Abstract
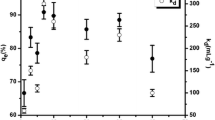
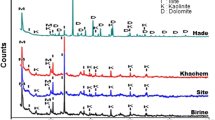
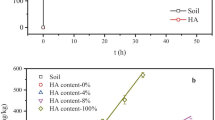
We explored the effect of the presence or absence of humic acid (HA) on the sorption behaviour of Sr onto soil. We examined three different experimental cases for Sr sorption: (1) sorption in the presence of only colloidal HA, (2) sorption in the presence of only soil and (3) sorption in the presence of both colloidal HA and soil (HS). A batch technique was used to study the influencing factors, including the amount of colloidal HA, solid content, pH, initial concentration of Sr and contact time. The experiments showed that the influencing factors significantly affected the sorption process. For example, in the case of soil and HS, the sorption percentage increased rapidly with increasing solid content at m/V < 20 g/L, changing from 8.35% and 37.54% to 49.09% and 77.03%, respectively. Moreover, scanning electron microscopy and Fourier transform infrared spectroscopy were used to characterize samples. The kinetics and isotherms of Sr were best described by the pseudo-second-order and Langmuir models, which indicated that the process was controlled by chemisorption and uniform monolayer sorption with constant energy on the outer surface. These findings provide valuable information for predicting strontium migration in radioactive waste disposal sites.







Similar content being viewed by others
References
Albarran N, Missana T, García-Gutiérrez M, Alonsoe U, Mingarro M (2011) Strontium migration in a crystalline medium: effects of the presence of bentonite colloids. J Contam Hydrol 122:76–85
Ali R, Hamad H, Hussein M, Malash G (2016) Potential of using green adsorbent of heavy metal removal from aqueous solutions: adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol Eng 91:317–332
Başçetin E, Atun G (2006) Adsorption behavior of strontium on binary mineral mixtures of montmorillonite and kaolinite. Appl Radiat Isotopes 64:957–964
Bhattacharyya KG, Gupta SS (2008) Adsorption of Fe(III), Co(II) and Ni(II) on ZrO–kaolinite and ZrO–montmorillonite surfaces in aqueous medium. Colloids Surf A Physicochem Eng Asp 317(1):71–79
Borah D, Satokawa S, Kato S, Kojima T (2009) Sorption of As (V) from aqueous solution using acid modified carbon black. J Hazard Mater 162:1269–1277
Deng D, Tam N (2016)Adsorption-uptake-metabolism kinetic model on the removal of BDE-47 by a Chlorella isolate. Environ Pollut 212:290–298
Fan Q, Wu W, Song X, Xu J, Hu J, Niu Z (2008) Effect of humic acid, fulvic acid, pH and temperature on the sorption-desorption of Th (IV) on attapulgite. Radiochim Acta 96:159–165
Fan Q, Shao D, Lu Y, Wu W, Wang X (2009) Effect of pH, ionic strength, temperature and humic substances on the sorption of Ni (II) to Na–attapulgite. Chem Eng J 150:188–195
Feng Q, Zhang Z, Chen Y, Liu L, Zhang Z, Chen C (2013) Adsorption and desorption characteristics of arsenic on soils: kinetics, equilibrium, and effect of Fe(OH)3 colloid, H2SiO3 colloid and phosphate. J Environ Sci 18:26–36
Gil-García CJ, Rigol A, Rauret G, Vidal M (2008) Radionuclide sorption–desorption pattern in soils from Spain. Appl Radiat Isotopes 66(2):126–138
Gil-García CJ, Rigol A, Vidal M (2011) The use of hard- and soft-modelling to predict radiostrontium solid–liquid distribution coefficients in soils. Chemosphere 85(8):1400–1405
Hanudin E, Sukmawati ST, Radjagukguk B, Yuwono N (2014) The effect of humic acid and silicic acid on P adsorption by amorphous minerals. Procedia Environ Sci 20:402–409
He Y, Chen YG, Ye WM (2016) Equilibrium, kinetic, and thermodynamic studies of adsorption of Sr (II) from aqueous solution onto GMZ bentonite. Environ Earth Sci 75(9):807
Helal AA, Aly HF, Imam DM, Khalifa SM (1998) Effect of some metal ions on the complexation of strontium with humic acid. J Radioanal Nucl Chem 227(1–2):49–53
HJ615-2011 (2011) Baidu. http://www.doc88.com/p-3197961161257.html
Hui C, Berndtsson R, Ma M, Zhu K (2009) Characterization of insolubilized humic acid and its sorption behaviors. Environ Geol 57:1847–1853
International Atomic Energy Agency (2003) Categorization of radioactive sources. IAEA-TECDOC-1344 IAEA, Vienna
Ivanova B, Spiteller M (2014) Adsorption of uranium composites onto, saltrock oxides—experimental and theoretical study. J Environ Radioact 135:75–83
Kaçan E, Kütahyalı C (2012) Adsorption of strontium from aqueous solution using activated carbon produced from textile sewage sludges. J Anal Appl Pyrol 97:149–157
Kaygun AK, Eral M, Erenturk SA (2017) Removal of cesium and strontium using natural attapulgite: evaluation of adsorption isotherm and thermodynamic data. J Radioanal Nucl Chem 311:1459–1464
Kütahyalı C, Cetinkaya B, Acar M, Is¸ ık, N, Cireli I (2012) Investigation of strontium sorption onto Kula volcanics using central composite design. J Hazard Mater 201–202:115–124
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöster stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 24(4):1–39
Li M, Liu H, Zhu H, Gao H, Zhang S, Chen T (2017) Kinetics and mechanism of Sr(II) adsorption by Al-Fe2O3: evidence from XPS analysis. J Mol Liq 233:364–369
Liu F, Liu Y, Xu Y, Ni L, Meng X, Hu Z, Zhong G, Meng M, Wang Y, Han J (2015) Efficient static and dynamic removal of Sr (II) from aqueous solution using chitosan ion-imprinted polymer functionalized with dithiocarbamate. J Environ Chem Eng 3:1061–1071
Luo X, Yu L, Wang C, Yin X, Mosa A, Lv J, Sune H (2017) Sorption of vanadium (V) onto natural soil colloids under various solution pH and ionic strength conditions. Chemosphere 169:609–617
Ma Z, Li Q, Yue Q, Gao B, Li W, Xu X, Zhong Q (2011) Adsorption removal of ammonium and phosphate from water by fertilizer controlled release agent prepared from wheat straw. Chem Eng J 171:1209–1217
Ma F, Li Z, Zhao H, Geng Y, Zhou W, Li Q, Zhang L (2017) Potential application of graphene oxide membranes for removal of Cs(I) and Sr(II) from high level-liquid waste. Sep Purif Technol 188:523–529
Missana T, Garcia-Gutierrez M, Alonso U (2008) Sorption of strontium onto illite/smectite mixed clays. Phys Chem Earth 33:156–162
Montoya V, Baeyens B, Glaus MA, Kupcik T, Fernandes MM, Laer LV, Bruggeman C, Maes N, Scha¨fer T (2018) Sorption of Sr, Co and Zn on illite: batch experiments and modelling including Co in-diffusion measurements on compacted samples. Geochim Cosmochim Acta 223:1–20
Murali MS, Mathur JN (2002) Sorption characteristics of Am (III), Sr (II) and Cs (I) on bentonite and granite. J Radioanal Nucl Chem 254:129–136
Nandi BK, Goswami A, Purkait MK (2009) Adsorption characteristics of brilliant green dye on kaolin. J Hazard Mater 161:387–395
Qi H, Liu H, Gao Y (2015) Removal of Sr (II) from aqueous solutions using polyacrylamide modified graphene oxide composites. J Mol Liq 208:394–401
Radwan E, Ghafar H, Moursy A, Langford C, Bedair A, Achari G (2015) Preparation and characterization of humic acid-carbon hybrid materials as adsorbents for organic micro-pollutants. Environ Sci Pollut Res 22:12035–12049
Rani R, Sasidhar P (2012) Geochemical and thermodynamic aspects of sorption of strontium on kaolinite dominated clay samples at Kalpakkam. Environ Earth Sci 65:1265–1274
Saleh AS, Lee JY, Jo Y, Yun J (2018) Uranium(VI) sorption complexes on silica in the presence of calcium and carbonate. J Environ Radioact 182:63–69
Schulz W, Gupta DK, Beate R, Georg S, Clemens W (2019) Sorption of radiostrontium on various soils. Appl Geochem 101:103–108
Shi T, Jia S, Chen Y, Wen Y, Du C, Guo H, Wang Z (2009) Adsorption of Pb (II), Cr (III), Cu (II), Cd (II) and Ni (II) onto a vanadium mine tailing from aqueous solution. J Hazard Mater 169:838–846
Singer DM, Guo H, Davis JA (2014) U(VI) and Sr(II) batch sorption and diffusion kinetics into mesoporous silica (MCM-41). Chem Geol 390:152–163
SL 237-068-1999(1999) Baidu. https://wenku.baidu.com/view/37bf65d233d4b14e8524683d.html
Sureda R, Martínez-Lladó X, Rovira M, Pablo J, Casas I, Giménez J (2010) Sorption of strontium on uranyl peroxide: implications for a high-level nuclear waste repository. J Hazard Mater 181:881–885
Tan L, Wang X, Tan X, Mei H, Chen C, Hayat T, Alsaedi A, Wen T, Lu S, Wang X (2017) Bonding properties of humic acid with attapulgite and its influence on U (VI) sorption. Chem Geol 464:91–100
Wallace S, Shaw SA, Morris KB, Smoll J, Fuller A, Burker I (2012) Effect of groundwater pH and ionic strength on strontium sorption in aquifer sediments: implications for 90Sr mobility at contaminated nuclear sites. Appl Geochem 27(8):1482–1491
Wang M, Xu L, Peng J, Zhai M, Li J, Wei G (2009) Adsorption and desorption of Sr (II) ions in the gels based on polysaccharide derivates. J Hazard Mater 171:820–826
Wang C, Yang X, Li C, Liu C (2015) The sorption interactions of humic acid onto Beishan granite. Colloid Surface A 484:37–46
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civ Engrs 89:31–59
Wissocq A, Beaucaire C, Latrille C (2017a) Ca and Sr sorption on Ca-illite: experimental study and modelling. Earth Planet Sci Lett 17:662–665
Wissocq A, Beaucaire C, Latrille C (2017b) Application of the multi-site ion exchanger model to the sorption of Sr and Cs on natural clayey sandstone. Appl Geochem 93:167–177. https://doi.org/10.1016/j.apgeochem.2017.12.010
Xiao J, Chen Y, Zhao W, Xu J (2013) Sorption behavior of U(VI) onto Chinese bentonite: effect of pH, ionic strength, temperature and humic acid. J Mol Liq 188(12):178–185
Yang S, Li J, Lu Y, Chen Y, Wang X (2009) Sorption of Ni (II) on GMZ bentonite: effects of pH, ionic strength, foreign ions, humic acid and temperature. Appl Radiat Isotopes 67:1600–1608
Yoon TH, Moon H, Park YJ, Park KK (1994) Investigation of metal binding sites on soil fulvic acid using Eu(III) luminescence spectroscopy. Environ Sci Technol 28(12):2139–2146
Yu S, Mei H, Chen X, Tan X, Ahmad B, Alsaedie A, Hayat T, Wang X (2015) Impact of environmental conditions on the sorption behavior of radionuclide 90 Sr (II) on Na-montmorillonite. J Mol Liq 203:39–46
Zhang H, Wang X, Liang H, Tan T, Wu W (2016) Adsorption behavior of Th (IV) onto illite: effect of contact time, pH value, ionic strength, humic acid and temperature. Appl Clay Sci 127–128:35–43
Zhao Y, Shao Z, Chen C, Hu J, Chen H (2014) Effect of environmental conditions on the adsorption behavior of Sr (II) by Na-rectorite. Appl Clay Sci 87:1–6
Zhao XG, Wang J, Chen F, Li P, Ma L, Xie J, Liu Y (2016) Experimental investigations on the thermal conductivity characteristics of Beishan granitic rocks for China’s HLW disposal. Tectonophysics 683:124–137
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Georg Steinhauser
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zuo, R., Chen, M., Lin, Y. et al. Effect of a humic acid colloid on the sorption behaviour of Sr onto soil in a candidate high-level radioactive waste geological disposal site. Environ Sci Pollut Res 26, 25235–25246 (2019). https://doi.org/10.1007/s11356-019-05545-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05545-9