei, W. (2003). Solubilization and mineralization of polycyclic aromatic hydrocarbons by Pseudomonas putida in the presence of surfactant. Journal of Hazardous Materials, 96(1), 15–27.
El-Sheikh, S. M., El-Sherbiny, S., Barhoum, A., & Deng, Y. (2013). Effects of cationic surfactant during the precipitation of calcium carbonate nano-particles on their size, morphology, and other characteristics. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 422, 44–49.
Farajzadeh, R., Barati, A., Delil, H. A., Bruining, J., & Zitha, P. L. J. (2007). Mass transfer of CO2 into water and surfactant solutions. Petroleum Science and Technology, 25(12), 1493–1511.
Gilbert, P., Allison, D. G., & McBain, A. J. (2002). Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? Journal of Applied Microbiology, 92(s1), 98S–110S.
Gonzalez-Munoz, M. T., Rodriguez-Navarro, C., Martinez-Ruiz, F., Arias, J. M., Merroun, M. L., & Rodriguez-Gallego, M. (2010). Bacterial biomineralization: new insights from Myxococcus-induced mineral precipitation. Geological Society, London, Special Publications, 336(1), 31–50.
Hammes, F., & Verstraete, W. (2002). Key roles of pH and calcium metabolism in microbial carbonate precipitation. Reviews in Environmental Science and Biotechnology, 1(1), 3–7.
Hanwright, J., Zhou, J., Evans, G. M., & Galvin, K. P. (2005). Influence of surfactant on gas bubble stability. Langmuir The ACS Journal of Surfaces and Colloids, 21(11), 4912–20.
Heywood, B. R., & Mann, S. (1994). Molecular construction of oriented inorganic materials: controlled nucleation of calcite and aragonite under compressed langmuir monolayers. Chemistry of Materials, 6(3), 311–318.
Hrenovic, J., & Ivankovic, T. (2007). Toxicity of anionic and cationic surfactant to Acinetobacter junii in pure culture. Central European Journal of Biology, 2(3), 405–414.
Huang, H. L., & Lee, W. M. (2001). Simultaneous absorption of vapor phase polycyclic aromatic hydrocarbon and carbon dioxide in anionic surfactant solutions. Journal of Environmental Science and Health Part A Toxic/Hazardous Substances & Environmental Engineering, 36(7), 1187–204.
Jansson, C., & Northen, T. (2010). Calcifying cyanobacteria—the potential of biomineralization for carbon capture and storage. Current Opinion in Biotechnology, 21(3), 365–71.
Jin, D., Jiang, X., Jing, X., & Ou, Z. (2007). Effects of concentration, head group, and structure of surfactants on the degradation of phenanthrene. Journal of Hazardous Materials, 144(1–2), 215–21.
Keith, D. W. (2009). Why capture CO2 from the atmosphere? Science (New York, N.Y.), 325(5948), 1654–5.
Klusman, R. W. (2003). Evaluation of leakage potential from a carbon dioxide EOR/sequestration project. Energy Conversion and Management, 44(12), 1921–1940.
Lee, D. M., Guckert, J. B., Ventullo, R. M., Davidson, D. H., & Belanger, S. E. (1997). Stream periphytic biodegradation of the anionic surfactant C12-alkyl sulfate at environmentally relevant concentrations. Ecotoxicology and Environmental Safety, 36(3), 288–96.
Lee, S.-W., Park, S.-B., Jeong, S.-K., Lim, K.-S., Lee, S.-H., & Trachtenberg, M. C. (2010). On carbon dioxide storage based on biomineralization strategies. Micron (Oxford, England 1993), 41(4), 273–82.
Lee, J., Kim, C. G., & Mahanty, B. (2013). Mineralization of gaseous CO2 by Bacillus megaterium in close environment system. Water, Air, & Soil Pollution, 225(1), 1787.
Li, J.-L., & Chen, B.-H. (2009). Surfactant-mediated biodegradation of polycyclic aromatic hydrocarbons. Materials, 2(1), 76–94.
Louvado, A., Coelho, F. J. R. C., Domingues, P., Santos, A. L., Gomes, N. C. M., Almeida, A., & Cunha, A. (2012). Isolation of surfactant-resistant pseudomonads from the estuarine surface microlayer. Journal of Microbiology and Biotechnology, 22(3), 283–91.
Mahanty, B., Kim, S., & Kim, C. G. (2014). Biokinetic modeling of ureolysis in Sporosarcina pasteurii and its integration into a numerical chemodynamic biocalcification model. Chemical Geology, 383, 13–25.
Marques, E., Miguel, M., Dias, R., Melnikov, S., Khan, A., & Lindman, B. (2000). Gel formation and association in systems of catanionic surfactant vesicles and oppositely charged polymers. American Chemical Society Polymer Preprints Division of Polymer Chemistry, 41(1), 737–738.
Mata-Sandoval, J. C., Karns, J., & Torrents, A. (2001). Influence of Rhamnolipids and triton X-100 on the biodegradation of three pesticides in aqueous phase and soil slurries. Journal of Agricultural and Food Chemistry, 49(7), 3296–3303.
McDonnell, G., & Russell, A. D. (1999). Antiseptics and disinfectants: activity, action, and resistance. Clinical Microbiology Reviews, 12(1), 147–79.
Mereghetti, L., Quentin, R., Marquet-Van Der Mee, N., & Audurier, A. (2000). Low sensitivity of Listeria monocytogenes to quaternary ammonium compounds. Applied and Environmental Microbiology, 66(11), 5083–6.
Mikkelsen, M., Jørgensen, M., & Krebs, F. C. (2010). The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy & Environmental Science, 3(1), 43.
Mitchell, A. C., Dideriksen, K., Spangler, L. H., Cunningham, A. B., & Gerlach, R. (2010). Microbially enhanced carbon capture and storage by mineral-trapping and solubility-trapping. Environmental Science & Technology, 44(13), 5270–5276.
Mösche, M., & Meyer, U. (2002). Toxicity of linear alkylbenzene sulfonate in anaerobic digestion: influence of exposure time. Water Research, 36(13), 3253–60.
Nielsen, A. D., Borch, K., & Westh, P. (2000). Thermochemistry of the specific binding of C12 surfactants to bovine serum albumin. Biochimica et Biophysica Acta (BBA) Protein Structure and Molecular Enzymology, 1479(1–2), 321–331.
Normile, D. (2009). Round and round: a guide to the carbon cycle. Science (New York, N.Y.), 325(5948), 1642–3.
Nye, J. V., Guerin, W. F., & Boyd, S. A. (1994). Heterotrophic activity of microorganisms in soils treated with quaternary ammonium compounds. Environmental Science & Technology, 28(5), 944–51.
Pacala, S., & Socolow, R. (2004). Stabilization wedges: solving the climate problem for the next 50 years with current technologies. Science (New York, N.Y.), 305(5686), 968–72.
Perito, B., & Mastromei, G. (2011). Molecular basis of bacterial calcium carbonate precipitation. Progress in Molecular and Subcellular Biology, 52, 113–39.
Plasynski, S. I., Litynski, J. T., McIlvried, H. G., & Srivastava, R. D. (2009). Progress and new developments in carbon capture and storage. Critical Reviews in Plant Sciences, 28(3), 123–138.
Rodrigues, A., Nogueira, R., Melo, L. F., & Brito, A. G. (2013). Effect of low concentrations of synthetic surfactants on polycyclic aromatic hydrocarbons (PAH) biodegradation. International Biodeterioration & Biodegradation, 83, 48–55.
Rodriguez-Navarro, C., Rodriguez-Gallego, M., Ben Chekroun, K., & Gonzalez-Munoz, M. T. (2003). Conservation of ornamental stone by myxococcus xanthus-induced carbonate biomineralization. Applied and Environmental Microbiology, 69(4), 2182–2193.
Rouse, J. D., Sabatini, D. A., Suflita, J. M., & Harwell, J. H. (1994). Influence of surfactants on microbial degradation of organic compounds. Critical Reviews in Environmental Science and Technology, 24(4), 325–370.
Rubin, E. S., Mantripragada, H., Marks, A., Versteeg, P., & Kitchin, J. (2012). The outlook for improved carbon capture technology. Progress in Energy and Combustion Science, 38(5), 630–671.
Sarda, D., Choonia, H. S., Sarode, D. D., & Lele, S. S. (2009). Biocalcification by Bacillus pasteurii urease: a novel application. Journal of Industrial Microbiology & Biotechnology, 36(8), 1111–1115.
Scott, V., Gilfillan, S., Markusson, N., Chalmers, H., & Haszeldine, R. S. (2012). Last chance for carbon capture and storage. Nature Climate Change, 3(2), 105–111.
Senthil Balan, S., Fathima, F., & Jayalakshmi, S. (2012). Characterization of urease enzyme from marine bacterium Klebsiella species. African Journal of Microbiology Research, 6(30), 5914–5923.
Slepecky, R. A., & Hemphill, H. E. (2006). The genus Bacillus—nonmedical. In: The prokaryotes (pp. 530–562). New York: Springer.
Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K. B., et al. (Eds.). (2007). Climate change 2007: the physical science basis: contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change (p. 996). New York: Cambridge University Press.
Spycher, N., Pruess, K., & Ennis-King, J. (2003). CO2-H2O mixtures in the geological sequestration of CO2. I. Assessment and calculation of mutual solubilities from 12 to 100 °C and up to 600 bar. Geochimica et Cosmochimica Acta, 67(16), 3015–3031.
Sutton, A. J., Feely, R. A., Sabine, C. L., McPhaden, M. J., Takahashi, T., Chavez, F. P., et al. (2014). Natural variability and anthropogenic change in equatorial Pacific surface ocean pCO2 and pH. Global Biogeochemical Cycles, 28(2), 131–145.
Szcześ, A., Chibowski, E., & Hołysz, L. (2007). Influence of ionic surfactants on the properties of freshly precipitated calcium carbonate. Colloids and Surfaces A Physicochemical and Engineering Aspects, 297(1–3), 14–18.
Tsomides, H. J., Hughes, J. B., Thomas, J. M., & Ward, C. H. (1995). Effect of surfactant addition on phenanthrene biodegradation in sediments. Environmental Toxicology and Chemistry, 14(6), 953–959.
Volkering, F., Breure, A. M., & Rulkens, W. H. (1997). Microbiological aspects of surfactant use for biological soil remediation. Biodegradation, 8(6), 401–417.
Wei, H., Shen, Q., Zhao, Y., Zhou, Y., Wang, D., & Xu, D. (2005). On the crystallization of calcium carbonate modulated by anionic surfactants. Journal of Crystal Growth, 279(3–4), 439–446.
Whiffin, V. S., van Paassen, L. A., & Harkes, M. P. (2007). Microbial carbonate precipitation as a soil improvement technique. Geomicrobiology Journal, 24(5), 417–423.
Williams, S. C., Hong, Y., Danavall, D. C. A., Howard-Jones, M. H., Gibson, D., Frischer, M. E., & Verity, P. G. (1998). Distinguishing between living and nonliving bacteria: evaluation of the vital stain propidium iodide and its combined use with molecular probes in aquatic samples. Journal of Microbiological Methods, 32(3), 225–236.
Yalçin, E., Çavuşoğlu, K., & Özen, E. (2011). Hydrocarbon degradation by a new Pseudomonas sp., strain RW-II, with polycationic surfactant to modify the cell hydrophobicity. Environmental Technology, 32(15), 1743–1747.
Yang, C., & Gu, Y. (2006). Accelerated mass transfer of CO2 in reservoir brine due to density-driven natural convection at high pressures and elevated temperatures. Industrial & Engineering Chemistry Research, 45(8), 2430–2436.
Yu, J., Zhao, X., Cheng, B., & Zhang, Q. (2005). Controlled synthesis of calcium carbonate in a mixed aqueous solution of PSMA and CTAB. Journal of Solid State Chemistry, 178(3), 861–867.
Yu, K. M. K., Curcic, I., Gabriel, J., & Tsang, S. C. E. (2008). Recent advances in CO2 capture and utilization. ChemSusChem. doi:10.1002/cssc.200800169.
Zamarreño, D. V., Inkpen, R., & May, E. (2009). Carbonate crystals precipitated by freshwater bacteria and their use as a limestone consolidant. Applied and Environmental Microbiology, 75(18), 5981–90.
Zeng, G., Fu, H., Zhong, H., Yuan, X., Fu, M., Wang, W., & Huang, G. (2007). Co-degradation with glucose of four surfactants, CTAB, Triton X-100, SDS and Rhamnolipid, in liquid culture media and compost matrix. Biodegradation, 18(3), 303–10.
Zhang, J., Han, B., Zhao, Y., Li, J., Hou, M., & Yang, G. (2011). CO2 capture by hydrocarbon surfactant liquids. Chemical Communications (Cambridge, England), 47(3), 1033–5.
Acknowledgments
This research was financially supported by NRF projects of development of biomimetic technology for acid soil rehabilitation using biocalcification process and partially supported by INHA University research grant.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 28 kb)
Supplementary Figure S1
Experimental and simulated pH profiles based on atmospheric CO2 dissolution kinetics into alkaline water system with or without surfactant. (GIF 49 kb)
ESM 2
High resolution image (TIFF 752 kb)
Rights and permissions
About this article
Cite this article
Cho, Y., Mahanty, B. & Kim, C.G. Effect of Surfactants on CO2 Biomineralization with Sporosarcina pasteurii and Bacillus megaterium . Water Air Soil Pollut 226, 2245 (2015). https://doi.org/10.1007/s11270-014-2245-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2245-x