Abstract
This work evaluated the degradation efficiency of the most used commercial pesticide chlorpyrifos (CP) by UV/H2O2 and UVC photolysis processes. Photodegradation was carried out with 200 μg L−1 of commercial CP for 30, 60, 90, 120, 240, 360, and 480 min. The samples were submitted to the liquid–liquid extraction technique and analyzed by HPLC–DAD. Bioassays were performed using two organisms, Daphnia magna and Aedes aegypti larvae, at all the degradation times. The degradation rate was 98% and 99% after 30 min of reaction for the UV/H2O2 process and UVC photolysis, respectively. Moreover, during treatment, the main CP by-product, chlorpyrifos oxon (CPO), was identified among other unknown by-products. Acute toxicity with D. magna showed a decrease in the immobility at 480 min by the UV/H2O2 process, while in UVC photolysis, 100% immobility was observed for up to 90 min of treatment, and the endpoint oscillated until the end of the process. Bioanalytical monitoring with A. aegypti showed no toxic effects on samples treated by the UV/H2O2 process at 60, 90, 120, and 480 min of degradation. Despite the detection of CPO after UVC photolysis from 60 min onwards, no toxicity was verified, indicating that the by-products generated were not toxic to this organism. Therefore, even though high CP degradation rates were reached, for both processes, it was noted that bioassays and the ecotoxicological effect after degradation effluent are important to complement analytical tools.
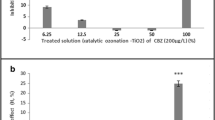
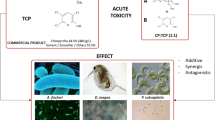
Graphical abstract





Similar content being viewed by others
Data and Materials Availability
Not applicable.
Code Availability
Not applicable.
References
Abe, F. R., Coleone, A. C., Machado, A. A., & Machado-Neto, J. G. (2014). Ecotoxicity and environmental risk assessment of larvicides used in the control of Aedes aegypti to Daphnia magna (crustacea, cladocera). Journal of Toxicology and Environmental Health - Part a: Current Issues, 77(1–3), 37–45. https://doi.org/10.1080/15287394.2014.865581
ABNT. (2016). NBR 12713 Ecotoxicologia aquática — Toxicidade aguda — Método de ensaio com Daphnia spp (Crustacea, Cladocera) Aquatic. ABNT NBR 12713, (4), 1–23. http://revistas.ufpr.br/pesticidas/article/view/7483
Álvarez, M., Du Mortier, C., Sokolic, T., & Cirelli, A. F. (2013). Studies on the persistence of a commercial formulation of chlorpyrifos on an agricultural soil from Provincia de Buenos Aires, República Argentina. Water, Air, and Soil Pollution, 224(5). https://doi.org/10.1007/s11270-013-1571-8
Amiri, H., Nabizadeh, R., Silva Martinez, S., Jamaleddin Shahtaheri, S., Yaghmaeian, K., Badiei, A., et al. (2018). Response surface methodology modeling to improve degradation of Chlorpyrifos in agriculture runoff using TiO2 solar photocatalytic in a raceway pond reactor. Ecotoxicology and Environmental Safety, 147(August 2017), 919–925. https://doi.org/10.1016/j.ecoenv.2017.09.062
Bar, A., & Andrew, J. (2013). Morphology and morphometry of Aedes aegypti larvae. Annual Review & Research in Biology, 3(1), 1–21.
Baumer, J. D., Valério, A., de Souza, S. M. A. G. U., Erzinger, G. S., Furigo, A., & de Souza, A. A. U. (2018). Toxicity of enzymatically decolored textile dyes solution by horseradish peroxidase. Journal of Hazardous Materials, 360(May), 82–88. https://doi.org/10.1016/j.jhazmat.2018.07.102
Boczkaj, G., & Fernandes, A. (2017). Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chemical Engineering Journal, 320, 608–633. https://doi.org/10.1016/j.cej.2017.03.084
Bonifacio, A. F., Ballesteros, M. L., Bonansea, R. I., Filippi, I., Amé, M. V., & Hued, A. C. (2017). Environmental relevant concentrations of a chlorpyrifos commercial formulation affect two neotropical fish species, Cheirodon interruptus and Cnesterodon decemmaculatus. Chemosphere, 188, 486–493. https://doi.org/10.1016/j.chemosphere.2017.08.156
Brandhuber, P. J., & Korshin, G. (2009). Methods for the Detection of Residual Concentrations of Hydrogen Peroxide in Advanced Oxidation Processes. Akexandria: Water Reuse Foundation.
Cáceres, T., He, W., Naidu, R., & Megharaj, M. (2007). Toxicity of chlorpyrifos and TCP alone and in combination to Daphnia carinata: The influence of microbial degradation in natural water. Water Research, 41(19), 4497–4503. https://doi.org/10.1016/j.watres.2007.06.025
Christophers, R. (1960). Aedes Aegypti: The yellow fever mosquito. Cambridge At The University Press, 10(1), 1–721.
de Oliveira, A. G., Ribeiro, J. P., De Oliveira, J. T., De Keukeleire, D., Duarte, M. S., & Do Nascimento, R. F. (2014). Degradation of the pesticide chlorpyrifos in aqueous solutions with UV/H2O2: Optimization and effect of interfering anions. Journal of Advanced Oxidation Technologies, 17(1), 133–138.
Demetrio, P. M., Bonetto, C., & Ronco, A. E. (2014). The effect of cypermethrin, chlorpyrifos, and glyphosate active ingredients and formulations on Daphnia magna (straus). Bulletin of Environmental Contamination and Toxicology, 93(3), 268–273. https://doi.org/10.1007/s00128-014-1336-0
Dhiraj, S. U. D., Kumar, J., Kaur, P., & Bansal, P. (2020). Toxicity, natural and induced degradation of chlorpyrifos. Journal of the Chilean Chemical Society, 65(2), 4807–4816. https://doi.org/10.4067/S0717-97072020000204807
Dutta, A., Chakraborty, I., Sarkar, D., & Chakrabarti, S. (2015). Sunlight-assisted photo-Fenton degradation of pesticide in wastewater: Ecotoxicological impact on Nostoc sp. Algae. Water, Air, and Soil Pollution, 226(12), 1–13. https://doi.org/10.1007/s11270-015-2661-6
Fatma, F., Verma, S., Kamal, A., & Srivastava, A. (2018). Phytotoxicity of pesticides mancozeb and chlorpyrifos: Correlation with the antioxidative defence system in Allium cepa. Physiology and Molecular Biology of Plants, 24(1), 115–123. https://doi.org/10.1007/s12298-017-0490-3
Femia, J., Mariani, M., Zalazar, C., & Tiscornia, I. (2013). Photodegradation of chlorpyrifos in water by UV/H2O2 treatment: Toxicity evaluation. Water Science and Technology, 68(10), 2279–2286. https://doi.org/10.2166/wst.2013.493
Ferrario, C., Parolini, M., De Felice, B., Villa, S., & Finizio, A. (2018). Linking sub-individual and supra-individual effects in Daphnia magna exposed to sub-lethal concentration of chlorpyrifos. Environmental Pollution, 235, 411–418. https://doi.org/10.1016/j.envpol.2017.12.113
Gar Alalm, M., Tawfik, A., & Ookawara, S. (2015). Comparison of solar TiO2 photocatalysis and solar photo-Fenton for treatment of pesticides industry wastewater: Operational conditions, kinetics, and costs. Journal of Water Process Engineering, 8, 55–63. https://doi.org/10.1016/j.jwpe.2015.09.007
Kumar, U., Berliner, J., Adak, T., Rath, P. C., Dey, A., Pokhare, S. S., et al. (2017). Non-target effect of continuous application of chlorpyrifos on soil microbes, nematodes and its persistence under sub-humid tropical rice-rice cropping system. Ecotoxicology and Environmental Safety, 135(May 2016), 225–235. https://doi.org/10.1016/j.ecoenv.2016.10.003
Lescano, M. R., Lopez, A. O., Romero, R. L., & Zalazar, C. S. (2021). Degradation of chlorpyrifos formulation in water by the UV/H2O2 process: Lumped kinetic modelling of total organic carbon removal. Journal of Photochemistry and Photobiology A: Chemistry, 404(September 2020), 112924. https://doi.org/10.1016/j.jphotochem.2020.112924
Lopez, B., Ponce, G., Gonzalez, J. A., Gutierrez, S. M., Villanueva, O. K., Gonzalez, G., et al. (2014). Susceptibility to chlorpyrifos in pyrethroid-resistant populations of Aedes aegypti (Diptera: Culicidae) from Mexico. Journal of Medical Entomology, 51(3), 644–649. https://doi.org/10.1603/ME13185
Marigoudar, S. R., Nagarjuna, A., Karthikeyan, P., Mohan, D., & Sharma, K. V. (2018). Comparative toxicity of chlorpyrifos: Sublethal effects on enzyme activities and histopathology of Mugil cephalus and Chanos chanos. Chemosphere, 211, 89–101. https://doi.org/10.1016/j.chemosphere.2018.07.137
Mesnage, R., & Antoniou, M. N. (2018). Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Frontiers in Public Health, 5(January), 1–8. https://doi.org/10.3389/fpubh.2017.00361
Murillo, R., Sarasa, J., Lanao, M., & Ovelleiro, J. L. (2010). Degradation of chlorpyriphos in water by advanced oxidation processes. Water Science and Technology: Water Supply, 10(1), 1–6. https://doi.org/10.2166/ws.2010.777
Neale, P. A., Altenburger, R., Aït-Aïssa, S., Brion, F., Busch, W., de Aragão Umbuzeiro, G., et al. (2017). Development of a bioanalytical test battery for water quality monitoring: Fingerprinting identified micropollutants and their contribution to effects in surface water. Water Research, 123, 734–750. https://doi.org/10.1016/j.watres.2017.07.016
Neale, P. A., Antony, A., Bartkow, M. E., Farré, M. J., Heitz, A., Kristiana, I., et al. (2012). Bioanalytical assessment of the formation of disinfection byproducts in a drinking water treatment plant. Environmental Science and Technology, 46(18), 10317–10325. https://doi.org/10.1021/es302126t
Palma, P., Palma, V. L., Fernandes, R. M., Soares, A. M. V. M., & Barbosa, I. R. (2008). Acute toxicity of atrazine, endosulfan sulphate and chlorpyrifos to Vibrio fischeri, Thamnocephalus platyurus and Daphnia magna, relative to their concentrations in surface waters from the Alentejo Region of Portugal. Bulletin Environmental Contamination and Toxicology, 81, 485–489. https://doi.org/10.1007/s00128-008-9517-3
Pelit, F. O., Pelit, L., Ertaş, H., & Nil Ertaş, F. (2012). Development of a gas chromatographic method for the determination of chlorpyrifos and its metabolite chlorpyrifos-oxon in wine matrix. Journal of Chromatography b: Analytical Technologies in the Biomedical and Life Sciences, 904, 35–41. https://doi.org/10.1016/j.jchromb.2012.07.006
Pereira, V. J., Linden, K. G., & Weinberg, H. S. (2007). Evaluation of UV irradiation for photolytic and oxidative degradation of pharmaceutical compounds in water. Water Research, 41(19), 4413–4423. https://doi.org/10.1016/j.watres.2007.05.056
Rebechi, D., Richardi, V. S., Vicentini, M., Guiloski, I. C., Silva de Assis, H. C., & Navarro-Silva, M. A. (2014). Low malathion concentrations influence metabolism in Chironomus sancticaroli (Diptera, Chironomidae) in acute and chronic toxicity tests. Revista Brasileira De Entomologia, 58(3), 296–301. https://doi.org/10.1590/S0085-56262014000300012
Rigueira, L. M. B., De L. Ribeiro, K., De Queiroz, M. E. L. R., Neves, A. A., Zambolim, L., & Oliveira, R. M. (2013). Determination of chlorpyrifos and thiamethoxam in potato tuber (Solanum tuberosum L.) and soil of Brazil using solid-liquid extraction with low temperature partitioning (SLE/LTP). Journal of the Brazilian Chemical Society, 24(12), 2042–2049. https://doi.org/10.5935/0103-5053.20130256
Rizzo, L. (2011). Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Research, 45(15), 4311–4340. https://doi.org/10.1016/j.watres.2011.05.035
Rizzo, L., Malato, S., Antakyali, D., Beretsou, V. G., Đolić, M. B., Gernjak, W., et al. (2019). Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Science of the Total Environment, 655(August 2018), 986–1008. https://doi.org/10.1016/j.scitotenv.2018.11.265
Slotkin, T. A., Seidler, F. J., Wu, C., MacKillop, E. A., & Linden, K. G. (2009). Ultraviolet photolysis of chlorpyrifos: Developmental neurotoxicity modeled in PC12 cells. Environmental Health Perspectives, 117(3), 338–343. https://doi.org/10.1289/ehp.11592
Solomon, K. R., Williams, W. M., Mackay, D., Purdy, J., Giddings, J. M., & Giesy, J. P. (2014). Properties and Uses of Chlorpyrifos in the United States In: Giesy J., Solomon K. (eds) Ecological risk assessment for chlorpyrifos in terrestrial and aquatic systems in the United States. Reviews of Environmental Contamination and Toxicology (Continuation (Vol. 231, pp. 119–162). Springer, Cham. https://doi.org/10.1007/978-3-319-03865-0_2
Sparling, D. W., & Fellers, G. (2007). Comparative toxicity of chlorpyrifos, diazinon, malathion and their oxon derivatives to larval Rana boylii. Environmental Pollution, 147(3), 535–539. https://doi.org/10.1016/j.envpol.2006.10.036
Thind, P. S., Kumari, D., & John, S. (2018). TiO2/H2O2 mediated UV photocatalysis of Chlorpyrifos: Optimization of process parameters using response surface methodology. Journal of Environmental Chemical Engineering, 6(3), 3602–3609. https://doi.org/10.1016/j.jece.2017.05.031
Utzig, L. M., Lima, R. M., Gomes, M. F., Ramsdorf, W. A., Martins, L. R. R., Liz, M. V., & Freitas, A. M. (2019). Ecotoxicity response of chlorpyrifos in Aedes aegypti larvae and Lactuca sativa seeds after UV/H 2 O 2 and UVC oxidation. Ecotoxicology and Environmental Safety, 169(October 2018), 449–456. https://doi.org/10.1016/j.ecoenv.2018.11.003
Vagi, M. C., & Petsas, A. S. (2020). Recent advances on the removal of priority organochlorine and organophosphorus biorecalcitrant pesticides defined by Directive 2013/39/EU from environmental matrices by using advanced oxidation processes: An overview (2007–2018). Journal of Environmental Chemical Engineering, 8(1), 102940. https://doi.org/10.1016/j.jece.2019.102940
Wang, J., & Wang, S. (2021). Toxicity changes of wastewater during various advanced oxidation processes treatment : An overview. Journal of Cleaner Production, 315(June), 128202. https://doi.org/10.1016/j.jclepro.2021.128202
WHO. (1981). Instruction for determining the susceptibility or resistance of mosquito larvae to insecticide (p. 1981). World Health Organization.
Zahrani, M. R. Al, Gharsan, F. N., Al-Ghamdi, K. M., Mahyoub, J. A., & Alghamdi, T. S. (2020). Toxicity of two groups of pesticides against the mosquito Aedes aegypti. GSC Biological and Pharmaceutical Sciences, 13(1), 148–155. https://doi.org/10.30574/gscbps.2020.13.1.0334
Zalizniak, L., & Nugegoda, D. (2006). Effect of sublethal concentrations of chlorpyrifos on three successive generations of Daphnia carinata. Ecotoxicology and Environmental Safety, 64(2), 207–214. https://doi.org/10.1016/j.ecoenv.2005.03.015
Acknowledgements
We are grateful to the multi-user laboratory of chemical analyses (LAMAQ) and the multi-user laboratory of equipment and environmental analyses (LAMEAA) for the chromatographic analyses and the Laboratory of Physiology and Control of Arthropod Vectors (LAFICAVE), Oswaldo Cruz Institute, RJ, in supplying the Aedes aegypti eggs.
Funding
The authors would like to thank the Federal University of Technology, Paraná (UTFPR), for financial support and the Fundação Araucária, Brazil, for the graduate scholarship. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES) (postdoctoral scholarship of PhD Liziê Prola) and National Council for Scientific and Technological (CNPq, protocol number 458362/2014–0).
Author information
Authors and Affiliations
Contributions
Suelen Angeli, Gabriella P. Masceno, Fernando R. Silva, and Liziê D. T. Prola were responsible to perform the degradation experiments and analyses of the results. Rúbia M. Lima and Suelen Angeli were responsible for the chromatographic analyses and validation methods. Eliane Adams and Larisa M. Utzig performed the ecotoxicological bioassays. Adriane M. de Freitas and Marcus V. de Liz supervised the project and revised the manuscript and responsible to funding acquisition. All authors contributed to the analysis and writing of the manuscript.
Corresponding author
Ethics declarations
Consent for Publication
The publication has been approved by all co-authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Angeli, S., Adams, E., Masceno, G.P. et al. Toxicity Assays of Commercial Chlorpyrifos Under UV/H2O2 and UVC Photolysis Treatments. Water Air Soil Pollut 232, 353 (2021). https://doi.org/10.1007/s11270-021-05314-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05314-w