Abstract
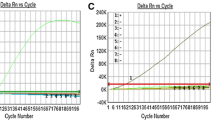
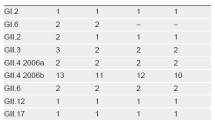
Norovirus (NoV) is the major etiological agent causing foodborne and waterborne outbreaks worldwide. We developed a novel duplex real-time quantitative RT-PCR assay designed for the simultaneous detection of and discrimination between NoV genogroups GI and GII, by targeting the short junction region between ORF1 and ORF2, with sensitivity and efficiency comparable to those of each simplex RT-PCR assay. This new duplex assay was evaluated against clinical stool (n = 82) and environmental (groundwater or surface water, n = 60) specimens from South Korea, and the results were compared with those of conventional RT-PCR (cRT-PCR) assays. The duplex assay detected more positive samples than did the cRT-PCR for both clinical (74 vs. 71) and, more strikingly, environmental (24 vs. 10) specimens. No cross-reactivity against specimens containing other enteric viruses such as rotavirus, adenovirus, and poliovirus were observed. These results suggest that this newly developed duplex real-time RT-PCR assay can be used for the sensitive and simultaneous genogroup-specific detection of NoV in both clinical and environmental specimens.


Similar content being viewed by others
References
D.J. Allen, J.J. Gray, C.I. Gallimore, J. Xerry, M. Iturriza-Gomara, PLoS One 3, e1485 (2008)
T. Ando, J.S. Noel, R.L. Fankhauser, J. Infect. Dis. 181(Suppl. 2), S336–S348 (2000)
R.L. Fankhauser, S.S. Monroe, J.S. Noel, C.D. Humphrey, J.S. Bresee, U.D. Parashar, T. Ando, R.I. Glass, J. Infect. Dis. 186, 1–7 (2002)
A.M. Hutson, R.L. Atmar, M.K. Estes, Trends Microbiol. 12, 279–287 (2004)
M.M. Patel, A.J. Hall, J. Vinje, U.D. Parashar, J. Clin. Virol. 44, 1–8 (2009)
A. Leiste, A. Skaletz-Rorowski, I. Venten, P. Altmeyer, N.H. Brockmeyer, J. Dtsch. Dermatol. Ges. 6, 563–565 (2008)
T. Kageyama, S. Kojima, M. Shinohara, K. Uchida, S. Fukushi, F.B. Hoshino, N. Takeda, K. Katayama, J. Clin. Microbiol. 41, 1548–1557 (2003)
T. Kageyama, M. Shinogara, K. Uchida, S. Fukushi, F.B. Hoshino, S. Kojima, R. Takai, T. Oka, N. Takeda, K. Katayama, J. Clin. Microbiol. 42, 2988–2995 (2004)
F. Loisy, R.L. Atmar, P. Guillon, P. Le Cann, M. Pommepuy, F.S. Le Guyader, J. Virol. Methods 123(1), 1–7 (2005)
A.K. Da Silva, J.C. Le Saux, S. Parnaudeau, M. Pommepuy, M. Elimelech, F.S. Le Guyader, Appl. Environ. Microbiol. 73(24), 7891–7897 (2007)
S. Butot, F.S. Le Guyader, J. Krol, T. Putallaz, R. Amoroso, G. Sánchez, J. Virol. Methods 167(1), 90–94 (2010)
R.L. Atmar, M.K. Estes, Clin. Microbiol. Rev. 14, 15–37 (2001)
R.I. Glass, J. Noel, T. Ando, R. Fankhauser, G. Belliot, A. Mounts, U.D. Parashar, J.S. Bresee, S.S. Monroe, J. Infect. Dis. 181(Suppl. 2), S254–S261 (2000)
J.J. Siebenga, M.F. Beersma, H. Vennema, P. Van Biezen, N.J. Hartwig, M. Koopmans, J. Infect. Dis. 198, 994–1001 (2008)
C.I. Gallimore, M. Iturriza-Gomara, J. Xerry, J. Adigwe, J.J. Gray, Arch. Virol. 152, 1295–1303 (2007)
B. Lopman, H. Vennema, E. Kohli, P. Pothier, A. Sanchez, A. Negredo, J. Buesa, E. Schreier, M. Reacher, D. Brown, J. Gray, M. Iturriza, C. Gallimore, B. Bottiger, K.O. Hedlund, M. Torven, C.H. Von Bonsdorff, L. Maunula, M. Poljsak-Prijatelj, J. Zimsek, G. Reuter, G. Szucs, B. Melegh, L. Svennson, Y. Van Duijnhoven, M. Koopmans, Lancet 363, 682–688 (2004)
J. Xerry, C.I. Gallimore, M. Iturriza-Gomara, D.J. Allen, J.J. Gray, J. Clin. Microbiol. 46, 947–953 (2008)
R.A. Bull, G.S. Hansman, L.E. Clancy, M.M. Tanaka, W.D. Rawlinson, P.A. White, Emerg. Infect. Dis. 11, 1079–1085 (2005)
K. Bok, E.J. Abente, M. Realpe-Quintero, T. Mitra, S.V. Sosnovtsev, A.Z. Kapikian, K.Y. Green, J. Virol. 83, 11890–11901 (2009)
M. Hoehne, E. Schreier, BMC Infect. Dis. 6, 69 (2006)
H. Lee, M. Kim, S.Y. Paik, C.H. Lee, W.H. Jheong, J. Kim, G. Ko, J. Water Health 9(1), 27–36 (2011)
S. Kojima, T. Kageyama, S. Fukushi, F.B. Hoshino, M. Shinohara, K. Uchida, K. Natori, N. Takeda, K. Katayama, J. Virol, Methods 100, 107–114 (2002)
I.L. Boxman, J.J. Tilburg, T.A. Te Loeke, H. Vennema, K. Jonker, E. De Boer, M. Koopmans, Int. J. Food Microbiol. 108, 391–396 (2006)
Y. Park, Y.H. Cho, Y. Jee, G. Ko, Appl. Environ. Microbiol. 74, 4226–4230 (2008)
R. Rasmussen, Quantification on the LightCycler, in Rapid Cycle Real-time PCR, Methods and Applications, ed. by S. Meuer, C. Wittwer, K.-I. Nakagawara (Springer, Heidelberg, 2001), pp. 21–34
S.H. Kim, D.S. Cheon, J.H. Kim, D.H. Lee, W.H. Jheong, Y.J. Heo, H.M. Chung, Y. Jee, J.S. Lee, J. Clin. Microbiol. 43, 4836–4839 (2005)
T.G. Phan, K. Kaneshi, Y. Ueda, S. Nakaya, S. Nishimura, A. Yamamoto, K. Sugita, S. Takanashi, S. Okitsu, H. Ushijima, J. Med. Virol. 79, 1388–1400 (2007)
N. Jothikumar, J.A. Lowther, K. Henshilwood, D.N. Lees, V.R. Hill, J. Vinje, Appl. Environ. Microbiol. 71, 1870–1875 (2005)
I.V. Kutyavin, I.A. Afonina, A. Mills, V.V. Gorn, E.A. Lukhtanov, E.S. Belousov, M.J. Singer, D.K. Walburger, S.G. Lokhov, A.A. Gall, R. Dempcy, M.W. Reed, R.B. Meyer, J. Hedgpeth, Nucleic Acids Res. 28, 655–661 (2000)
R. Morales-Rayas, P.F.G. Wolffs, M.W. Griffiths, Int. J. Food Microbiol. 139, 48–55 (2010)
J. Nordgren, F. Bucardo, O. Dienus, L. Svensson, P.E. Lindgren, J. Clin. Microbiol. 46, 164–170 (2008)
G.S. Hansman, K. Natori, H. Shirato-horikoshi, S. Ogawa, T. Oka, K. Ktayama, T. Tanaka, T. Miyoshi, K. Sakae, S. Kobayashi, M. Shinohara, K. Uchida, N. Sakurai, K. Shinozaki, M. Okada, Y. Seto, K. Kamata, N. Nagata, K. Tanaka, T. Miyamura, N. Takeda, J. Gen. Virol. 87, 909–919 (2006)
J.J. Siebenga, H. Vennema, D.P. Zheng, J. Vinje, B.E. Lee, X.L. Pang, E.C. Ho, W. Lim, A. Choudekar, S. Broor, T. Halperin, N.B. Rasool, J. Hewitt, G.E. Greening, M. Jin, Z.J. Duan, Y. Lucero, M. O’Ryan, M. Hoehne, E. Schreier, R.M. Ratcliff, P.A. White, N. Iritani, G. Reuter, M. Koopmans, J. Infect. Dis. 200, 802–812 (2009)
D. Häfliger, M. Gilgen, J. Luthy, P. Hubner, Int. J. Food Microbiol. 37, 27–36 (1997)
C. Lee, S.J. Kim, Water Res. 42(17), 4477–4484 (2008)
D.J. Allen, J.J. Gray, C.I. Gallimore, J. Xerry, M. Iturriza-Gómara, PLoS One 3(1), e1485 (2008)
S. Ishida, S. Yoshizumi, T. Ikeda, M. Miyoshi, M. Okano, T. Okui, J. Med. Virol. 80, 913–920 (2008)
Acknowledgment
This research was supported by the Agriculture Research Center program of the Ministry of Food, Agriculture, Forestry, and Fisheries, and National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2011-0000177).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, Y., Cho, YH. & Ko, G. A duplex real-time RT-PCR assay for the simultaneous genogroup-specific detection of noroviruses in both clinical and environmental specimens. Virus Genes 43, 192–200 (2011). https://doi.org/10.1007/s11262-011-0626-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-011-0626-4