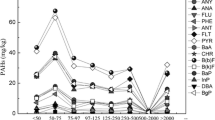
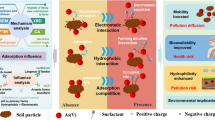
Abstract
Purpose
The ionic and nonionic surfactants have different adsorption-desorption models for polycyclic aromatic hydrocarbons (PAHs) in soil-water system, due to the difference in the composition and charge of the hydrophilic groups. Surfactant eluents retained in the soil may also have a secondary effect on the soil environment. Thus, the aim of the study was to investigate sorption-desorption mechanisms and environmental toxicity of different surfactants in enhancing remediation of soil contaminated with PAHs.
Materials and methods
The distribution of PAHs between different surfactants and soil is influenced by the surfactant bilayer formation. The average molecular density, Sips isotherm, critical micelle concentration (CMC), and critical washing concentration (CWC) models were used to explore the mechanisms involved in the adsorption-desorption of cationic (cetyltrialkylammonium bromide, CTAB), anionic (sodium dodecyl benzene sulfonate, SDBS), and nonionic (TritonX-100, TX100) surfactants. These models were associated with the surfactant-enhanced remediation (SER) of naphthalene-contaminated soil. The mean concentrations and activity of indigenous bacteria were used to detect the toxic effect of the above surfactants on soil environment.
Results and discussion
The results of SER experiments showed that there are critical washing points (SDBS = 2.4 CMC, TX100 = 4.6 CMC, CTAB = 3.3 CMC) for different surfactants washing naphthalene (Nap). The values of CWC corresponding to critical washing points were key variables driving the need for added surfactants to remove or immobilize Nap. The CWC of different surfactants could be conveniently predicted by a model based on the surfactant-derived organic carbon. In addition, the mean concentration of viable bacteria in biological culture experiment was highest for TX100, followed by CTAB and SDBS.
Conclusions
This study revealed the effects of average molecular density and surfactant-derived organic carbon on the distribution of Nap, and suggested a model for calculating CWC of different surfactants, to optimize SER technology. Biological culture experiment indicated that the high concentration and ionic surfactants had a significantly toxic effect on indigenous bacteria.





Similar content being viewed by others
References
Arab P, Araújo T, Pejon O (2015) Identification of clay minerals in mixtures subjected to differential thermal and thermogravimetry analyses and methylene blue adsorption tests. Appl Clay Sci 114:133–140
Boyd S, Mortland M, Chiou C (1988) Sorption characteristics of organic compounds on hexadecyltrimethylammonium-smectite. Soil Sci Soc Am J 52:652–657
Bramwell D, Laha S (2000) Effects of surfactant addition on the biomineralization and microbial toxicity of phenanthrene. Biodegradation 11:263–277
Chen B, Zhu L, Lin B, Tao S (2004) Enhancement of cationic surfactant on immobilizing P-nitrophenol phenol in soil. Acta Pedol Sin 41(01):148–151 (In Chinese)
Chen B, Huang W, Mao J, Lv S (2008) Enhanced sorption of naphthalene and nitroaromatic compounds to bentonite by potassium and cetyltrimethylammonium cations. J Hazard Mater 158(1):116–123
Chiou C, Peters L, Freed V (1979) A physical concept of soil-water equilibria for nonionic organic compounds. Science 206:831–832
Chiou C, Mcgroddy S, Kile D (1998) Partition characteristics of polycyclic aromatic hydrocarbons on soils and sediments. Environ Sci Technol 32:264–269
Coulon F, Jones K, Li H, Hu Q, Gao J, Li F, Chen M, Zhu Y, Liu R, Liu M (2016) China’s soil and groundwater management challenges: lessons from the UK’s experience and opportunities for China. Environ Int 91:196–200
Edwards D, Luithy R, Liu Z (1991) Solubilization of polycyclic aromatic hydrocarbons in micellar nonionic surfactant solutions. Environ Sci Technol 25:127–133
Ghosh I, Mukherji S (2016) Diverse effect of surfactants on pyrene biodegradation by a Pseudomonas strain utilizing pyrene by cell surface hydrophobicity induction. Int Biodeterior Biodegradation 108:67–75
Hwang S, Cutright T (2002) Impact of clay minerals and DOM on the competitive sorption/desorption of PAHs. Soil Sediment Contam 11:269–291
Jafvert C, Patricia L, Heath J (1994) Solubilization of non-polar compounds by non-ionic surfactant micelles. Water Res 28:1009–1017
Jian L, Wong Y, Tam N (2009) Static and dynamic sorption of phenanthrene in mangrove sediment slurry. J Hazard Mater 168:1422–1429
Jian L, Ming H, Wong Y, Tam N (2011) Modeling sorption and biodegradation of phenanthrene in mangrove sediment slurry. J Hazard Mater 190:409–415
Jin H, Zhou W, Zhu L (2013) Utilizing surfactants to control the sorption, desorption, and biodegradation of phenanthrene in soil-water system. J Environ Sci 25(6):1355–1361
Jones D, Willett V (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38(5):991–999
Kanaly R, Harayama S (2000) Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol 182:2059–2067
Karickhoff S, Brown D, Scott T (1979) Sorption of hydrophobic pollutants on natural sediments. Water Res 13:241–248
Khan S, Cao Q (2012) Human health risk due to consumption of vegetables contaminated with carcinogenic polycyclic aromatic hydrocarbons. J Soils Sediments 12(2):178–184
Kim J, Kim M, Hyun S, Kim JG, Ok YS (2012) Sorption of acidic organic solute onto kaolinitic soils from methanol-water mixtures. J Environ Sci Health B 47(1):22–29
Kuppusamy S, Thavamani P, Venkateswarlu K, Yong B, Naidu R, Megharaj M (2016) Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: technological constraints, emerging trends and future directions. Chemosphere 168:944
Laha S, Tansel B, Ussawarujikulchai A (2009) Surfactant–soil interactions during surfactant-amended remediation of contaminated soils by hydrophobic organic compounds: a review. J Environ Manag 90:95–100
Lamichhane S, Bal Krishna K, Sarukkalige R (2017) Surfactant-enhanced remediation of polycyclic aromatic hydrocarbons: a review. J Environ Manag 199:46–61
Lee J, Liao P, Kuo C, Yang H, Chiou C (2000) Influence of a nonionic surfactant (Triton X-100) on contaminant distribution between water and several soil solids. J Colloid Interface Sci 229:445–452
Liang X, Guo C, Liao C, Liu S, Wick L, Peng D, Yi X, Lu G, Yin H, Lin Z (2017) Drivers and applications of integrated clean-up technologies for surfactant-enhanced remediation of environments contaminated with polycyclic aromatic hydrocarbons (PAHs). Environ Pollut 225:129–140
Liu G, Jiang N, Zhan L, Liu Z (1996) Soil physical and chemical analysis description of soil profiles. China Standard Methods Press, Beijing (In Chinese)
Lu S, Kunjappu J, Somasundaran P, Zhang L (2008) Adsorption of a double-chain surfactant on an oxide. Colloids Surf A Physicochem Eng Asp 324:65–70
Mulligan C, Yong N, Gibbs F (2001) Surfactant-enhanced remediation of contaminated soil : a review. Eng Geol 60:371–380
Paria S, Khilar K (2004) A review on experimental studies of surfactant adsorption at the hydrophilic solid–water interface. Adv Colloid Interface Sci 110:75–95
Peluffo M, Pardo F, Santos A, Romero A (2016) Use of different kinds of persulfate activation with iron for the remediation of a PAH-contaminated soil. Sci Total Environ 563-564:649–656
Qiao M, Huang S, Wang Z (2008) Partitioning characteristics of PAHs between sediment and water in a shallow lake. J Soils Sediments 8(2):69–73
Rao P, He M (2006) Adsorption of anionic and nonionic surfactant mixtures from synthetic detergents on soils. Chemosphere 63:1214–1221
Rhue R, Mansell R (1988) The effect of pH on sodium-calcium and potassium-calcium exchange selectivity for Cecil soil. Soil Sci Soc Am J 52:641–647
Somasundaran P, Krishnakumar S (1997) Adsorption of surfactants and polymers at the solid-liquid interface. Colloids Surf A Physicochem Eng Asp 123–124:491–513
Sun S, Inskeep W, Boyd S (1995) Sorption of nonionic compounds in soil-water systems containing a micelle-forming surfactant. Environ Sci Technol 29(4):903–913
Tian S, Zhu L, Shi Y (2004) Characterization of sorption mechanisms of VOCs with organobentonites using a LSER approach. Environ Sci Technol 38:489–495
Tsomides H, Hughes J, Thomas J, Ward C (2010) Effect of surfactant addition on phenanthrene biodegradation in sediments. Envrion Toxicol Chem 14:953–959
Ukalska-Jaruga A, Smreczak B, Klimkowicz-Pawlas A (2019) Soil organic matter composition as a factor affecting the accumulation of polycyclic aromatic hydrocarbons. J Soils Sediments 19:1890–1900
Wahab M, Jellali S, Jedidi N (2010) Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour Technol 101:5070–5075
Wick L, Colangelo T, Harms H (2001) Kinetics of mass transfer-limited bacterial growth on solid PAHs. Environ Sci Technol 35:354–361
Yang Y, Zhang N, Xue M, Tao S (2010) Impact of soil organic matter on the distribution of polycyclic aromatic hydrocarbons (PAHs) in soils. Environ Pollut 158:2170–2174
Yang X, Lu G, She B, Liang X, Yin R, Guo C, Yi X, Zhi D (2015) Cosolubilization of 4,4′-dibromodiphenyl ether, naphthalene and pyrene mixtures in various surfactant micelles. Chem Eng J 260:74–82
Zhang R, Somasundaran P (2006) Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Adv Colloid Interf Sci 123-126:213–229
Zhao S, Huang G, An C, Wei J, Yao Y (2015) Enhancement of soil retention for phenanthrene in binary cationic gemini and nonionic surfactant mixtures: characterizing two-step adsorption and partition processes through experimental and modeling approaches. J Hazard Mater 286:144–151
Zhou W, Zhu L (2005) Distribution of polycyclic aromatic hydrocarbons in soil-water system containing a nonionic surfactant. Chemosphere 60:1237–1245
Zhou Y, Liu R, Tang H (2004) Sorption interaction of phenanthrene with soil and sediment of different particle sizes and in various CaCl2 solutions. J Colloid Interface Sci 270(1):37–46
Zhu L (2012) Controlling technology of interfacial behaviors of organic pollutants and its application. Acta Sci Circumst 32(11):2641–2649 (In Chinese)
Zhu L, Chen B, Tao S, Chiou C (2003) Interactions of organic contaminants with mineral-adsorbed surfactants. Environ Sci Technol 37:4001–4006
Zhu R, Zhu L, Xu L (2007) Sorption characteristics of CTMA–bentonite complexes as controlled by surfactant packing density. Colloids Surf A Physicochem Eng Asp 294(1–3):221–227
Funding
The authors would like to thank the National Natural Science Foundation of China (41230314), the Key Laboratory of Degraded and Unused Land Consolidation Engineering of the Ministry of Land and Resources of China (SXDJ2017-6), and the Fund Project of Shaanxi Key Laboratory of Land Consolidation (2018-ZD04) for funding this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Responsible editor: Xilong Wang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 806 kb)
Rights and permissions
About this article
Cite this article
Zhao, S., Li, Y., Cao, Z. et al. Sorption-desorption mechanisms and environmental friendliness of different surfactants in enhancing remediation of soil contaminated with polycyclic aromatic hydrocarbons. J Soils Sediments 20, 2817–2828 (2020). https://doi.org/10.1007/s11368-020-02640-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-020-02640-0