Abstract
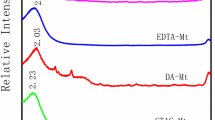
In this study, the characterization and adsorption properties of montmorillonite (MMT) and organomontmorillonites with different surfactant/cationic exchange capacity (CEC) ratios (1 and 2) of tetradecyl trimethylammonium (TDTMA+) and hexadecyl trimethylammonium (HDTMA+) cations were evaluated. The particle apparent diameter, determined by laser and scanning electron microscopy showed aggregate formation, which varied with loading and cation length of the surfactant used. X-ray diffraction analysis revealed the formation of a pseudotrilayer arrangement of both surfactants in the interlayer space, FTIR showed the characteristic bands of the surfactants and micelle formations, and zeta potential determinations indicated neutral or negative surface charge values, except for sample obtained with one CEC concentration exchanged with HDTMA+ (HDTMA1-MMT) where a charge reversal to positive was found. Higher adsorption amounts of humic acid (HA) were found for HDTMA1-MMT and TDTMA1-MMT samples than for MMT sample, while the increase in the loading of both surfactants decreased the amounts of HA adsorbed could be assigned to a higher micelle formation and different packing density of alkyl chain, in the external surface. The correlation found between the total surface area and negative zeta potential values and the HA adsorption rate, within each surfactant, indicated the strong influence that these properties have on HA adsorption.







Similar content being viewed by others
References
Anirudhan, T. S., & Ramachandran, M. (2007). Surfactant-modified bentonite as adsorbent for the removal of humic acid from wastewaters. Applied Clay Science, 35, 276–281.
Avisar, D., Primor, O., Gozlan, I., & Mamane, H. (2010). Sorption of sulfonamides and tetracyclines to montmorillonite clay. Water, Air, and Soil Pollution, 29, 439–450.
Bianchi, A. E., Fernández, M., Pantanetti, M., Viña, R., Torriani, I., Torres Sánchez, R. M., et al. (2013). ODTMA and HDTMA Organomontmorillonites characterization: new insight by WAXS, SAXS and surface charge. Applied Clay Science, 83–84, 280–285.
Bojemueller, E., Nennemann, A., & Lagaly, G. (2001). Enhanced pesticide adsorption by thermally modified bentonites. Applied Clay Science, 18, 277–284.
Daifullah, A. A., Girgis, B. S., & Gad, H. M. (2004). A study of the factors affecting the removal of humic acid by activated carbon prepared from biomass material. Colloids and Surfaces A, 235, 1–10.
Dekov, V. M., Komy, Z., Araújo, F., Van Put, A., & Van Grieken, R. (1997). Chemical composition of sediments, suspended matter, river water and ground water of the Nile (Aswan-Sohag traverse). Science of the Total Environment, 201(3), 195–210.
Doulia, D., Leodopoulos, C., Gimouhopoulos, K., & Rigas, F. (2009). Adsorption of humic acid on acid-activated Greek bentonite. Journal of Colloid and Interface Science, 340, 131–141.
Fernández, M. A. (2012).Thesis. Utilización de arcillas naturales argentinas y órgano arcillas para la retención de compuestos derivados de la materia orgánica de suelo. Univ. Nac. de Quilmes.
Fernández, M., Cosp, J., Pérez Recuerda, R., Assiego Larriva, M., & Torres Sánchez, R. M. (2011). Caracterización y evaluación de una bentonita argentina, sus productos de tratamientos mecánicos y una española para la potabilización de aguas. In A. M. da Silva, G. Galindo, & J. L. Fernandez Turiel (Eds.), Aguas, suelos y vegetación en Iberoamérica (pp. 85–98). Salamanca: Soc. Iberoamericana de Física y Química Ambiental.
Frimmel, F. H., & Christman, R. F. (1988). Humic substances and their role in the environment. N York: John Wiley and Sons.
Froehner, S., Fernandes Martins, R., Furukawa, W., & Risso Errera, M. (2009). Water remediation by adsorption of phenol onto hydrophobic modified clay. Water, Air, and Soil Pollution, 199, 107–113.
Fuguet, E., Ráfols, E., Martí Rosés, C., & Bosch, E. (2005). Critical micelle concentration of surfactants in aqueous buffered and unbuffered systems. Analytica Chimica Acta, 548, 95–100.
Furukawa, K., & Takahashi, Y. (2008). Effect of complexation with humic substances on diffusion of metal ions in water. Chemosphere, 73, 1272–1278.
Gieseking, J. E. (1939). The mechanism of cation exchange in the montmorillonite-beidellite-nontronite type of clay minerals. Soil Science, 47, 1–13.
Goldani, E., Moro, C. C., & Maia, S. M. (2013). A study employing differents clays for Fe and Mn removal in the treatment of acid mine drainage. Water, Air, and Soil Pollution, 224, 1401–1404.
He, H., Frost, R. L., Bostrom, T., Yuan, P., Duong, L., Yang, D., et al. (2006). Changes in the morphology of organoclays with HDTMA surfactant loading. Applied Clay Science, 31, 262–271.
He, H., Ma, Y., Zhu, J., Yuan, P., & Qing, Y. (2010). Organoclays prepared from montmorillonites with different cation exchange capacity and surfactant configuration. Applied Clay Science, 48, 67–72.
Jiang, J.-Q., Zeng, Z., & Pearce, P. (2004). Preparation and use of modified clay coagulants for wastewater treatment. Water, Air, and Soil Pollution, 158, 53–65.
Klepsch, S., Aquino, A. J. A., Haas, U., Tunega, D., Haberhauer, G., Gerzabek, M. H., et al. (2011). Sorption of selected aromatic substances-application of kinetic concepts and quantum mechanical modeling. Water, Air, and Soil Pollution, 215(1–4), 449–464.
Lagaly, G., & Weiss, A. (1970). Anordnung und Orientierung kationischer Tenside auf Silicatoberflächen. Teil I. Darstellung der n-Alkylammoniumderivate von glimmerartigen Schichtsilicaten. Kolloid-Zeitschrift & Zeitschrift für Polymere, 237, 266–273.
Li, H., Sheng, G., Teppen, B. J., Johnston, C. T., & Boyd, S. A. (2003). Sorption and desorption of pesticides by clay minerals and humic acid-clay complexes. Soil Science Society of America Journal, 67(1), 122–131.
Li, Z., Jiang, W.-T., & Hong, H. (2008). An FTIR investigation of hexadecyltrimethyl ammonium intercalation into rectorite. Spectrochimica Acta Part A, 71, 1525–1534.
Lombardi, B., Torres Sánchez, R. M., Eloy, P., & Genet, M. (2006). Interaction of thiabendazole and benzimidazole with montmorillonite. Applied Clay Science, 33, 59–65.
Magnoli, A. P., Tallone, L., Rosa, C. A. R., Dalcero, A. M., Chiacchiera, S. M., & Torres Sanchez, R. M. (2008). Commercial bentonites as detoxifier of broiler feed contaminated with aflatoxin. Applied Clay Science, 40, 63–71.
Michot, L. J., & Villieras, F. (2006). Surfac area and porosity. In F. Bergaya, B. K. Theng, & G. Lagaly (Eds.), Handbook of clay science (pp. 965–978). Amsterdam: Elsevier.
Mishael, Y. G., Undabeytia, T., Rytwo, G., Papahadjopoulos-Sternberg, B., Rubin, B., & Nir, S. (2002). Sulfometuron incorporation in cationic micelles adsorbed on montmorillonite. Journal of Agricultural and Food Chemistry, 50, 2856–2863.
Naranjo, P., Sham, E. L., Rodríguez Castellón, E., Torres Sánchez, R. M., & Farfán Torres, E. M. (2013). Identification and quantification of the interaction mechanisms between the cationic surfactant HDTMA-Br and montmorillonite. Clays and Clay Minerals, 61(2), 98–106.
Noudeh, G. D., Housaindokht, M., & Fazly Bazzaz, B. S. (2007). The effect of temperature on thermodynamic parameters of micellization of some surfactants. Applied Clay Science, 7, 47–52.
Park, Y., Ayoko, G. A., & Frost, R. L. (2011). Characterisation of organoclays and adsorption of p-nitrophenol: environmental application. Journal of Colloid and Interface Science, 360, 440–456.
Pospíšil, M., Capková, P., Merínská, Weiss, Z., Maláč, S., & Simoníkz, J. (2002). Intercalation of octadecylamine into montmorillonite: molecular simulations and XRD analysis. Journal of Colloid and Interface Science, 245, 126–132.
Praus, P., Turicová, M., Študentová, S., & Ritz, M. (2006). Study of cetyltrimethylammonium and cetylpyridinium adsorption on montmorillonite. Journal of Colloid and Interface Science, 304, 29–36.
Radian, A., Carmeli, M., Zadaka-Amir, D., Nir, S., Wakshal, E., & Mishael, Y. G. (2011). Enhanced removal of humic acid from water by micelle-montmorillonite composites: comparison to granulated activated carbon. Applied Clay Science, 54, 258–263.
Ruiz-Hitzky, E., Aranda, P., Darder, M., et al. (2010). Hybrid materials based on clays for environmental and biomedical applications. Journal of Materials Chemistry, 20, 9306–9321.
Shah, K. J., Mishra, M. K., Shukla, A. D., Imae, T., & Shah, D. O. (2013). Controlling wettability and hydrophobicity of organoclays modified with quaternary ammonium surfactants. Journal of Colloid and Interface Science, 407, 493–499.
Stevenson, F. J., & Goh, K. M. (1971). IR spectra of humic acids and related substances. Geochimia et Cosmochimia Acta, 35, 471–483.
Theng, B. K., Churchman, G. J., Gates, W. P., & Yuan, G. (2008). Organically modified clays for pollutant uptake and environmental protection. In Q. Huang, P. M. Huang, & A. Violante (Eds.), Soil mineral-microbe-organic interactions: theories and applications (pp. 145–174). Berlin: Springer.
Thomas, F., Michot, L. J., Vantelon, D., Montargès, E., Prélot, E. B., Cruchaudet, M., et al. (1999). Layer charge and electrophoretic mobility of smectites. Colloids and Surfaces, A, 159, 351–358.
Torres Sánchez, R. M., & Falasca, S. (1997). Specific surface and surface charges of some Argentinian soils. Zeitschrift für Pflanzenernährung und Bodenkunde, 160, 223–226.
Tschapek, M., Torres Sánchez, R. M., & Wasowski, C. (1989). Handy methods for determining the isoelectric point of soils. Zeitschrift für Pflanzenernährung und Bodenkunde, 152, 73–76.
van Wijk, D., Gyimesi-van den Bos, M., Garttener-Arends, I., Geurts, M., Kamstra, J., & Thomas, P. (2009). Bioavailability and detoxification of cationics: I. Algal toxicity of alkyltrimethyl ammonium salts in the presence of suspended sediment and humic acid. Chemosphere, 75, 303–309.
Vermeer, W. P., van Riemsdijk, W. H., & Koopal, L. K. (1998). Adsorption of humic acid to mineral particles. 1. Specific and electrostatic interactions. Langmuir, 14, 2810–2819.
White, G. C. (1999). Handbook of chlorination and alternative disinfectants, chap. 12. New York: Wiley-Interscience Publication.
Zadaka, D., Radian, A., & Mishael, Y. G. (2010). Applying zeta potential measurements to characterize the adsorption on montmorillonite of inorganic, cations as monomers, micelles or polymers. Journal of Colloid and Interface Science, 352, 171–177.
Zhan, Y., Zhu, Z., Lin, J., Qiu, Y., & Zhao, J. (2010). Removal of humic acid from aqueous solution by cetylpyridinum bromide modified zeolite. Journal of Environmental Science, 22(9), 1327–1334.
Zhang, X., & Bai, R. (2003). Mechanisms and kinetics of humic acid adsorption onto chitosan-coated granules. Journal of Colloid and Interface Science, 264, 30–38.
Zhu, J., He, H., Guo, J., Yang, D., & Xie, X. (2003). Arrangement models of alkylammonium cations in the interlayer of HDTMA+ pillared montmorillonites. Chinese Science Bulletin, 48, 368–372.
Zhu, J., He, H., Zhu, L., Wen, X., & Deng, F. (2005). Characterization of organic phases in the interlayer of montmorillonite using FTIR and 13C NMR. Journal of Colloid and Interface Science, 286, 239–244.
Acknowledgments
The authors acknowledge the technical help from G. Guzman and ANPCyT through Project PICT 1360/2006 and FONARSEC FS Nano-008/2010 for financial support of this work. MF acknowledges a fellowship research grant from MINCyT-ANPCyT and CONICET.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 112 kb)
Rights and permissions
About this article
Cite this article
Fernández, M., Curutchet, G. & Torres Sánchez, R.M. Removal of Humic Acid by Organo-Montmorillonites: Influence of Surfactant Loading and Chain Length of Alkylammonium Cations. Water Air Soil Pollut 225, 1987 (2014). https://doi.org/10.1007/s11270-014-1987-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-1987-9