Abstract
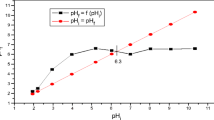
Natural palygorskite was used as an adsorbent for the removal of copper, cobalt and nickel from an aqueous solution. All assays were performed under controlled conditions to establish the adsorption capacity of the solid. Initially, the clay was characterized by chemical analysis, XRD, infrared spectroscopy and thermogravimetry. Adsorption experiments for the ions in aqueous solution were carried out by a batch method through which the reaction time, initial concentration of cations, temperature and pH of the aqueous solution were systematically varied. First-order, pseudo-second-order and intraparticle diffusion models were used to describe the kinetic data. The results show that the processes were fitted well by the pseudo-second-order model. Moreover, the equilibrium solid–cation systems followed the Langmuir isotherm model. The results indicate that raw palygorskite could be employed as a low-cost material for the removal of heavy metals from aqueous solution.







Similar content being viewed by others
References
Adamson, A. W. (1990). Physical Chemistry of Surfaces. New York: Wiley.
Al-Degs, Y. S., El-Barghouthi, M. I., Issa, A. A., Khraisheh, M. A., Majeda, A., & Walker, G. M. (2006). Sorption of Zn(II), Pb(II), and Co(II) using natural sorbents: equilibrium and kinetic studies. Water Research, 40(14), 2645–2658.
Álvarez-Ayuso, E., & Garcia-Sánchez, A. (2003). Palygorskite as a feasible amendment to stabilize heavy metal polluted soils. Environmental Pollution, 125(3), 337–344.
Álvarez-Ayuso, E., & Garcia-Sánchez, A. (2007). Removal of cadmium from aqueous solutions by palygorskite. Journal of Hazardous Materials, 147(1–2), 594–600.
Bache, B. W. (1976). The measurement of cation exchange capacity of soils. Journal Science Food Agriculture, 27(3), 273–280.
Bailey, S. W. (1980). Structure of layer silicates. In G. W. Brindley & G. Brown (Eds.), Crystal structures of clay minerals and their X-Ray identification (pp. 1–123). London: Mineralogical Society.
Bailey, S. E., Olin, T. J., Bricka, R. M., & Adrian, D. D. (1999). A review of potentially low-cost sorbents for heavy metals. Water Research, 33(11), 2469–2479.
Balistrieri, L. S., & Murray, J. W. (1981). The surface chemistry of goethite (−FeOOH) in major ion seawater. American Journal Science, 281(5), 788–806.
Bhattacharyya, K. G., & Gupta, S. S. (2008). Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Advanced Colloid Interface Science, 40(2), 114–131.
Bradl, H. B. (2004). Adsorption of heavy metal ions on soils and soils constituents. Journal of Colloid and Interface Science, 277(1), 1–18.
Bradley, W. F. (1940). The structural scheme of atalpugite. American Mineralogist, 25(6), 405–410.
Cai, Y., Xue, J., & Polya, D. A. (2007). A Fourier transform infrared spectroscopic study of Mg-rich, Mg-poor and acid leached palygorskites. Spectrochimica Acta A, 66(2), 282.
Carrado, K. A., Decarreau, A., Petit, S., Bergaya, F., & Lagaly, G. (2006). Synthetic clay minerals and purification of natural clays. In F. Bergaya, B. K. G. Theng, & G. Lagaly (Eds.), Handbook of Clay Science (Vol. 1, pp. 115–140). Oxford: Elsevier.
Chen, H., & Wang, A. (2007). Kinetic and isothermal studies of lead ion adsorption onto palygorskite clay. Journal of Colloid Interface Science, 307(2), 309–316.
Chen, H., & Wang, A. (2009). Adsorption characteristics of Cu(II) from aqueous solution onto poly(acrylamide)/attapulgite composite. Journal of Hazardous Materials, 165(1–3), 223–231.
Chen, H., Zhao, Y., & Wang, A. (2007). Removal of Cu (II) from aqueous solution by adsorption onto acid-activated palygorskite. Journal of Hazardous Materials, 149(2), 346–354.
Cheng, H., Yang, J., & Frost, R. L. (2011). Thermogravimetric analysis-mass spectrometry (TG-MS) of selected Chinese palygorskites—implications for structural water. Thermochimimica Acta, 512(1–2), 202–207.
Fan, Q., Li, Z., Zhao, H., Jia, Z., Xu, J., & Wu, W. (2009). Adsorption of Pb(II) on palygorskite from aqueous solution: effects of pH, ionic strength and temperature. Applied Clay Science, 43(3), 111–116.
Freundlich, H. M. F. (1906). Uber die adsorption in losungen. Zeitschrift für Physikalische Chemie, 57A, 385–470.
Frost, R. L., & Ding, Z. (2003). Controlled rate thermal analysis and differential scanning calorimetry of sepiolites and palygorskites. Thermochimica Acta, 397(1–2), 119–128.
Frost, R. L., Locos, O. B., Ruan, H., & Kloprogge, J. T. (2001). Near-infrared and middle infra-red spectroscopic study of sepiolites and palygorsquite. Vibrational Spectroscopy, 27(1), 1–13.
Guggenheim, S., & Krekeler, P. S. (2011). The structure and microtextures of the palygorskite–sepiolite group minerals. In A. Singer & E. Galan (Eds.), Developments in Palygorskite-Sepiolite Research A New Outlook on these Nanomaterials (pp. 3–29). Oxford: Elsevier.
Handen, W. L., & Schwint, I. A. (1967). Attapulgite its properties and applications. Industrial Engineering Chemistry, 59(9), 59–69.
Ho, Y. S., & Mckay, G. (1999). Pseudo-second order model for sorption process. Process Biochemistry, 34(5), 451–465.
Hua, M., Zhang, S., Pan, B., Zhang, W., Lv, L., & Zhang, Q. (2012). Heavy metal removal from water/wastewater by nanosized metal oxides: a review. Journal of Hazardous Materials, 211–212, 317–331.
Jeffery, P. G., & Hutchison, D. (1981). Chemical methods of rock analysis. Oxford: Pergamon Press.
Jiratova, K. (1981). Isoelectric point of modified alumina. Applied Catalysis, 1(1), 165–167.
Khan, N. A., Hasan, Z., & Jhung, S. H. (2013). Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): a review. Journal of Hazardous Materials, 244–245, 444–456.
Kobya, M., Demirbas, E., Sinter, E., & Ince, M. (2005). Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresource Technology, 96(13), 1518–1521.
Koedrith, P., & Seo, Y. R. (2011). Advances in carcinogenic metal toxicity and potential molecular markers. International Journal of Molecular Science, 12(12), 9576–9595.
Kong, Y., Wei, J., Wang, Z., Sun, T., Yao, C., & Chen, Z. (2011). Heavy metals removal from solution by polyaniline/palygorskite composite. Journal of Applied Polymer Science, 122(3), 2054–2059.
Krestov, G. A. (1991). Thermodynamics of solvation: Solution and dissolution; ions and solvents; structure and energetics. London: Ellis Horwood.
Lagergren, S. (1898). Zur theorie der sogenannten adsorption gelöster stoffe Kungliga Svenska Vetenskapsakademiens. Handlingar, 24(4), 1–39.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of American Chemical Society, 40(9), 1361–1403.
Lazarevic, S., Jankovic´-Castvan, I., Djokic, V., Radovanovic, Z., Janackovic, D., & Petrovic, R. (2010). Iron-modified sepiolite for Ni2+ sorption from aqueous solution: an equilibrium, kinetic, and thermodynamic study. Journal of Chemical Engineering Data, 55(12), 5681–5689.
Lee, T. (2012). Removal of heavy metals in storm water runoff using porous vermiculite expanded by microwave preparation. Water, Air, & Soil Pollution, 223(6), 3399–3408.
Li, Z., Zhang, L., Zang, Z. P., Chang, X. J., & Zou, X. J. (2010). Attapulgite modified with 2-hydroxy-1-naphthaldehyde as selective solid-phase extractant for determination of copper(II) in environmental samples by ICP-OES. Microchimica Acta, 171(1–2), 161–168.
Mendelovici, E. (1973). Infrared study of attalpugite and HCl-treated attalpugite. Clay Clays Minerals, 21(2), 115–119.
Post, J. L., & Crawford, S. (2007). Varied forms of palygorskite and sepiolite from different geologic systems. Applied Clay Science, 36(4), 232–244.
Potgieter, J. H., Potgieter-Vermaak, S. S., & Kalibantonga, P. D. (2006). Heavy metals removal from solution by palygorskite clay. Minerals Engineering, 19(5), 463–470.
Sari, A., Tuzen, M., Citak, D., & Soylak, M. (2007). Adsorption characteristics of Cu(II) and Pb(II) onto expanded perlite from aqueous solution. Journal of Hazardous Materials, 148(1–2), 387–394.
Sdiri, A., Higashi, T., Chaabouni, R., & Jamoussi, F. (2012). Competitive removal of heavy metals from aqueous solutions by montmorillonitic and calcareous clays. Water, Air, & Soil Pollution, 223(3), 1191–1204.
Sheikhhosseini, A., Shirvani, M., & Shariatmadari, H. (2013). Competitive sorption of nickel, cadmium, zinc and copper on palygorskite and sepiolite silicate clay minerals. Geoderma, 192, 249–253.
Shirvani, M., Kalbasi, M., Shariatmadari, H., Nourbakhsh, F., & Najafi, B. (2006). Sorption–desorption of cadmium in aqueous palygorskite, sepiolite, and calcite suspensions: isotherm hysteresis. Chemosphere, 65(11), 2178–2184.
Shuali, U., Nir, S., & Rytwo, G. (2011). Adsorption and surfactants, dyes and cationic herbicides on sepiolite and palygroskite: Modifications, applications and modeling. In E. Singer & E. Galan (Eds.), Developments in palygorskite-sepiolite research, a new outlook on these nanomaterials (pp. 351–369). Oxford: Elsevier.
Srasra, N. F., & Srasra, E. (2010). Acid treatment of south Tunisian palygorskite: removal of Cd (II) from aqueous and phosphoric acid solutions. Desalination, 250(1), 26–34.
Tan, L., Jin, Y., Chen, J., Cheng, X., Wu, J., & Feng, L. (2011a). Sorption of radiocobalt (II) from aqueous solutions to Na-attapulgite. Journal of Radioanalytical and Nuclear Chemistry, 289(2), 601–610.
Tan, L., Jin, Y., Chen, J., Cheng, X., Wu, J., & Feng, L. (2011b). Sorption of radioeuropium onto attapulgite: effect of experimental conditions. Journal of Radioanalytical and Nuclear Chemistry, 3, 575–585.
Weber, W. J., Jr., & Morris, J. C. (1963). Kinetics of adsorption on carbon from solution. Journal of Sanitary Engineering Division, 89(2), 31–60.
Yao, C., Xu, Y., Kong, Y., Liu, W., Wang, W., Wang, Z., et al. (2012). Polypyrrole/palygorskite nanocomposite: a new chromate collector. Applied Clay Science, 67–68, 32–35.
Zhao, D., Zhou, J., & Liu, N. (2006). Preparation and characterization of Mingguang palygorskite supported with silver and copper for antibacterial behavior. Applied Clay Science, 33(3–4), 161–170.
Zhou, S., Xue, A., Zhao, Y., Wang, Q., Chen, Y., Li, M., et al. (2011). Competitive adsorption of Hg2+, Pb2+ and Co2+ ions on polyacrylamide/attapulgite. Desalination, 270(1–3), 269–274.
Acknowledgments
The authors thank CNPq and CAPES for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliveira, A.M.B.M., Coelho, L.F.O., Gomes, S.S.S. et al. Brazilian Palygorskite as Adsorbent for Metal Ions from Aqueous Solution—Kinetic and Equilibrium Studies. Water Air Soil Pollut 224, 1687 (2013). https://doi.org/10.1007/s11270-013-1687-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1687-x