Abstract
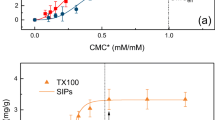
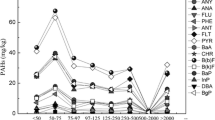
The high hydrophobicity of polycyclic aromatic hydrocarbons (PAHs) is the main limiting factor for the remediation of soils and aquifers. Surfactants are amphiphilic substances which encourage the transfer of hydrophobic compounds from the solid to the liquid phase. While the interaction between organic matter and surfactants has been widely studied, there is a lack of knowledge concerning the relationship between surfactant efficiency and the granulometry of soil and/or geologic material. In this paper, three non-ionic surfactants (Tween 80, Gold Crew, and BS-400) were used to study the desorption of pyrene, chosen as a representative PAH, in soils with different grain size proportions (1%, 5%, 10%, and 20% of clay and silt) and no organic matter (<0.1%). The best quantity of surfactant to apply is closely related to the proportion of fine materials. Tween 80 gave better maximum desorption than Gold Crew and BS-400 (89%, 40%, and 36%, respectively). As an important proportion of aquifers show fine material above 1%, the effective critical micellar concentration obtained when applying surfactants to this type of geologic materials has to be higher than 150 mg L−1 for Tween 80, and higher than 65 mg L−1, and 100 mg L−1 for Golf Crew and BS 400, respectively. Furthermore, results indicate that carrying out simple laboratory tests before the use of surfactants on a field scale is necessary to improve the efficiency and minimize the financial and environmental impact of its application.




Similar content being viewed by others
References
Bamforth, S. M., & Singleton, I. (2005). Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions. J Chem Technol Biot, 80, 723–736.
Cheng, K. Y., & Wong, J. W. C. (2006). Combined effect of nonionic surfactant Tween 80 and DOM on the behaviors of PAHs in soil–water system. Chemosphere, 62, 1907–1916.
Chu, W., & Chan, K. H. (2003). The mechanism of the surfactant-aided soil washing system for hydrophobic and partial hydrophobic organics. The Science of the Total Environment, 307, 83–92.
Duffield, A. R., Ramamurthy, R. S., & Campanelli, J. R. (2003). Surfactant enhanced mobilization of mineral oil within porous. Water, Air and Soil Pollution, 143, 111–122.
Edwards, D. A., Luthy, R., & Liu, Z. (1991). Solubilization of polycyclic aromatic hydrocarbons in micellar nonionic surfactant solutions. Environmental Science & Technology, 25, 127–133.
Garon, D., Krivobok, S., Wouessidjew, D., & Seigle-Murandi, F. (2002). Influence of surfactants on solubilization and fungal degradation of fluorene. Chemosphere, 47, 303–309.
Gonen, Y., & Rytwo, G. (2006). Using the dual-mode model to describe adsorption of organic pollutants onto an organoclay. Journal of Colloid and Interface Science, 299, 95–101.
Grinberg, S., Stringfellow, W., & Aitken, M. (1996). Quantifying the biodegradation of phenanthrene by Pseudomonas stutzeri P16 in the presence of a nonionic surfactant. Applied and Environmental Microbiology, 62, 2387–2392.
Gurein, W. F., & Jones, G. E. (1988). Mineralization of phenanthrene by Mycobacterium sp. Applied and Environmental Microbiology, 54, 937–944.
Guha, P. R., Jaffe, C., & Peters, M. (1998). Solubilization of PAH mixtures by nonionic surfactant. Environmental Science & Technology, 32, 930–954.
Hwang, S., Ramirez, N., Cutright, T. J., & Ju, L.-K. (2003). The role of soil properties in pyrene sorption and desorption. Water, Air, and Soil Pollution. doi:10.1023/A:1022863015709.
Jahan, K., Ahmed, T., & Maier, W. J. (1999). Modeling the influence of nonionic surfactants on biodegradation of phenanthrene. Water Research, 33, 2181–2193.
Jönsonn, B., Holmberg, K., & Kronberg, B. (1998). Surfactants and polymers in aqueous solution. Chichester: Wiley.
Joshi, M. M., & Lee, S. (1996). Optimization of surfactant-aided remediation of industrially contaminated soils. Energy Sources, 18, 291–301.
Karichoff, S. W. (1984). Organic pollutant sorption in aquatic systems. Journal of Hydraulic Enginnering, 6, 707–735.
Kim, I. S., & Park, J. (2001). Enhanced biodegradation of polycylic aromatic hydrocarbons using nonionic surfactants in soil slurry. Applied Geochemistry, 16, 1519–1528.
Laha, S., & Luthy, R. G. (1992). Inhibition of phenanthrene mineralization by nonionic surfactants in soil–water systems. Environmental Science & Technology, 25, 1290–1930.
Laha, S., Tansel, B., & Ussawarujikulchai, A. (2009). Surfactant-soil interactions during surfactant-amended remediation of contaminated soils by hydrophobic organic compounds: a review. J Environ Mang, 90, 95–100.
Lippold, H., Gottschalch, U., & Kupsch, H. (2008). Joint influence of surfactants and humic matter on PAH solubility. Are mixed micelles formed? Chemosphere, 70, 1979–1986.
Martinez-Bofill, J., Corominas, J., Soler, A. (2008). Approach to the relationship between durability and petrological characteristics of weak rocks. In II European Conference of the International Association for Engineering Geology. EuroEnGeo. The City and its Subterranean Environment. Ed. AEGAIN Asociación Española de Geología Aplicada a la Ingeniería and IAEG International Association for Engineering Geology (national groups of Spain, Portugal, France). Madrid. Abstract pp. 63. CD. pp. 1–6.
Mulligan, C. N., Yong, R. N., & Gibbs, B. F. (2001). Surfactant-enhanced remediation of contaminated soil: a review. Engineering Geology, 60, 371–380.
Pennell, K.D., Karagunduz, A., Yeh, D.H., Martin, C.A., Chang, E.K., Pavlostathis, S.G. (2000). Influence of nonionic surfactants on the bioavailability of chlorinated benzenes for microbial reductive dechlorination. Presented at the 220th ACS National Meeting, Washington, DC. p. 724.
Peters, R. W., Montemagno, C. D., Shem, L., & Lewis, B. (1992). Surfactant screening of diesel-contaminated soil. Hazardous Waste and Hazardous Materials, 9, 2035–2042.
Pinto, L., & Moore, M. (2000). Release of polycyclic aromatic hydrocarbons from contamiated soils by surfactant and remediation of the effluent by Penicillum sp. Environmental Toxicology and Chemistry, 19, 2387–2392.
Rosen, M. J. (1989). Surfactants and interfacial phenomena. New York: Wiley & Sons.
Sánchez-Martín, M. J., Dorado, M. C., Del Hoyo, C., & Rodríguez-Cruz, M. S. (2008). Influence of clay mineral structure and surfactant nature on the adsorption capacity of surfactants by clays. Journal of Hazardous Materials, 150, 115–123.
Schwarzenbach, R., & Giger, W. (1985). Behavior and fate of halogenated hydrocarbons in ground water. In C. H. Ward, W. Giger, & P. L. McCarty (Eds.), Ground water quality. New York: Wiley-Interscience.
Teixeira, S. C. G., Lourenço Ziolli, R., da Costa Marques, M., & Vidal Pérez, D. (2010). Study of pyrene adsorption on two brazilian soils. Water, Air, and Soil Pollution. doi:10.1007/s11270-010-0707-3.
Tielmes, A. (1994). Degradation of polycylic aromatic hydrocarbons in the presence of syntethic surfactants. Applied and Environmental Microbiology, 60, 258–263.
Volkering, F., Breure, A., Andel, J., & Rulkens, W. (1995). Influence of nonionic surfactants on bioavailability and biodegradation of polycylic aromatic hydrocarbons. Applied and Environmental Microbiology, 61, 1699–1705.
Yalkowsky, S. H. (1999). Solubilization in aqueous media. Amercian Chemical Society, Washington, DC. New York: Oxford University Press.
Yeon, I. T., Ghosh, M. M., Cox, C. D., & Robinson, K. G. (1995). Micellar solubilization of polynuclar aromatic hydrocarbon in coal tarcontaminated soils. Environmental Science & Technology, 29, 3015–3021.
Ying, G. G. (2006). Fate, behavior and effects of surfactants and their degradation products in the environment. Environment International, 32, 417–431.
Yuan, S. Y., Wei, S. H., & Chang, B. V. (2000). Biodegradation of polycyclic aromatic hydrocarbons by a mixed culture. Chemosphere, 41, 1463–1468.
Zhao, B., Zhu, L., Li, W., & Chen, B. (2005). Solubilization and biodegradation of phenanthrene in mixed anionic–nonionic surfactant solutions. Chemosphere, 58, 33–40.
Zhou, W., & Zhu, L. (2008). Enhanced soil flushing of phenanthrene by anionic–nonionic mixed surfactant. Water Research, 42, 101–108.
Zhou, W., & Zhu, L. (2007). Efficiency of surfactant-enhanced desorption for contaminated soils depending on the component characteristics of soil-surfactant–PAHs system. Environmental Pollution, 147, 66–73.
Acknowledgments
We acknowledge the initial contributions from Georgina Vidal from d D’ENGINY biorem. This work was supported by the CICYT project CGL2008-06373-C03-03/BTE and the Spanish National R&D + i Plan, project number CTM2007-60971. The Department of Chemical Engineering of the Universitat Autònoma de Barcelona is the Biochemical Engineering Unit of the Network for Research in Biotechnology (XRB) established by the government of Catalonia. Teresa Vicent is a member of a consolidated research group in Catalonia (2009-SGR-656).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodriguez-Escales, P., Sayara, T., Vicent, T. et al. Influence of Soil Granulometry on Pyrene Desorption in Groundwater Using Surfactants. Water Air Soil Pollut 223, 125–133 (2012). https://doi.org/10.1007/s11270-011-0844-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-011-0844-3