Abstract
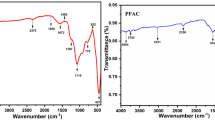
Cr(VI) adsorption from aqueous solutions on peanut husk modified with formaldehyde (PeH-F) and peanut husk modified with formaldehyde and Fe (PeH-FFe) was evaluated as a function of shaking time, initial pH, chromium concentration, and temperature. Results showed that the Cr(VI) is preferentially adsorbed by PeH-FFe at pH 2 than pH 6. It also was found that the chromate equilibrium sorption capacity for PeH-FFe is at least six times higher than for PeH-F. The optimum pH to remove chromium is 2 for both materials; however, PeH-FFe has a higher efficiency for the chromium removal. Finally, Cr(VI) adsorption also depends on chromium concentration and temperature. The adsorption data as a function of concentration obey Linear, Freundlich, and Langmuir isotherms at pH 2 and 6. The Cr(VI) maximum capacity of PeH-FFe at pH 2 was 33.11 mg Cr(VI)/g, slightly higher than that at pH 6 (31.75 mg Cr(VI)/g). The linear isotherm shows that the pH affect the Cr(VI) distribution into the aqueous/solid phases. The negative value of ΔH° and positive values of ΔG° indicate that the chromium adsorption process is an exothermic and non-spontaneous process. The characterization of the peanut husk modified with formaldehyde and peanut husk modified with formaldehyde and Fe by scanning electron microscopy, Raman, and IR spectroscopies as well as the textural characteristics of the no-living biomasses were also considered in this work.







Similar content being viewed by others
References
Al-Othman, Z. A., Ali, R., & Nauhad, M. (2012). Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: adsorption kinetics, equilibrium and thermodynamic studies. Chemical Engineering Journal, 184, 238–247.
Aryal, M., Ziagova, M., & Liakopoulou-Kyriakides, M. (2011). Comparison of Cr(VI) and As(V) removal in single and binary mixtures with Fe(III)-treated Staphylococcus xylosus biomass: thermodynamic studies. Chemical Engineering Journal, 169, 100–106.
Chakravarti, A. K., Chowdhury, S., Chakrabarty, S., & Mukherjee, D. C. (1995). Liquid membranes multiple emulsion process of chromium (VI) separation from wastewater. Colloid Surface A, 103, 59–71.
Cimino, G., Passerini, A., & Toscano, G. (2000). Removal of toxic cations and Cr(VI) from aqueous solution by hazelnut shell. Water Research, 34, 2955–2962.
Daneshvar, N., Salari, D., & Aber, S. (2002). Chromium adsorption and Cr(VI) reduction to trivalent chromium in aqueous solution by soya cake. Journal of Hazardous Materials, 94, 49–61.
Dziwulska, U., Bajguz, A., & Zylkiewicz, B. (2004). The use of algae Chlorella vulgaris immobilized on cellex T support for separation/preconcentration of trace amounts of platinum and palladium before GFAAS determination. Analytical Letters, 37, 2189–2203.
El-Zahrani, H. A., & El-Saied, A. I. (2011). Bioremediation of heavy metal toxicity from factory effluents by transconjugants bacteria. Journal of the Egyptian Society of Parasitology, 41, 641–650.
Gode, F., & Pehlivan, E. (2005). Removal of Cr(VI) from aqueous solution by two Lewatit-anion exchange resins. Journal of Hazardous Materials, 119, 175–182.
Granados-Correa, F., & Serrano-Gómez, J. (2009). CrO4 2− ions adsorption by Fe-modified pozzolane. Separation Science and Technology, 44, 924–936.
Hasany, M. S., Saeed, M. M., & Ahmed, M. (2000). Adsorption isotherms and thermodynamic profile of Co(II)–SCN complex uptake on polyurethane foam. Separation Science and Technology, 35, 379–394.
Juang, R. S., & Shiau, R. C. (2000). Metal removal from aqueous solutions using chitosan enhanced membrane filtration. Journal of Membrane Science, 21, 1091–1097.
Kowalsky, Z. (1994). Treatment of chromic tannery wastes. Journal of Hazardous Materials, 37, 137–144.
Liu, B., & Huang, Y. (2011). Polyethyleneimine modified eggshell membrane as a novel biosorbent for adsorption and detoxification of Cr(VI) from water. Journal of Materials Chemistry, 21, 17413–17418.
Machado, M. D., Santos, M. S. F., Gouveia, C., Soares, H. M. V. M., & Soares, E. V. (2008). Removal of heavy metals using a Brewer's yeast strain of Saccharomyces cerevisiae: the flocculation as a separation process. Bioresource Technology, 99, 2107–2115.
Malik, P. K. (2004). Dye removal from waste water using activated carbon developed from sawdust: adsorption equilibrium and kinetics. Journal of Hazardous Materials, 113, 81–88.
Marandi, R. (2011). Biosorption of hexavalent chromium from aqueous solution by dead fungal biomass of Phanerochaete crysosporium: batch and fixed bed studies. Canadian Journal of Chemical Engineering, 2, 8–22.
Owland, M., Arou, M. K., Daud, W. A. W., & Baroutian, S. (2009). Removal of hexavalent chromium-contaminated water and wastewater: a review. Water, Air, and Soil Pollution, 200, 59–77.
Qadeer, R., Hanif, J., Saleem, M., & Afzal, M. (1993). Surface characterization and thermodynamics of adsorption of Sr2+, Ce3+, Sm3+, Gd3+, Th4+, UO2 2+ on activated charcoal from aqueous solution. Colloid & Polymer Science, 271, 83–90.
Randall, J. M., Hautala, E., & McDonald, G. (1978). Binding of heavy metal Ions by formaldehyde-polymerized peanut skins. Journal of Applied Polymer Science, 22, 379–387.
Rengaraj, S., Yeon, K. H., & Moon, S. H. (2001). Removal of chromium from water and wastewater by ion exchange resins. Journal of Hazardous Materials, 87, 273–287.
Ricordel, S., Taha, S., Cisse, I., & Dorange, G. (2001). Heavy metals removal by adsorption onto peanut husks carbon: characterization, kinetic study and modeling. Separation and Purification Technology, 24, 389–401.
Saeed, M. M. (2003). Adsorption profile and thermodynamic parameter of the preconcentration of Eu(III) on 2-thenoyltrifluroroacetone loaded polyurethane (PUR) foam. Journal of Radioanalytical and Nuclear Chemistry, 256, 73–80.
Saha, B., & Orvig, C. (2010). Biosorbents for hexavalent chromium elimination from industrial and municipal effluents. Coordination Chemistry Reviews, 254, 2959–2972.
Serrano-Gómez, J., López-González, H., Olguín, M. T., & Bulbulian, S. (2010). As (V) adsorption by uunmodified and Iron modified pozzolane. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 67, 153–158.
Shaik, B., Murthy, Z. V. P., & Jha, B. (2008). Biosorption of hexavalent chromium by chemically modified seaweed, Cystoseira indica. Chemical Engineering Journal, 137, 480–488.
Tewari, N., Vasudevan, P., & Guha, B. K. (2005). Study on biosorption of Cr(VI) by Mucor hiemalis. Biochemical Engineering Journal, 23, 185–192.
Ucun, H., Bayhan, K. Y., & Kaya, Y. (2008). Kinetic and thermodynamic studies of the biosorption of Cr(VI) by Pinus silvestri Linn. Journal of Hazardous Materials, 153, 52–59.
Varga, M., Takács, M., Záray, G., & Varga, I. (2013). Comparative study of sorption kinetic and equilibrium of chromium (VI) on charcoals prepared from different low-cost materials. Michrochemical Journal, 107, 25–30.
Witek-Krowiak, A., Szafran, R. G., & Modelski, S. (2011). Biosorption of heavy metals from aqueous solutions onto peanut shell a low-cost biosorbent. Desalination, 265, 126–134.
Acknowledgments
M. T. Olguín thanks CONACyT project 131174-Q for partial financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olguín, M.T., López-González, H. & Serrano-Gómez, J. Hexavalent Chromium Removal From Aqueous Solutions by Fe-Modified Peanut Husk. Water Air Soil Pollut 224, 1654 (2013). https://doi.org/10.1007/s11270-013-1654-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1654-6