Abstract
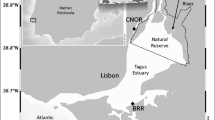
The availability of metals to plants is a complex function of numerous environmental factors. Many of these factors are interrelated, and vary seasonally and temporally. The current study intended to understand the influence of seasonal fluctuations and the vegetation of salt marsh plants (SMPs; Halimione portulacoides, Juncus maritimus) on sediment’s mercury (Hg) and its pH and redox potential (Eh), as well as their cumulative effect on the plant’s Hg-accumulation and Hg-partitioning potential. The area selected for the study was Laranjo Basin at Ria de Aveiro lagoon (Portugal) where a known Hg gradient was existed due to chlor-alkali plant discharge. Three sampling sites (L1, L2 and L3) were selected along a transect defined by the distance from the main Hg source. Samples were also collected from the Hg-free site (R). Irrespective of the plant vegetation, Hg in sediments gradually increased with a decreasing distance towards Hg-point source. The sediment colonised by J. maritimus showed more Hg concentration compared with H. portulacoides irrespective of the season. As a whole, J. maritimus accumulated Hg more than H. portulacoides at all the sampling sites, whereas in root, stem and leaf, the concentration was ranked as: L1 > L2 > L3 in both the plant species and was differentially influenced by seasonal changes. Moreover, root of both plants exhibited highest Hg concentration compared with stem and leaf. In addition, the leaf of H. portulacoides exhibited more Hg than leaves of J. maritimus. Bioaccumulation and translocation factors and dry weight were differentially influenced by seasonal changes. Taking together the results, the physico-chemical properties of sediment especially the sediment-Eh seems to be influnced by the type of plant vegetation and seasonal changes which in turn may have influenced the chemistry of sediments; thus, it enfluences the bioavalability of Hg and the Hg-retention capacity of both salt marsh sediments (SMSs) and SMPs (bioaccumulation factor). Moreover, SMSs vegetated by J. maritimus exhibited a stronger capacity for the retention and phytostabilization of Hg belowground (in sediments and/or roots) than those dominated by H. portulacoides. Conversely, those SMSs extensively vegetated by H. portulacoides are expected to translocate more Hg to aboveground parts, acting as a potential source of this metal to the marsh ecosystem. Therefore, J. maritimus and H. portulacoides may be used repectively for phytostabilization (in rhizosediments) and phytoextraction (by accumulation in aboveground plant tissue for subsequent plant removal).



Similar content being viewed by others
References
Alloway, B. J., Thornton, L., Smart, G., Sherlock, J. C., & Quinn, M. J. (1988). Metal availability. The Science of the Total Environment, 75, 41–69.
Almeida, C. M. R., Mucha, A. P., & Vasconcelos, M. T. S. D. (2004). Influence of the sea rush Juncus maritimus on metal concentration and speciation in estuarine sediment colonized by the plant. Environmental Science & Technology, 38, 3112–3118.
Almeida, C. M. R., Mucha, A. P., & Vasconcelos, M. T. S. D. (2006a). Comparison of the role of the sea club-rush Scirpus maritimus and the sea rush Juncus maritimus in terms of concentration, speciation and bioaccumulation of metals in the estuarine sediment. Environmental Pollution, 142, 151–159.
Almeida, C. M. R., Mucha, A. P., & Vasconcelos, M. T. S. D. (2006b). Variability of metal contents in the sea rush Juncus maritimus-estuarine sediment system through one year of plant’s life. Marine Environmental Research, 61, 424–438.
Anjum, N. A., Umar, S., Ahmad, A., Iqbal, M., & Khan, N. A. (2008a). Ontogenic variation in response of Brassica campestris L. to cadmium toxicity. Journal of Plant Interactions, 3, 189–198.
Anjum, N. A., Umar, S., Ahmad, A., Iqbal, M., & Khan, N. A. (2008b). Sulphur protects mustard (Brassica campestris L.) from cadmium toxicity by improving leaf ascorbate and glutathione. Plant Growth Regulation, 54, 271–279.
Anjum, N. A., Umar, S., Ahmad, A., & Iqbal, M. (2008c). Responses of components of antioxidant system in moongbean [Vigna radiata (L.) Wilczek] genotypes to cadmium stress. Communications in Soil Science and Plant Analysis, 39, 2469–2483.
Anjum, N. A., Umar, S., Iqbal, M., & Khan, N. A. (2011). Cadmium causes oxidative stress in moongbean [Vigna radiata (L.) Wilczek] by affecting antioxidant enzyme systems and ascorbate-glutathione cycle metabolism. Russian Journal of Plant Physiology, 58, 92–99.
Anjum, N. A., Umar, S., & Ahmad, A. (2011). Oxidative stress in plants: Causes, consequences and tolerance. New Delhi: IK International Publishing House.
Ansari, M. K. A., Ahmad, A., Umar, S., & Iqbal, M. (2009). Mercury-induced changes in growth variables and antioxidative enzyme activities in Indian mustard. Journal of Plant Interactions, 4, 31–36.
Bouchard, V., Creach, V., Lefeuvre, J. C., Bertru, G., & Mariotti, A. (1998). Fate of plant detritus in a European salt marsh dominated by Atriplex portulacoides (L.), Aellen. Hydrobiologia, 373(374), 75–87.
Brekken, A., & Steinnes, E. (2004). Seasonal concentrations of cadmium and zinc innative pasture plants: consequences for grazing animals. The Science of the Total Environment, 326, 181–195.
Burke, D. J., Weis, J. S., & Weis, P. (2000). Release of metals by the leaves of the salt marsh grasses Spartina alterniflora and Phragmites australis. Estuarine, Coastal and Shelf Science, 51, 153–159.
Caçador, I., & Vale, C. (2001). Retention of heavy metals in salt marshes. In M. N. V. Prasad (Ed.), Metals in the environment: Analysis by biodiversity (pp. 95–116). New York: Marcel Dekker.
Caçador, I., Vale, C., & Catarino, F. (1996a). Accumulation of Zn, Pb, Cu and Ni in sediments between roots of the Tagus estuary salt marshes, Portugal. Estuarine, Coastal and Shelf Science, 42, 393–403.
Caçador, I., Vale, C., & Catarino, F. (1996b). The influence of plants on concentration and fractionation of Zn, Pb, and Cu in salt marsh sediments (Tagus Estuary, Portugal). Journal of Aquatic Ecosystem Health, 5, 193–198.
Caçador, I., Vale, C., & Catarino, F. (2000). Seasonal variation of Zn, Pb, Cu and Cd concentrations in the roots-sediment system of Spartina maritima and Halimione portulacoides from Tagus estuary salt marshes. Marine Environmental Research, 49, 279–290.
Caçador, I., Caetano, M., Duarte, B., & Vale, C. (2009). Stock and losses of trace metals from salt marsh plants. Marine Environmental Research, 67, 75–82.
Capiomont, A., Piazzi, L., & Pergent, G. (2000). Seasonal variations of total mercury in foliar tissues of Posidonia oceanica. Journal of the Marine Biological Association (United Kingdom), 80, 1119–1123.
Castro, R., Pereira, S., Lima, A., Corticeiro, S., Válega, M., Pereira, E., et al. (2009). Accumulation, distribution and cellular partitioning of mercury in several halophytes of a contaminated salt marsh. Chemosphere, 76, 1348–1355.
Chapman, P. M., & Wang, F. (2001). Assessing sediment contamination in estuaries. Environmental Toxicology and Chemistry, 20, 3–22.
Coelho, J. P., Pereira, M. E., Duarte, A. C., & Pardal, M. A. (2009). Contribution of primary producers to mercury trophic transfer in estuarine ecosystems: possible effects of eutrophication. Marine Pollution Bulletin, 58, 358–365.
Costley, C., Mossop, K., Dean, J., Garden, L., Marshall, J., & Carroll, J. (2000). Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Analytica Chimica Acta, 405, 179–183.
Crowder, A. (1991). Acidification, metals and macrophytes. Environmental Pollution, 71, 171–203.
Drifmeyer, J. E., & Redd, B. (1981). Geographic variability in trace element levels in Spartina alterniflora. Estuarine, Coastal and Shelf Science, 13, 709–716.
Duman, F., Obali, O., & Demirezen, D. (2006). Seasonal changes of metal accumulation and distribution in shining pondweed (Potamogeton lucens). Chemosphere, 65, 2145–2151.
EPA (2009). Mercury Human Exposure. US Environmental Protection Agency (2008). Retrieved February 8, 2009 from www.epa.gov/mercury/exposure.htm.
Ernst, W. H. O. (1990). Element allocation and (re)translocation in plants and its impact on representative sampling. In H. Lieth & B. Markert (Eds.), Element concentration cadasters in ecosystems (pp. 17–40). Weinheim: VCH Verlagsgesellschaft.
Fitzgerald, E. J., Caffrey, J. M., Nesaratnam, S. T., & McLoughlin, P. (2003). Copper and Pb concentrations in salt marsh plants on the Suir Estuary, Ireland. Environmental Pollution, 123, 67–74.
Greger, M. (2004). Metal availability, uptake, transport and accumulation in plants. In M. N. V. Prasad (Ed.), Heavy metal stress in plants—From biomolecules to ecosystems (2nd ed., pp. 1–27). Berlin: Springer.
Homyog, K., Pokethitiyooka, P., Kruatrachue, M., Chaiyarat, R., & Ngernsansaruay, C. (2008). Spatial and seasonal variations in lead content of plants colonizing the Bo Ngam lead mine, Thailand. ScienceAsia, 34, 169–178.
Jacob, D. L., & Otte, M. L. (2003). Conflicting processes in the wetland plant rhizosphere: metal retention or mobilization? Water, Air, and Soil Pollution, 3, 91–104.
Jing, Y. D., He, Z. L., & Yang, X. E. (2007). Effects of pH, organic acids, and competitive cation on mercury desorption in soils. Chemosphere, 69, 1662–1669.
Kamnev, A. A., & van der Lelie, D. (2000). Chemical and biological parameters as tools to evaluate and improve heavy metal phytoremediation. Bioscience Reports, 20, 239–258.
Lytle, J. S., & Lytle, T. F. (2001). Use of plants for toxicity assessment of estuarine ecosystems. Environmental Toxicology and Chemistry, 20, 68–83.
Martin, M., & Coughtrey, P. (1982). Biological monitoring of heavy metal pollution. London/New York: Applied Sciences Publications.
Matheus, D. J., Moran, B. M., McCabe, P. F., & Otte, M. L. (2004). Zinc tolerance, uptake, accumulation and distribution in plant and protoplasts of five European populations of the wetland grass Glyceria fluitans. Aquatic Botany, 80, 39–52.
Meagher, R. (2000). Phytoremediation of toxic elemental and organic pollutants. Current Opinion in Plant Biology, 3, 153–162.
Mitsch, W. J., & Gosselink, J. G. (2000). Wetlands. New York: Wiley.
Mucha, A. P., Almeida, C. M. R., Bordalo, A. A., & Vasconcelos, M. T. S. D. (2005). Exudation of organic acids by a marsh plant and implications on trace metal availability in the rhizosphere of estuarine sediments. Estuarine, Coastal and Shelf Science, 65, 191–198.
Otero, X. L., & Macías, F. (2002). Variation with depth and season in metal sulfides in salt marsh soils. Biogeochemistry, 61, 247–268.
Pacyna, E. G., Pacyna, J. M., Sundseth, K., Munthe, J., Kindbom, K., Wilson, S., et al. (2010). Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmospheric Environment, 44, 2487–2499.
Pereira, M. E. (1997). Distribuição, Reactividade e Transporte do Mercúrio na Ria de Aveiro. PhD Thesis, Aveiro University, Portugal.
Pereira, M. E., Duarte, A. C., Millward, G. E., Vale, C., & Abreu, S. N. (1998). Tidal export of particulate mercury from the most contaminated area of Aveiro’s Lagoon, Portugal. The Science of the Total Environment, 213, 157–163.
Pereira, M. E., Lillebø, A. I., Pato, P., Válega, M., Coelho, J. P., Lopes, C. B., et al. (2009). Mercury pollution in Ria de Aveiro (Portugal): a review of the system assessment. Environmental Monitoring and Assessment, 155, 39–49.
Ragsdale, H. L., & Thorhaug, A. (1980). Trace metal cycling in the U.S. coastal zone: a synthesis. American Journal of Botany, 67, 1102–1112.
Reboreda, R., & Caçador, I. (2007). Halophyte vegetation influences in salt marsh retention capacity for heavy metals. Environmental Pollution, 146, 147–154.
Reimer, P., & Duthie, H. C. (1993). Concentrations of zinc and chromium in aquatic macrophytes from the Sudbury and Muskoka regions of Ontario, Canada. Environmental Pollution, 79, 261–265.
Robereda, R., Caçador, I., Pedro, S., & Almeida, P. R. (2008). Mobility of metals in salt marsh sediments colonised by Spartina maritima (Tagus estuary, Portugal). Hydrobiologia, 606, 129–137.
Rodrigues, S. M., Henriques, B., Coimbra, J., Ferreira da Silva, E., Pereira, E., & Duarte, A. C. (2010). Water-soluble fraction of mercury, arsenic and other potentially toxic elements in highly contaminated sediments and soils. Chemosphere, 78, 1301–1312.
Rozema, J., Otte, R., Broekman, R., & Punte, H. (1985). Accumulation of heavy metals in estuarine salt marsh sediment and uptake of heavy metals by salt marsh halophytes. In T. Lekkas (Ed.), Proceedings of international conference on heavy metals in the environment (Vol. 1) (pp. 545–547). Edinburgh: CEP Consultants.
Sousa, A., Lillebo, A., Caçador, I., & Pardal, M. (2008). Contribution of Spartina maritima to the reduction of eutrophication in estuarine systems. Environmental Pollution, 156, 628–635.
Sundby, B., Caetano, M., Vale, C., Gobeil, C., Luther, G., & Nuzzio, D. (2005). Root-induced cycling of lead in salt marsh sediments. Environmental Science & Technology, 39, 2080–2086.
Válega, M., Lillebø, A. I., Pereira, M. E., Duarte, A. C., & Pardal, M. (2008a). Long term effects of mercury in a salt marsh: hysteresis in the distribution of vegetation following recovery from contamination. Chemosphere, 71, 765–772.
Válega, M., Lillebo, A., Pereira, M., Caçador, I., Duarte, A., & Pardal, M. (2008b). Mercury mobility in a salt marsh colonized by Halimione portulacoides. Chemosphere, 72, 1607–1613.
Válega, M., Lima, A. I. G., Figueira, E. M. A. P., Pereira, E., Pardal, M. A., & Duarte, A. C. (2009). Mercury intracellular partitioning and chelation in a salt marsh plant, Halimione portulacoides (L.) Aellen: strategies underlying tolerance in environmental exposure. Chemosphere, 74, 530–536.
Weis, J., & Weis, P. (2004). Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environmental International, 30, 685–700.
Weis, P., Windham, L., Burke, D. J., & Weis, J. S. (2002). Release into the environment of metals by two vascular salt marsh plants. Marine Environental Research, 54, 325–329.
Williams, J. (2002). Phytoremediation in wetland ecosystems: progress, problems, and potential. Critical Reviews in Plant Science, 21, 607–635.
Williams, T. P., Bubb, J. M., & Lester, J. N. (1994). Metal accumulation within salt marsh environments: a review. Marine Pollution Bulletin, 28, 277–290.
Windham, L., Weis, J. S., & Weis, P. (2003). Uptake and distribution of metals in two dominant salt marsh macrophytes, Spartina alterniflora (cordgrass) and Phragmites australis (common reed). Estuarine, Coastal and Shelf Science, 56, 63–72.
Acknowledgements
The financial supports from FCT (Government of Portugal) provided through contract (SFRH/BPD/64690/2009) and by the Aveiro University Research Institute/CESAM are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anjum, N.A., Ahmad, I., Válega, M. et al. Impact of Seasonal Fluctuations on the Sediment-Mercury, its Accumulation and Partitioning in Halimione portulacoides and Juncus maritimus Collected from Ria de Aveiro Coastal Lagoon (Portugal). Water Air Soil Pollut 222, 1–15 (2011). https://doi.org/10.1007/s11270-011-0799-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-011-0799-4