Abstract
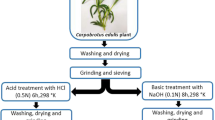
Adsorption potentials of native and amine-modified plant biomass of Alyssum caricum for the removal of Reactive Green 19 (RG-19) and Reactive Red 2 (RR-2) dyes from aqueous solutions were studied. The adsorbents were characterized before and after modification process using Fourier transform infrared spectroscopy (FTIR), Brunauer–Emmett–Teller (BET) and potentiometric titration analysis. Modification of the surface of A. caricum biomass with hexamethylenediamine (HMDA) showed an increase of 1.18-fold in its surface area. Batch studies illustrated that dye adsorption were highly dependent on different process variables, pH, initial dye concentration of solution, adsorbent dosage, and temperature. The maximum adsorption capacities of the native and amine-modified adsorbents were 27.6 and 63.4 mg/g adsorbent for RG-19 dye and 16.5 and 36.8 mg/g adsorbent for RR-2 dye, respectively. The adsorption of both dyes on the native and amine-modified plant biomass correlated well with the Langmuir and Temkin isotherm equations as compared to Freundlich and D-R equations. The calculated thermodynamic parameters for both native and amine-modified adsorbents showed that the adsorption was feasible, spontaneous, and exothermic. The information gained from these studies was expected to indicate whether native and amine-modified adsorbents can have potential to be used for the removal of other dyes from wastewaters.










Similar content being viewed by others
References
Akar, T., Celik, S., & Tunali Akar, S. (2010). Biosorption performance of surface modified biomass obtained from Pyracantha coccinea for the decolorization of dye contaminated solutions. Chemical Engineering Journal, 160, 466–472.
Akar, T., Ozcan, A. S., Tunali, S., & Ozcan, A. (2008). Biosorption of a textile dye (Acid Blue 40) by cone biomass of Thuja orientalis: estimation of equilibrium, thermodynamic and kinetic parameters. Bioresource Technology, 99, 3057–3065.
Anbia, M., & Salehi, S. (2012). Removal of acid dyes from aqueous media by adsorption onto amino-functionalized nanoporous silica SBA-3. Dyes and Pigments, 94, 1–9.
Arica, M. Y., & Bayramoglu, G. (2007). Biosorption of Reactive Red-120 dye from aqueous solution by native and modified fungus biomass preparations of Lentinus sajor-caju. Journal of Hazardous Materials, 149, 499–507.
Bayramoglu, G., & Arica, M. Y. (2011). Preparation of a composite biosorbent using Scenedesmus quadricauda biomass and alginate/polyvinyl alcohol for removal of Cu(II) and Cd(II) ions: isotherms, kinetics, and thermodynamic studies. Water, Air, and Soil Pollution, 221, 391–403.
Bayramoglu, G., Celik, G., & Arica, M. Y. (2006). Biosorption of Reactive Blue 4 dye by native and treated fungus Phanerocheate chrysosporium: batch and continuous flow system studies. Journal of Hazardous Materials, 137, 1689–1697.
Bayramoglu, G., Gursel, I., Tunalı, Y., & Arica, M. Y. (2009). Funalia trogii pellets for removal of phenol and 2-chlorophenol. Bioresource Technolology, 100, 2685–2691.
Celekli, A., Ilgun, G., & Bozkurt, H. (2012). Sorption equilibrium, kinetic, thermodynamic, and desorption studies of Reactive Red 120 on Chara contraria. Chemical Engineering Journal, 191, 228–235.
Couto, S. R. (2009). Dye removal by immobilized fungi. Biotechnology Advance, 27, 227–235.
Du, L. N., Wang, B., Li, G., Wang, S., Crowley, D. E., & Zhao, Y.-H. (2012). Biosorption of the metal-complex dye Acid Black 172 by live and heat-treated biomass of Pseudomonas sp. strain DY1: kinetics and sorption mechanisms. Journal of Hazardous Materials, 205–206, 47–54.
Farkas, V., Felinger, A., Hegedusova, A., Dekany, I., & Pernyeszi, T. (2013). Comparative study of the kinetics and equilibrium of phenol biosorption on immobilized white-rot fungus Phanerochaete chrysosporium from aqueous solution. Colloids Surfaces B, 103, 381–390.
Gercel, O., Gercel, H. F., Koparal, A. S., & Ogutveren, U. B. (2008). Removal of disperse dye from aqueous solution by novel adsorbent prepared from biomass plant material biomass of Thuja orientalis. Journal of Hazardous Materials, 160, 668–674.
Ho, Y. S., & Mckay, G. (1998). Sorption of dye from aqueous solution by peat. Chemical Engineering Journal, 70, 115–124.
Krishnan, K., Meng, X., Christodoulatos, C., & Boddu, V. M. (2008). Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. Journal of Hazardous Materials, 153, 1222–1234.
Low, L. W., Teng, T. T., Ahmad, A., Morad, N., & Wong, Y. S. (2011). A novel pretreatment method of lignocellulosic material as adsorbent and kinetic study of dye waste adsorption. Water, Air, and Soil Pollution, 218, 293–306.
Mahmoodia, N. M., Najafib, F., Khorramfara, S., Aminia, F., & Aramic, M. (2011). Synthesis, characterization and dye removal ability of high capacity polymeric adsorbent: polyaminoimide homopolymer. Journal of Hazardous Materials, 198, 87–94.
Mengoni, A., Baker, A. J. M., Bazzicalupo, M., Reeves, R. D., Adigüzel, N., Chianni, E., Galardi, F., Gabbrielli, R., & Gonnelli, C. (2003). Evolutionary dynamics of nickel hyper accumulation in Alyssum revealed by ITS nrDNA analysis. New Phytologist, 159, 691–699.
Mittal, A., Mittal, J., Malviya, A., & Gupta, V. K. (2009). Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. Journal of Colloids Interface Science, 340, 16–26.
Prasad, M. N. V., & Freitas, H. (2000). Removal of toxic metals from solution by leaf, stem and root phytomass of Quercus ilex L. Environmental Pollution, 110, 277–283.
Reeves, R. D., & Adiguzel, N. (2004). Rare plants and nickel accumulators from Turkish serpentine soils, with special reference to Centaurea species. Turkish Journal of Botany, 28, 147–153.
Robens, J. F., Dill, G. S., Ward, J. M., Joiner, J. R., Griesemer, R. A., & Douglas, J. F. (1980). Thirteen-week subchronic toxicity studies of Direct Blue 6, Direct Black 38 and Direct Brown 95 dyes. Toxicology and Applied Pharmacology, 54, 431–442.
Roy, A., Chakraborty, S., Kundu, S. P., Adhikari, B., & Majumder, S. B. (2012). Adsorption of anionic-azo dye from aqueous solution by lignocellulose-biomass jute fiber: equilibrium, kinetics, and thermodynamics study. Industrial and Engineering Chemistry Research, 51, 12095–12106.
Safa, Y., & Bhatti, H. N. (2011a). Adsorptive removal of direct textile dyes by low cost agricultural waste: application of factorial design analysis. Chemical Engineering Journal, 167, 35–41.
Safa, Y., & Bhatti, H. N. (2011b). Kinetic and thermodynamic modeling for the removal of Direct Red-31 and Direct Orange-26 dyes from aqueous solutions by rice husk. Desalination, 272, 313–322.
Saha, P. (2010). Assessment on the removal of methylene blue dye using tamarind fruit shell as biosorbent. Water, Air, and Soil Pollution, 213, 287–299.
Shah, K., & Nongkynrih. (2007). Metal hyperaccumulation and bioremediation. Biologia Plantarum, 51, 618–634.
Sidney, S. (1967). Quantitative organic analysis (3rd ed.). New York: Wiley.
Tang, H., Zhou, W., & Zhang, L. (2012). Adsorption isotherms and kinetics studies of malachite green on chitin hydrogel. Journal of Hazardous Materials, 209, 218–225.
Trevisan, C. W., Foletto, E. L., & Meili, L. (2013). Removal of tannery dye from aqueous solution using papaya seed as an efficient natural biosorbent. Water, Air, and Soil Pollution, 224, 1427.
Venturini, S., & Tamaro, M. (1979). Mutagencity of anthraquinone and azo dyes in Ames Salmonella typhimurium test. Mutation Research, 68, 307–312.
Weber, C. T., Meili, E. L., & Foletto, L. (2013). Removal of tannery dye from aqueous solution using papaya seed as an efficient natural biosorbent. Water, Air, and Soil Pollution, 224, 1427–1438.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayramoglu, G., Adiguzel, N., Ersoy, G. et al. Removal of Textile Dyes from Aqueous Solution using Amine-Modified Plant Biomass of A. caricum: Equilibrium and Kinetic Studies. Water Air Soil Pollut 224, 1640 (2013). https://doi.org/10.1007/s11270-013-1640-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1640-z