Abstract
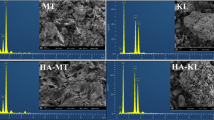
The inorganic and organic components of soils from a forest and from a semiarid region were separated and characterized by different techniques. The kinetic adsorption behavior of lanthanum ions by the inorganic components of the soils indicated that the systems behave according to the kinetic model of pseudo-second order, so 3 h of contact was sufficient to achieve equilibrium. The sorption isotherm data, q e vs. C e, were best adjusted to the Langmuir model for the soil of the forest; this means that a monolayer is formed and that the energy of adsorption is the same in all adsorption sites so the material is homogeneous. The data for the soil of the semiarid area were adjusted to the Freundlich model; this indicates that the material is heterogeneous and multilayers are formed. The competition between the lanthanum sorption by the inorganic components and the formation of a complex with humic acids was shown by a decrease of q e. The method of Schubert was used to determine the stoichiometric coefficient of the complex La:(HA) n , which was 1:1 for both soils, whereas the \( \log {\beta}_{La,{(HA)}_n}^{app} \) was 5.1 ± 0.2 for the soil of the forest and 7 ± 1 for the semiarid soil.






Similar content being viewed by others
References
Bhattacharyya, K. G., & Gupta, S. S. (2008). Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Advances in Colloid and Interface Science, 140, 114–131.
Chegrouche, S., Mellah, A., & Telmoune, S. (1997). Removal of lanthanum from aqueous solutions by natural bentonite. Water Research, 31, 1733–1737.
Chin, Y., Alken, G., & O’Loughlin, E. (1994). Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environmental Science and Technology, 28, 1853.
Choppin, G. R. (1988). Humic and radionuclides migration. Radiochimica Acta, 44, 23–28.
Darehkordi, A., Hosseini, S. S., & Tahmooresi, M. (2012). Montmorillonite modified as an efficient and environment friendly catalyst for one-pot synthesis of 3, 4-dihydropyrimidine-2(1H) ones. Iranian Journal of Materials Science & Engineering, 9, 49–57.
Dierkcx, A., Maes, A., & Vancluysen, J. (1994). Mixed complexes formation of Eu3+ with humic acids and a competing ligand. Radiochimica Acta, 66, 149–156.
Eren, E., & Afsin, B. (2008). An investigation of Cu(II) adsorption by raw and acid-activated bentonite: a combined potentiometric, thermodynamic, XRD, IR, DTA study. Journal of Hazardous Materials, 151, 682–691.
Grenthe, I., & Puigdomench, I. (Eds.). (1997). Modelling in aquatic chemistry. Paris: OECD Publications.
Haghseresht, F., Wang, S., & Do, D. D. (2009). A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters. Applied Clay Science, 46, 369–375.
Hlavay, J., Jonas, K., Elek, S., & Inczedy, J. (1978). Characterization of the particle size and the crystallinity of certain minerals by ir spectrophotometry and other instrumental methods: II, Investigations on quartz and feldspar. Clays and Clay Minerals, 26, 139–143.
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34, 451–465.
Jiménez-Reyes, M., & Solache-Ríos, M. (2012). The influence of pH on the stability constants of lanthanum and europium complexes with humic acids. Journal of Radioanalytical and Nuclear Chemistry, 293, 273–278.
Jiménez-Reyes, M., Solache-Ríos, M., & Rojas-Hernández, A. (1999). Behaviour of europium (III) and its carbonate complexes in a solvent extraction system with HDBM in 2M NaCl at 303 K. Radiochimica Acta, 87, 125–133.
Kornilovich, B., Pshinko, G., Spasenova, L., & Kovalchuk, I. (2000). Influence of humic substances on the sorption interactions between lanthanide and actinide ions and clay minerals. Adsorption Science & Technology, 18, 873–880.
Licona Sánchez, T.J., Álvarez Romero, G.A., & Páez Hernández, M.E. (2008). Influencia del método de extracción sobre las propiedades fisicoquímicas de los ácidos húmicos. Congreso Nacional de Química Analítica, Memorias in extenso. Edited by AMQA, Mexico. 113–118.
Lopez-Capel, E., Sohi, S. P., Gaunt, J. L., & Manning, D. A. C. (2005). Use of thermogravimetric scanning calorimetry to characterize modelable soil organic matter fractions. Soil Science Society of American Journal, 69, 136–140.
Marquadt, C. M. (Ed.). (2000). Influence of humic acids on the migration behaviour of radioactive and non-radioactive substances under conditions close to nature: final report. Karlsruhe: Forschungszentrum Karlsruhe GmbH.
McDonnell, R. M., Holden, N. M., Ward, S. M., Collins, J. F., Farell, E. P., & Hayes, M. H. B. (2001). Characteristics of humic substances in Heathland and forested peat soils of the Wicklow Mountains. Biology and Environment: Proceedings of the Royal Irish Academy, 101B, 187–197.
Montalvon, G., Markai, S., Andrés, Y., & Grambow, B. (2002). Complexation studies of Eu(III) with alumina-bound polymaleic acid: effecto of organic polymer loading and metal ion concentration. Environmental Science & Technology, 36, 3303–3309.
Norma Oficial Mexicana (2002). NOM-021-RECNAT-2000. Diario Oficial (31 de diciembre de 2002)18–20.
Pandey, A. K., Pandey, S. D., & Mishra, V. (2000). Stability constants of metal-humic acid complexes and its role in environmental detoxification. Ecotoxicology and Environmental Safety, 47, 195–200.
Pepper, S. E., Hull, L. C., Bottenus, B. N., & Clark, S. B. (2006). Adsorption of lanthanum to goethite in the presence of gluconate. Radiochimica Acta, 94, 229–237.
Puigdomenech, I. (2010). Program MEDUSA (Make equilibrium diagrams using sophisticated algorithms). Royal Institute of Technology. Inorganic Chemistry. 10644 Stockholm Sweden, ignasi@inorg.kth.se.
Rupiasih, N. N., & Vidyasagar, P. B. (2008). Humic substances: structure, function, effects and applications. Asian Journal of Water, Environment and Pollution, 5, 39–47.
Schubert, J. (1948). The use of ion exchangers for the determination of physical–chemical properties of substances, particularly radiotracers, in solution: 1. Theoretical. The Journal of Physical Chemistry, 52, 340–350.
Sheng, G., Dong, H., & Li, Y. (2012). Characterization of diatomite and its application for the retention of radiocobalt: role of environmental parameters. Journal of Environmental Radioactivity, 113, 108–115.
Sheng, G., Dong, H., Shen, R., & Li, Y. (2013a). Microscopic insights into the temperature-dependent adsorption of Eu(III) onto titanate nanotubes suited by FTIR, XPS, XAFS and batch techniques. Chemical Engineering Journal, 217, 486–494.
Sheng, G., Shen, R., Dong, H., & Li, H. (2013b). Colloidal diatomite, radionickel and humic substance interaction: a combined batch, XPS and EXAFS investigation. Environmental Science and Pollution Research, 20, 3708–3717.
Sheng, G., Shao, X., Li, Y., Li, J., Dong, H., Cheng, W., et al. (2014a). Enhanced removal of U(VI) by nanoscale zerovalent iron supported on Na-bentonite and an investigation of mechanism. Journal of Physical Chemistry, 118, 2952–2958.
Sheng, G. D., Yang, S. T., Li, Y. M., Gao, X., Huang, Y. Y., Hu, J., et al. (2014b). Retention mechanisms and microstructures of Eu(III) on manganese dioxide studied by batch and high resolution EXAFS technique. Radiochimica Acta, 102, 155–167.
Sheng, G., Yang, Q., Peng, F., Li, H., Gao, X., & Huang, Y. (2014c). Determination of colloidal pyrolusite, Eu(III) and humic substance interaction: a combined batch and EXAFS approach. Chemical Engineering Journal, 245, 10–16.
Sheng, G., Ye, L., Li, Y., Dong, H., Li, H., Gao, X., et al. (2014d). EXAFS study of the interfacial interaction of nickel(II) on titanate nanotubes: role of contact time, pH and humic substances. Chemical Engineering Journal, 248, 71–78.
Spencer, K. L., James, S. L., Taylor, J. A., & Kearton-Gee, T. (2007). Sorption of lanthanum onto clay minerals: a potential tracer for fine sediment transport in the coastal marine environment. Geological Society, London, Special Publications, 274, 17–24.
Stevenson, F. J., & Goh, K. M. (1971). Infrared spectra of humic acids and related substances. Geochimica et Cosmochimica Acta, 35, 471–483.
Sun, Y., Li, J., & Wang, X. (2014). The retention of uranium and europium onto sepiolite investigated by macroscopic, spectroscopic and modeling techniques. Geochimica et Cosmochimica Acta, 140, 621–643.
Swift, R. S. (1996). Organic matter characterization (Chap. 35. In D. L. Sparks (Ed.), Methods of soil analysis. Part 3. Chemical methods (pp. 1018–1020). Madison USA: Soil Science Society of America. Book Series 5.
Tan, X. L., Wang, X. K., Geckeis, H., & Rabung, T. (2008). Sorption of Eu(III) on humic acid or fulvic acid bound to hydrous alumina studied by SEM-EDS, XPS, TRLFS, and batch techniques. Environmental Science & Technology, 42, 6532–6537.
Tang, J., & Johannesson, K. H. (2010). Ligand extraction of rare earth elements from aquifer sediments: implications of rare earth element complexation with organic matter in natural waters. Geochimica et Cosmochimica Acta, 74, 6690–6705.
Wasay, S. A., Haran, M. J., & Tokunaga, S. (1996). Adsorption of fluoride, phosphate, and arsenate ions on lanthanum-impregnated silica gel. Water Environment Research, 68, 295–300.
White, J. L. (1971). Interpretation of infrared spectra of soil minerals. Soil Science, 112, 22–31.
Yang, S. T., Zong, P. F., Sheng, G. D., Ren, X. M., Huang, Y. Y., & Wang, X. K. (2014). New insight inti Eu(III) sorption mechanism at alumina/water interface by batch technique and EXAFS analysis. Radiochimica Acta, 102, 155–167.
Ackowledgments
We thank F. Monroy Guzmán and E. Fernández Ramírez for the donation of the samples of the semiarid zone soil. The technical support of E. Morales Moreno, I. Z. López Malpica, J. Muñoz Lujano, and M. Villa Tomasa was much appreciated as well as the participation of the students: M. I. Valencia Flores, A. Hernández Jiménez, and B. Portillo Rodríguez.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiménez-Reyes, M., Solache-Ríos, M. Chemical Behavior of Lanthanum in the Presence of Soils Components: Adsorption and Humate Complexes. Water Air Soil Pollut 225, 2213 (2014). https://doi.org/10.1007/s11270-014-2213-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2213-5