Abstract
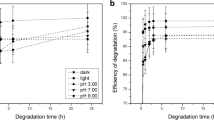
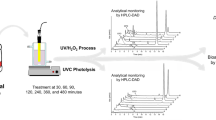
The behavior of several pesticides in aqueous solution, namely bifenthrin, amethrin (pyrethroid insecticides), endosulfan and endosulfan sulfate (organochlorine pesticides), disulfoton, methyl pyrimiphos, and phorate (organophosphorus pesticides), submitted to the conditions typically employed in water treatment stations was investigated. Continuous pesticide depletion was monitored by solid-phase microextraction sampling followed by gas chromatography–mass spectrometry analysis. The influence of major parameters (sodium hypochloride concentration, solution pH, and exposure time to ultraviolet (UV) light) was, thus, adequately established via two complementary approaches: factorial (23, three variables—two levels) and Doehlert designs. Hence, the sodium hypochloride concentration and the solution pH produced distinct effects depending on the pesticide evaluated (for instance, acidic and basic media caused increasing rates of degradation for the organophosphorus/pyrethroid and organochlorine pesticides, respectively). Conversely, higher rates of degradation were achieved for all of the pesticides investigated when increased exposure times to UV radiation were employed. Finally, the exposure time to UV radiation that lead to complete degradation of disulfoton and endosulfan sulfate (organophosphorus and organochlorine pesticides, respectively) in aqueous media under ordinary conditions employed in water treatment stations was established; disulfoton and endosulfan sulfate were completely degraded after 10 and 40 h, respectively.




Similar content being viewed by others
References
Acero, J. L., Benite, F. J., Real, F. J., & Gonzalez, M. (2008). Chlorination of organophosphorus pesticides in natural waters. Journal of Hazardous Materials, 153, 320–328.
Bali, U., Catalkaya, E. C., & Senguel, F. (2003). Photochemical degradation and mineralization of phenol; a comparative study. Journal of Environmental Science and Health, Part A: Toxic/Hazardous Substances amd Environmental Engineering, A38(10), 2259–2275.
Ballesteros Martín, M. M., Sánchez Pérez, J. A., García Sánchez, J. L., Montes de Oca, L., Casas López, J. L., Oller, I. M., et al. (2008). Degradation of alachlor and pyrimethanil by combined photo-Fenton and biological oxidation. Journal of Hazardous Materials, 155(1–2), 342–349.
Baxter, R. M., & Carey, J. H. (1983). Evidence for photochemical generation of superoxide ion in humic waters. Nature, 306, 575–578.
Capobiango, H. L. V., & Cardeal, Z. L. (2005). A solid-phase microextraction method for the chromatographic determination of organophosphorus pesticides in fish, potatoes, guava and coffee. Journal of the Brazilian Chemical Society, 16(5), 907–914.
Cardeal, Z. L., & Paes, C. M. C. (2006). Analysis of organophosphorus pesticides in whole milk by solid phase microextraction gas chromatography method. Journal of Environmental Science and Health. Part B: Pesticides, Food Contaminants, and Agricultural Wastes, 41(4), 369–375.
Chiron, S., Fernadez-Alba, A. R., & Rodriguez, A. (1997). Pesticide chemical oxidation processes: an analytical approach. TrAC, Trends in Analytical Chemistry, 16(9), 518–527.
Doong, R., & Chang, W. (1997). Photoassisted titanium dioxide mediated degradation of organophosphorus pesticides by hydrogen peroxide. Journal of Photochemistry and Photobiology, A: Chemistry, 107(1–3), 239–244.
Duirk, S. E., & Collete, T. W. (2006). Degradation of chlorpyrifos in aqueous chlorine solutions: pathways, kinetics, and modeling. Environmental Science & Technology, 40(2), 546–551.
Fernandez-Alvarez, M., Llompart, M., Lores, M., Garcia-Jares, C., Cela, R., & Dagnac, T. (2008). The photochemical behaviour of five household pyrethroid insecticides and a synergist as studied by photo solid phase microextraction. Analytical and Bioanalytical Chemistry, 388(5–6), 1235–1247.
Gratzel, C. K., Jirousek, M., & Gratzel, M. (1990). Decomposition of organophosphorus compounds on photoactivated TiO2 surfaces. Journal of Molecular Catalysis, 60(3), 375–387.
Ince, N. H., & Gonenc, D. T. (1997). Treatability of a textile azo dye by UV/H2O2. Environmental Technology, 18(2), 179–185.
Kerzhentsev, M., Guilhard, C., Hermann, J. M., & Pichat, P. (1996). Photocatalytic pollutant removal in water at room temperature: case study of the total degradation of the insecticide fenitrothion (phosphorothioic acid o, o-dimethyl-o-(3-methyl-4-nitro-phenyl) ester). Catalysis Today, 27(1–2), 215–220.
Legrini, O., Oliveros, E., & Braun, A. M. (1993). Photochemical processes for water treatment. Chemical Reviews, 93, 671–698.
Liao, C. H., & Gurol, M. D. (1995). Chemical oxidation by photolytic decomposition of hydrogen peroxide. Environmental Science & Technology, 29(12), 3007–3014.
Liu, Y. H., Liu, Y., Chen, Z. S., Lian, J., Huang, X., & Chung, Y. C. (2004). Purification and characterization of a novel organophosphorus pesticide hydrolase from Penicillium lilacinum BP 303. Enzyme and Microbial Technology, 34(3–4), 297–303.
Lunak, S., & Sedlak, P. (1992). Photoinitiated reactions of hydrogen peroxide in the liquid phase. Journal of Photochemistry and Photobiology A: Chemistry, 68(1), 1–33.
Malato, S., Blanco, J., Richter, C., Milow, B., & Maldonado, M. I. (1999). Solar photocatalytic mineralization of commercial pesticides: Methamidophos. Chemosphere, 38(5), 1145–1156.
Masten, S. J., & Davies, H. R. (1994). The use of ozonation to degrade organic contaminants in wastewaters. Environmental Science & Technology, 28(4), A180–A185.
Miltner, R. J., Baker, D. B., Speth, T. F., & Fronk, C. A. (1989). Treatment of seasonal pesticides in surface waters. Journal American Water Works Association, 81(1), 43–52.
Oancea, P., & Oncescu, T. (2008). The Photocatalytic degradation of dichlorvos under solar irradiations. Journal of Photochemistry and Photobiology A: Chemistry, 199(1), 8–13.
Pelizzetti, E. (1995). Concluding remarks on heterogeneous solar photocatalysis. Solar Energy Materials and Solar Cells, 38(1–4), 453–457.
Rosenfeldt, E. J., & Linden, K. G. (2007). The ROH-UV concept you characterize the model UV/H2O2 process in natural waters. Environmental Science & Technology, 41(7), 2548–2553.
Saien, J., & Khezrianjoo, S. (2008). Degradation of the fungicide carbezin in aqueous solutions with UV/TiO2 process optimization, kinetcs and toxicity studies. Journal of Hazardous Materials, 157(2–3), 269–276.
Silva, F. C., Carvalho, C. R., & Cardeal, Z. L. (1999). Determination of organophophorus pesticides in water using SPME-GC-MS. Química Nova, 22(2), 197–200.
Tago, K., Yonezawa, S., Ohkouchi, T., Hashimoto, M., & Hayatsu, M. (2006). Purification and characterization of fenitrothion hydrolase from Burkholderia sp. NF 100. Journal of Bioscience and Bioengineering, 101(1), 80–82.
Yu, B., Zeng, J., Cong, L., Zhang, M., Zhang, L., & Chen, X. (2007). Investigation of the photocatalytic degradation of organochlorine pesticides on a nano-TiO2 coated film. Talanta, 72(5), 1667–1674.
Zhang, Q., & Pehkonen, S. O. (1999). Oxidation of diazinon by aqueous chloride: kinetics, mechanisms and product studies. Journal of Agricultural and Food Chemistry, 47(4), 1760–1766.
Acknowledgments
Funding for this study was provided by the Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Souza, A.G., Costa, L.M., Augusti, R. et al. Degradation of Prototype Pesticides Submitted to Conventional Water Treatment Conditions: The Influence of Major Parameters. Water Air Soil Pollut 211, 427–434 (2010). https://doi.org/10.1007/s11270-009-0311-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0311-6