Abstract
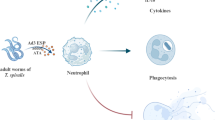
The protozoan parasite Tritrichomonas foetus (T. foetus) is the causative organism of bovine trichomonosis (also referred to as trichomoniasis), a sexually-transmitted infection that reduces fertility in cattle. Efforts to control trichomonosis on cattle farms are hindered by the discouragement of antibiotic use in agriculture, and the incomplete, short-lived protection conferred by the current vaccines. A more complete mechanistic understanding of what effective immunity to T. foetus entails could enable the development of more robust infection control strategies. While neutrophils, the primary responders to infection, are present in infected tissues and have been shown to kill the parasite in vitro, the mechanism they use for parasite killing has not been established. Here, we show that primary bovine neutrophils isolated from peripheral blood rapidly kill T. foetus in vitro in a dose-dependent manner, and that optimal parasite killing is reduced by inhibitors of trogocytosis. We also use imaging to show that bovine neutrophils surround T. foetus and trogocytose its membrane. These findings are consistent with killing via trogocytosis, a recently described novel neutrophil antimicrobial mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bovine trichomonosis is a sexually-transmitted infection with global distribution. The infection reduces fertility and increases spontaneous abortion rates in infected cows, thereby contributing to a reduction in the births of viable calves in affected herds (BonDurant 1997, Rhyan et al. 1995). The standard line of treatment for human trichomonosis is the imidazole family of antibiotics, however the use of these is prohibited in agriculture in the United States (Love et al. 2017). Therefore, separation of cows from bulls with fencing, testing and slaughter of infected bulls, and artificial insemination are recommended to reduce the incidence of trichomonosis on farms. In the United States, a patchwork of state regulations has resulted in under-reporting of the infection, but infected bulls are more common in western states, particularly California and Texas, where free-ranging is used (Martin et al. 2021). A whole-cell inactivated vaccine called TrichGuard™ exists for bovine trichomonosis, requiring two annual boosters before each breeding season. Vaccines have been reported to induce anti-T.foetus antibodies, and increase conception rates, (Corbeil et al. 2003, 2001; Kvasnicka et al. 1992, Ortega-Mora et al. 2022). However, they do not completely prevent infection in vaccinated individuals, confer long-term immunological memory, or completely prevent abortion (Edmondson et al. 2017). Overall, more research into the correlates of protection for bovine trichinosis is needed (Baltzell et al. 2013). A more complete understanding of cellular and molecular immunity against bovine trichomonosis could enable the rational design of more robust vaccines and treatments for the infection.
The causative agent of bovine trichomonosis is the parasite Tritrichomonas foetus, which is named for its effects on fetal weight and mortality (Rae and Crews 2006). T. foetus is a unicellular, flagellated protozoan parasite. It infects the prepuce in males, producing no symptoms or pathology, and does not get cleared. Therefore, bulls are long-term asymptomatic carriers (Rae and Crews 2006). In females, it infects the vagina, travelling up the cervix, and colonizing the uterus within two weeks (Parsonson et al. 1976, Singh et al. 2004). Unlike males, infected females usually clear the infection naturally. However, there is considerable variability in the timing and efficiency of clearance from cow-to-cow. Furthermore, even in cows that can clear the parasite, no long-term immunity is formed: cows may become infected again during the next breeding season (Collántes-Fernández et al. 2018; Rae and Crews 2006).
Immune responses against T. foetus have been extensively characterized at the organismal level, and cattle are known to make antibodies against T. foetus (BonDurant et al. 1993; Corbeil et al. 1998, 2001; Ikeda et al. 1995; Voyich et al. 2001). Moreover, neutrophils, also known as Polymorphonuclear Cells (PMNs), are abundantly recruited to the site of infection (Corbeil et al. 2003), and have been shown to kill T. foetus in vitro (Aydintug et al. 1993). However, PMNs have four known modes by which they can kill targets (Deniset and Kubes 2016; Kolaczkowska and Kubes 2013; Matlung et al. 2018; Mercer et al. 2018), and the specific mechanism that PMNs use to kill T. foetus is not known.
We have recently shown that human PMNs rapidly kill the human-infective trichomonad parasite, Trichomonas vaginalis, using a novel PMN antimicrobial mechanism called trogocytosis (Mercer et al. 2018). Trogocytosis is a cellular phenomenon in which plasma membrane is transferred from a donor cell to a recipient cell in a cell–cell contact-dependent manner, and has been increasingly observed to occur in more and more cell types, ranging from immune cells, to protozoans, to embryonic cells (Bettadapur et al. 2020; Uribe-Querol and Rosales 2021). While some forms of trogocytosis involve swapping of membrane proteins between target and recipient cells (i.e. cellular gnawing) (Ochs et al. 2023; Samer et al. 2023; Schriek and Villadangos 2023), other forms of trogocytosis involve engulfment of trogocytosed material by the recipient cell (i.e. nibbling) (Bettadapur et al. 2020; Gilmartin et al. 2017; Ralston et al. 2014; Steele et al. 2016). It is unclear whether just membrane, or additional cytosolic components and organelles, can be trogocytosed by neutrophils from T. vaginalis (Mercer and Johnson 2018). Furthermore, while we have previously shown that at least some membrane fragments of T. vaginalis do become internalized by neutrophils following trogocytosis (Mercer et al. 2018), the relative amount of “gnawed” versus “nibbled” parasite material, and which process causes more damage to the parasite, has not been formally tested. In some cases, trogocytosis results in death of the trogocytosed cell (Matlung et al. 2018; Mercer et al. 2018; Olivera-Valle et al. 2020; Ralston et al. 2014). Human PMNs have recently been observed to use trogocytosis to kill not only T. vaginalis, but also cancer cells (Matlung et al. 2018), and sperm cells (Olivera-Valle et al. 2020). A leading hypothesis is that PMNs use trogocytosis in lieu of phagocytosis (whole cell engulfment), when viable targets are too large to engulf whole (Bhakta et al. 2020).
We therefore hypothesize that, similar to human PMN killing of the human-infective trichomonad parasite T. vaginalis, bovine PMNs use trogocytosis to kill the bovine-infective trichomonad parasite T. foetus. As PMNs are the first responders to infections and are abundant at the infection site during bovine trichomonosis, the knowledge gained in this study will serve as a first step towards a more complete characterization of immune mechanisms at play during bovine trichomonosis, at a cellular and molecular level.
Materials & methods
Animals and blood products
Cattle housed at the California State Polytechnic University, Pomona (Cal Poly Pomona) cattle unit were blood donors. The protocol for bovine whole blood collection was approved by the Cal Poly Pomona Institutional Animal Care and Use Committee (protocol 20.031). Whole bovine blood was obtained via jugular venipuncture and collected into 10 mL K2EDTA vacutainer tubes (Becton Dickinson) and transported at room temperature. Bovine PMN were then promptly isolated as previously described (Czuprynski and Hamilton 1985; Roth and Kaeberle 1981). Briefly, blood was transferred into conical tubes, and centrifuged at 1000G for 20 min with no brake. The plasma, buffy coat, and ¼ of the top of the red blood cell pellet was aspirated and discarded. ACK lysis buffer (ThermoFisher) was added to the remaining ¾ of the red blood cell pellet and incubated for 15 min, to lyse erythrocytes, followed by an additional 5 min in fresh ACK lysis buffer if needed. Tubes were then centrifuged at 200G for 10 min and washed 3 times with PBS (Gibco). The remaining leukocyte pellet was resuspended in complete bovine RPMI + HEPES media containing 1% Glutamax, 1% pen/strep and 10% heat-inactivated bovine serum (all from Gibco), and passed through a 70uM cell strainer to remove any clumped cells. Functional experiments were commenced promptly. Alternatively, for isolation of PBMCs, blood was diluted 1:2 in HBSS (Gibco) and fractionated using Ficoll-paque PLUS (GE Healthcare) according to the manufacturer’s instructions. The buffy coat was removed and washed with PBS.
For collection of antiserum, one heifer was vaccinated and boosted with TrichGuard™ (Boehringer Ingelheim Vetmedica, Inc.) subcutaneously according to the manufacturer’s instructions. Antiserum was collected as previously described (Liu et al. 2018). Briefly, blood was collected into serum separator tubes (Becton Dickenson), allowed to clot at room temperature for 30 min, and then tubes were spun at 4000G for 10 min in a precooled 4 °C centrifuge. The top layer containing serum was collected and passed through a 0.22 μm filter. Aliquots were stored at -20 °C and thawed gradually on ice prior to use in each functional experiment.
Annexin V staining
Cells were stained with Annexin V APC (Biolegend) according to the manufacturer’s instructions and analyzed on a MACS quant flow cytometer.
Parasite strains and cultures
Tritrichomonas foetus strain KV-1 (ATCC) was grown in Diamond’s medium (Clark and Diamond 2002). Briefly, TYM medium was supplemented with 10% of heat-inactivated horse serum, and iron solution, and either left untouched (pH6.8), or adjusted to a pH of 6.2 or 7.2. Cultures were maintained at 37OC between 0.5 × 104 to 2 × 106 cells/mL, and passaged into fresh media daily in sealed conical tubes. Before passaging or using for experiments, cultures were placed on ice for 15 min to release parasites that had adhered to the plastic conical tube.
Antibody staining
Isolated leukocytes were stained with anti- bovine GR-1 antibody to confirm PMN identity, as previously described (Etchevers et al. 2023; Lietaer et al. 2021; Lopes et al. 2021; Oliveira et al. 2020). Briefly, cells were stained with mouse anti-bovine granulocyte (GR-1) monoclonal IgM primary antibody (clone-CH138A, Kingfisher Biotech), or isotype control (clone-COLIS52A2, Kingfisher Biotech) at a concentration of 1.0 ug/mL, followed by a FITC goat anti-mouse IgM polyclonal secondary antibody (ThermoFisher) at a concentration of 1.5 ug/mL. Stained cells were then run on a MACSQuant® Analyzer 10 Flow cytometer (Miltenyi Biotec Inc.) and data was analyzed using Flowjo (Becton Dickenson).
Cytotoxicity assays
Modified flow cytometry-based cytotoxicity assays previously established to evaluate PMN killing of trichomonads (Mercer et al. 2018) were used. For cytotoxicity assays in Figs. 2 and 3, a total of 3 × 105 PMNs and 3 × 104 Cell Tracker Green (CTG) labelled T. foetus were co-cultured in RPMI with 10% T. foetus antiserum in a 96 well plate at 37° and 5% CO2 for the indicated times. Co-cultures were then washed in Annexin V binding buffer (Biolegend) and resuspended in Zombie Violet (Biolegend), at a 1:1000 dilution from the stock, in Annexin V binding buffer and incubated for 30 min at room temperature. Samples were then washed and resuspended in Annexin V binding buffer before running on a MACS Quant 10 flow cytometer (Miltenyi Biotech). Live T. foetus (CTG + Zombie Violet- cells) were gated on, as shown in Figure S1A, and percent parasite death was determined using the following calculation: ([ number of live T. foetus in negative (parasites only) control – number of T. foetus in coculture condition] / number of T. foetus in negative control) × 100. Cytotoxicity assays that received trogocytosis inhibitors (Fig. 3) followed the same protocol, but PMNs were pre-incubated with 1.25 ug/mL Cytochalasin D (Tocris) or vehicle (DMSO) or 3.2ug/mL wortmannin (Tocris) or vehicle control (DMSO) for 20 min prior to adding T. foetus.
For the alternative cytotoxicity assay (Fig. S3), bovine PMNs were co-cultured with T. foetus in complete RPMI containing 10% T. foetus antiserum in a 96-well-v-bottom plate for 1 h at 37° in 5% CO2. All conditions contained 1.5 × 104 parasites, and the number of PMNs was varied according to the indicated ratios. After incubation, co-cultures were set on ice for 15 min and then centrifugated at 3200 rpm for 10 min at 4 °C, and decanted. Wells were then resuspended in 300uL of pre-warmed complete Diamonds media and left to incubate overnight in sealed plastic bags at 37°. The following day, cocultures were placed on ice for 15 min before reading on a MACS Quant 10 flow cytometer to determine parasite count in each well. Percent killing was calculated as ([ number of T. foetus in negative (parasites only) control – number of T. foetus in coculture condition] / number of T. foetus in negative control) × 100.
Imaging
To visualize PMNs following isolation, 10ul of isolated cells were used to create a smear on a glass slide and allowed to dry, followed by Leishman staining, in which 8 drops of the stain were added to the slide, left for 2 min, followed by 16 drops of water for 15 min, followed by gentle washing in water. Slides were imaged on a Leica light microscope with the 40 × objective. To visualize trogocytosis, parasites were surface-labelled with EZ-Link Sulfo-NHS-SS-Biotin (ThermoFisher) for 30 min on ice followed by 5ug/mL Streptavidin-Alexa 488 (Biolegend) for 5 min. For 3D imaging, PMNs were pre-labelled with Cell Tracker Red (Molecular Probes) according to the manufacturer’s instructions. A total of 3.6 × 106 PMNs, or Jurkat cells (Fig. S5), were plated in RPMI with 10% T. foetus antiserum in a 12-well plate and allowed to settle down on glass coverslips pre-coated with 0.01% poly-L-lysine (MilliporeSigma). Then, 3.6 × 105 labelled T. foetus were added in a dropwise manner slowly around the well and incubated for the indicated timepoints. Wells were then gently aspirated, washed twice with PBS and then fixed with 900uL of 4% PFA added gently to the side of the plate to minimize disruption of cellular aggregates. After 10 min, PFA was gently removed, and coverslips were dipped twice in PBS, and once in ddH2O before being placed with parasite-PMN aggregates facing down on slides with 20uL of Prolong Gold© anti-fade mounting media (Thermo Fisher). Slides were left flat in the dark to dry overnight before a thin layer of clear coat nail polish was applied around the edges of the coverslip to prevent contamination. Slides were stored at -20 °C and images were obtained using a Nikon Confocal Microscope using the 100 × objective with oil. Images were then analyzed by identifying each intact parasite in the image, and visually counting the number of PMNs touching that parasite, and the number of green fragments localized to PMNs, from each parasite. For 3D imaging, z-stacks were taken every 0.13 um and 3D reconstructions were made using volume viewer in FIJI software.
Results
Isolation of PMNs from bovine peripheral blood
To test whether and how bovine neutrophils, otherwise known as polymorphonuclear cells (PMN), kill T. foetus, we first isolated bovine PMNs from bovine blood. Using previously established centrifugation protocols (Roth and Kaeberle 1981), we isolated Peripheral Blood Mononuclear Cells (PBMC), and PMNs. After staining our fractionated PBMC or PMN cell fractions using an anti- bovine granulocyte monoclonal antibody (GR-1) (Etchevers et al. 2023; Lietaer et al. 2021; Lopes et al. 2021; Oliveira et al. 2020), we found that our PMN fraction was viable, and had a PMN identity, as anti-GR-1 reactivity was very high in all of the PMN cells, but few of the PBMC cells (Fig. 1A). Furthermore, the PMNs displayed a characteristic multi-lobed nuclear morphology (Fig. 1B). As expected, the PMNs were short-lived, as they became apoptotic by the following day (Fig. S1).
Isolation of PMNs from bovine blood. A PMNs or PBMCs were stained with anti-GR-1 antibody and analyzed using Flow Cytometry. PMNs show higher granularity, as assessed by side-scatter (ssc), and higher expression of Granulocyte Receptor-1 (right panels) than PBMCs. Data shown are representative of triplicate samples run in three independent experiments from three different donors. B PMN display characteristic multi-lobed nuclei, as visualized using Leishman staining. Image shown is representative of 16 images taken from PMNs isolated from 4 different donors over 2 independent experiments
Bovine PMNs rapidly kill T. foetus in a dose- dependent manner
We next sought to test whether our isolated bovine PMNs were able to kill T. foetus in vitro. To test this, we adapted an in vitro cytotoxicity assay previously used to test human PMN killing of the human-infective trichomonad parasite T. vaginalis (Mercer et al. 2018). Before the assay, T. foetus were pre-labelled with Cell Tracker Green (CTG) to discern them from PMNs following the co-culture. After the co-culture, all cells were spun down, stained with cell-death marker Zombie Violet, and then analyzed using flow cytometry, to identify and count live parasites (CTG + , Zombie Violet- cells) (Fig. 2A). Since a ratio of 10:1 PMN: trichomonad efficiently killed T. vaginalis (Mercer et al. 2018), we first started by co-culturing bovine PMNs with T. foetus at a 10:1 ratio. To test how rapidly the killing occurred, we analyzed various timepoints, and found that killing occurred rapidly, with low levels of parasite death occurring as early as five minutes, with about half of the parasites dead by ten minutes (Fig. 2B). All of the parasites were killed by 1 h of the co-culture (Fig. 2B). Next, to determine how many PMNs are required for optimal killing of T. foetus, we tested bovine PMN killing of T. foetus at various PMN: T. foetus ratios. Since we found killing to be complete within one hour (Fig. 2B), we used a one-hour timepoint, and set-up co-cultures of T. foetus with descending ratios of PMNs (Fig. 2C). We found that only moderate killing of T. foetus was observed at PMN: parasite ratios of 1:1 and 1:2, but that as PMN numbers increased, the parasites were killed more efficiently, with about half of the parasites killed at a 4:1 ratio, and very high percentages of parasites killed at 8:1 and 16:1 ratios (Fig. 2C). Therefore, bovine PMNs kill T. foetus in vitro rapidly, starting at five minutes, with most killing occurring within fifteen minutes, and completing within one hour. Maximum T. foetus killing required multiple neutrophils, with efficient killing requiring at least 4 PMN: 1 parasite, suggesting that bovine PMNs work in aggregates to rapidly attack single trichomonads.
Bovine PMNs rapidly kill Tritrichomonas foetus in a dose-dependent manner. (A) Representative gating strategy for assessing live parasite using flow cytometry. Cells (upper panel) were gated on, and all gated events were assessed for Cell Tracker Green (CTG) and Zombie violet, to identify live parasite (CTG + , Zombie violet- events). (B, C) Cytotoxicity assays. Live parasite counts were used to determine percent parasite death, by comparing the amount of live parasites remaining in the co-culture condition to a parasite-alone condition at each timepoint. Bargraphs show the average of three independent experiments with three different donors, all performed in triplicate. Error bars show standard deviations
To test an alternative method of assessing PMN killing of parasites, we also wanted to allow the parasites to grow following these co-cultures; any remaining live parasites should expand. Therefore, after determining that the optimal pH of the growth media for T. foetus was pH 6.8 (Fig. S2), we co-cultured bovine PMNs and T. foetus for one hour to allow killing to proceed, and then spun down the co-cultures and resuspended them in T. foetus growth media pH 6.8 for overnight culture (Fig. S3). We then counted parasites the following day and determined the percentage of parasites killed by PMN, by comparing numbers of parasites present in each PMN condition to a T. foetus only (no PMN) control. We found the calculated percentages of parasites killed using this alternative method were similar to that seen with the traditional method (Fig. 2C, Fig. S3E). Therefore, we show two valid methods to test parasite killing by PMN in vitro, one with the advantage of being completing in 1 day (Fig. 2), and one that avoids having to pre-label parasites with CTG (Fig. S3), and thus is a cheaper option.
Bovine neutrophil killing of T. foetus is reduced in the presence of trogocytosis inhibitors
We also observed a rapid killing requiring aggregates of PMNs to attack a single parasite in human PMN killing of the human-infective trichomonad parasite T. vaginalis, and found that the PMNs used a novel antimicrobial mechanism termed trogocytosis in this killing (Mercer et al. 2018). We therefore next sought to determine whether bovine PMN killing of T. foetus proceeds through trogocytosis. As opposed to phagocytosis, in which PMNs engulf targets whole, trogocytic killing involves PMNs acquiring small fragments of target cells, eventually leading to the target cell’s death (Matlung et al. 2018; Mercer et al. 2018). Trogocytosed material from target cells has been found to localize both to the cell surface (Ochs et al. 2023; Samer et al. 2023; Schriek and Villadangos 2023) as well as inside the trogocytosing cells (Bettadapur et al. 2020; Uribe-Querol and Rosales 2021). To test whether bovine PMNs kill T. foetus using trogocytosis, we therefore performed our in vitro cytotoxicity assay as described above (Fig. 2), but first pre-incubated bovine PMNs in two inhibitors: cytochalasin D, which inhibits actin polymerization, and wortmannin, which inhibits PI3K signaling. Both of these inhibitors have previously been shown to inhibit trogocytosis (Aucher et al. 2008; Matlung et al. 2018; Mercer et al. 2018; Olivera-Valle et al. 2020; Pham et al. 2011; Ralston et al. 2014; Shin et al. 2021), including trogocytosis of T. vaginalis (Mercer et al. 2018). While neither inhibitor demonstrated any inherent toxicity to the cells (Fig. S4), we found that both inhibitors reduced bovine PMNs killing of T. foetus, indicating that PMN trogocytosis of T. foetus could be involved in the killing we observed (Fig. 3).
Bovine PMN killing of Tritrichomonas foetus is reduced in the presence of trogocytosis inhibitors. Bovine PMNs were pre-incubated with either 1.25 ug/ml of actin polymerization inhibitor Cytochalasin D (CD) (A, B) or 3.2ug/ml of PI3K inhibitor wortmannin (WRT) (C, D) for 20 min, before co-culture with cell-tracker green labelled T. foetus at a ratio of 16 PMN: 1 parasite for 1 h. Then, cultures were stained with zombie violet to determine surviving cells. Numbers of surviving T. foetus (cell tracker green + , zombie violet- cells) were quantified using flow cytometry, and percent killing was calculated by comparing co-culture conditions to a parasite alone control for each condition. Panels A and C show averages of triplicate wells, and panels B and D show composite data from at least 3 independent experiments from at least 3 different blood donors, with triplicate wells each time. Error bars show the standard deviations, and p-values were calculated using a two-tailed T- test
Bovine neutrophils surround and acquire fragments of T. foetus’ membrane
Having observed that bovine killing of PMNs occurs rapidly and is reduced in the presence of actin polymerization and PI3K signaling inhibitors, we next sought to determine whether this rapid killing proceeds through phagocytosis (whole target cell engulfment) or trogocytosis (acquisition of fragments of cells), as both processes are inhibited when actin polymerization and PI3K signaling are impaired. Since bovine PMN killing of T. foetus was only efficient when PMN: T. foetus ratios were higher than 2:1, we hypothesized that killing proceeds through trogocytosis, where multiple PMNs surround one parasite and acquire fragments of it, whereas phagocytosis (whole parasite engulfment by a single PMN) should occur efficiently at a 1:1 PMN: parasite ratio. Furthermore, PMNs killing of T. vaginalis, a related trichomonad parasite, proceeds through trogocytosis (Mercer et al. 2018). In addition, T. foetus’ large size (10-20uM) (Bandeira et al. 2023) compared to bovine PMNs (less than 10uM) (Fingerhut et al. 2020) suggests that whole parasite engulfment by a single PMN is unlikely.
To test whether bovine PMN killing of T. foetus proceeds through phagocytosis or trogocytosis, we performed an imaging assay, in which T. foetus’ plasma membrane was stably labelled, and parasites were added to coverslips containing lawns of bovine PMNs. After allowing the cells to interact for up to fifteen minutes, the timepoint by which a majority of parasites were killed (Fig. 2B), we fixed the coverslips onto slides and imaged them using confocal fluorescent microscopy (Fig. 4). We found that by five minutes, aggregates of PMNs had surrounded individual parasites, and that small fragments of the parasite’s membrane had begun to colocalize with the PMNs. By ten minutes, more fragments accumulated, and by fifteen minutes, we were only able to identify very few intact parasites on our coverslips, but did find many PMNs with green fragments. We never observed transfer of parasite membrane to a control, non-phagocytic cell line (Fig. S5), indicating that the transfer was an active process driven by the PMNs. Higher resolution imaging in 3D revealed that while fragments of T. foetus’ membrane were found both inside PMNs and on the surface of PMN, a majority of fragments were surface localized (Fig. 5). Therefore, PMNs appear to surround individual parasites and trogocytose them, which leads to parasite death. However, we saw almost no instances of whole parasite engulfment. Similar to human PMN trogocytosis of T. vaginalis, we found that while the number of PMNs surrounding individual parasites varied, on average, we observed around four PMNs surrounding each parasite (Fig. 4B).
Bovine neutrophils surround and acquire fragments of Tritrichomonas foetus’ membrane. (A) T. foetus was surface-labelled (Alexa 488) and added to lawns of bovine PMNs on coverslips in the presence of 10% anti-T. foetus antiserum for the indicated timepoints, and then coverslips were fixed and mounted on glass slides for imaging. A representative parasite from an image taken at each timepoint is shown. Altogether, 33 parasites were imaged at time 0, 102 parasites were imaged at 5 min, 55 parasites were imaged at 10 min, and 42 images were taken at 15 min. (B, C) 102 individual parasites across triplicate coverslips for each of three independent experiments with unique blood donors were visually analyzed for (B) the number of PMNs physically touching each individual parasite, and (C) the number of green fragments observed to be trogocytosed from each parasite at the 5 min timepoint
Trogocytosed T. foetus membrane fragments localize mostly externally on PMNs. Imaging experiments performed as in Fig. 4, except PMNs were pre-labelled with Cell Tracker Red (red) and samples were imaged as z-stacks using the 100 × objective. The top view, and two cross sections through the image are shown. White arrows indicate T. foetus membrane fragments that are located internally in PMNs (not visible from the top view), while fragments located on the PMNs’ surface are left unmarked. The image shown is representative of at least 12 parasites imaged over three independent experiments with triplicate coverslips each
Discussion
Despite the serious impacts of Tritrichomonas foetus infection on bovine reproduction, control strategies have fallen short of eradicating infection or eliminating disease outcomes. A more complete understanding of how the parasite interacts with the bovine immune system at a cellular and molecular level may potentiate the future design of more robust control or mitigation strategies. As a first step towards characterizing immunity to T. foetus at the cellular level, we examined the interaction of T. foetus with bovine PMNs, the most abundant immune cells at the site of infection, and the canonical first responder leukocytes. Here, we show that bovine PMNs can rapidly kill T. foetus in vitro, in a dose-dependent manner, which occurs efficiently when multiple PMNs can attack a single parasite. Since killing is decreased in the presence of actin polymerization and PI3K signaling inhibitors previously shown to inhibit trogocytosis, and the acquisition of small fragments of T. foetus’ membrane by PMNs precedes parasite death, these data support a model where bovine PMNs kill T. foetus using trogocytosis, a recently described PMN antimicrobial mechanism used against large target cells.
While trogocytosis is not always lethal (Bettadapur et al. 2020; Uribe-Querol and Rosales 2021), trogocytosis as a mode by which PMNs kill targets has been established for target cells including cancer cells (Matlung et al. 2018), sperm cells (Olivera-Valle et al. 2020), and the human-infective trichomonad parasite, Trichomonas vaginalis (Mercer et al. 2018). Given the similarities between T. vaginalis and T. foetus in size and morphology (Vilela and Benchimol 2013), as well as similarities in their host-cell attachment and pathogenesis (Vilela and Benchimol 2013), it is logical that PMNs would combat both parasites similarly.
As we found T. foetus to be killed rapidly, with fragments of T. foetus’ membrane acquired by PMNs preceding parasite death, and with this killing decreased in the presence of trogocytosis inhibitors, we conclude that bovine PMNs use trogocytosis at least in part, to kill T. foetus, similarly to how human PMNs use trogocytosis to kill T. vaginalis. We found several similarities in PMN killing of both T. foetus and T. vaginalis, such as the timing, and the ratios at which the killing occurred most efficiently. Killing of both T. foetus and T. vaginalis was rapid, with substantial parasite death occurring as early as 5–10 min following PMN- trichomonad contact (Fig. 2B) (Mercer et al. 2018), and with substantial killing requiring aggregates of PMN to surround parasites; ratios of at least four PMN:one trichomonad were required to kill greater than 50% of the trichomonads present (Fig. 2C) (Mercer et al. 2018).
PMNs have three other established mechanisms by which they kill targets: phagocytosis (whole-cell engulfment), extracellular degranulation (exocytosis of toxic granules), and the casting of Neutrophil Extracellular Traps (NETosis), in which pathogens become ensnared in a web of chromatin released extracellularly from the PMN nucleus (Kolaczkowska and Kubes 2013). While NETosis may also be useful against large targets that are too big to engulf whole, it is generally viewed as a suicide mission, employed as a last-ditch effort when other modes have failed; NETs are released in vitro after 2–4 h of stimulation (Mercer et al. 2018; Ramirez-Ledesma et al. 2022). In contrast, the trichomonad killing we observed both here, and in our previous studies, occurred rapidly, with trogocytosis observed almost immediately following PMN-trichomonad contact (Figs. 2B and 4) (Mercer et al. 2018), and a majority of trichomonad death following within the next 10–15 min (Figs. 2B and 4) (Mercer et al. 2018). Furthermore, in our previous studies, we ruled out the role of NETosis in PMNs killing of T. vaginalis by performing cytotoxicity assays in the presence of DNAse that degraded PMN NETs, and saw no effect on killing within a two-hour timeframe (Mercer et al. 2018). However, it is possible that NETosis may play a role in killing of trichomonads at later time-points, for those parasites that were resistant to the initial trogocytic attempts, or under conditions that are unfavorable for trogocytosis, such as when parasites are highly clumped and therefore inaccessible to direct PMN contact, or in the absence of opsonins (Bhakta et al. 2020; Ramirez-Ledesma et al. 2022).
Of the remaining two PMN antimicrobial mechanisms, phagocytosis can be ruled out as a trichomonad killing mechanism utilized by PMNs because visually, whole parasite engulfment is not observed (Fig. 4) (Mercer et al. 2018); however, the role of extracellular degranulation is still in question. In our previous studies, we showed that exocytosis of PMN toxic granules into the extracellular space is not toxic to T. vaginalis, because parasite death only occurred when the PMNs and parasites came into contact; separation of parasites and PMNs in separate chambers of a transwell plate sharing continuous media prevented killing, even when PMNs were stimulated with a strong agonist to induce degranulation (Mercer et al. 2018). However, it is not known whether the exocytosis of PMN toxic granules directly onto the trichomonas surface when the cells are in direct contact could contribute to degradation of the parasite’s plasma membrane into fragments. In fact, we and others identified PMN serine proteases as a potential driver of trogocytosis (Mercer et al. 2018; Olivera-Valle et al. 2020), and many PMN serine proteases are present in PMN toxic granules (Kettritz 2016; Pham 2006). Furthermore, studies imaging the interaction between T. foetus and rat neutrophils showed that neutrophil granule contents were observed adjacent to the parasite following contact (De Azevedo and De Souza 1993). Therefore, while phagocytosis and NETosis can be ruled out as killing mechanisms that bovine PMNs in this rapid killing of T. foetus, the role of PMN extracellular degranulation either independently of, or in conjunction with trogocytosis, should still be investigated.
Furthermore, the subcellular localization of trogocytosed parasite material and downstream immunological consequences of PMN killing of targets using trogocytosis are yet unknown. Some instances of trogocytosis merely involve display of plasma membrane proteins from the trogocytosed cell to the recipient cell’s membrane (Daubeuf et al. 2010; Joly and Hudrisier 2003), while other instances have demonstrated trogocytosed fragments internalized into the recipient cell (Mercer et al. 2018; Ralston et al. 2014). When macrophages engage in trogocytosis, the engulfed material ends up in a double-membraned vesicle inside the recipient macrophage (Steele et al. 2016, 2019), indicating that the trogocytosed material may enter the endocytic pathway rather than directly enter the cytosol. In trogocytosis by E. histolytica, sustained trogocytosis and target killing requires lysosomal digestion of the initial bites (Gilmartin et al. 2017), suggesting a role for the lysosome in processing trogocytosed material. Here, we report that T. foetus’ membrane was found both internally, and on the surface of PMNs (Fig. 5), however it is not yet- known which specific subcellular pathways the trogocytosed material follows to arrive at these locations. Nonetheless, the substantial amount of trogocytosed material that localizes to the PMN surface suggests a role for plasma membrane display of parasite antigen in downstream immune responses (Herbst et al. 2022).
PMN are generally short-lived cells, however, macrophages that efferocytose (Bratton and Henson 2011; Martin et al. 2014) dead PMN-parasite complexes and rare populations of long-lived PMNs (Shim et al. 2022) may play a role in presenting trogocytosed material to T-cells. Therefore, future studies to determine which downstream immune responses are triggered following PMN trogocytic killing of trichomonads, and why, are warranted.
Bovine trichomonosis is a stubborn and persistent problem in cattle, with deleterious effects on reproduction. Improved understanding of cellular and molecular immune mechanisms against the parasite may help to shed light on why infection results in infertility and abortion, and why natural immunity and immunity induced by the existing vaccines is weak and short-lived. As PMN are both the major immune cells present in infected tissues, and play major roles in mediating inflammation, understanding how they interact with the parasite is an important first step towards delineating mechanisms of parasite clearance, persistence, and collateral damage. Understanding the interaction of immune cells with T. foetus in sufficient molecular detail may inform intervention strategies that can prolong immunity and/or minimize pathology. Furthermore, trogocytosis is a novel antimicrobial mechanism, and this study expands the range of targets seen to be killed by PMNs using trogocytosis, pointing to the importance of trogocytosis as a fundamental cell process to further explore in its molecular mechanisms, downstream effects, and broader impacts on host–pathogen interactions.
References
Aucher A, Magdeleine E, Joly E, Hudrisier D (2008) Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood 111:5621–5628
Aydintug MK, Widders PR, Leid RW (1993) Bovine polymorphonuclear leukocyte killing of Tritrichomonas foetus. Infect Immun 61:2995–3002
Baltzell P, Newton H, O’Connor AM (2013) A critical review and meta-analysis of the efficacy of whole-cell killed Tritrichomonas foetus vaccines in beef cattle. J Vet Intern Med 27(4):760–70. https://doi.org/10.1111/jvim.12112
Bandeira PT, Ortiz SFDN, Benchimol M, de Souza W (2023) Expansion microscopy of trichomonads. Exp Parasitol 4:108629. https://doi.org/10.1016/j.exppara.2023.108629
Bettadapur A, Miller HW, Ralston KS (2020) Biting off what can be chewed: trogocytosis in health, infection, and disease. Infect Immun 88(7):e00930–19. https://doi.org/10.1128/IAI.00930-19
Bhakta SB, Moran JA, Mercer F (2020) Neutrophil interactions with the sexually transmitted parasite Trichomonas vaginalis: implications for immunity and pathogenesis. Open Biol 10:200192
BonDurant RH (1997) Pathogenesis, diagnosis, and management of trichomoniasis in cattle. Vet Clin North Am Food Anim Pract 13(2):345–361. https://doi.org/10.1016/s0749-0720(15)30346-7
BonDurant RH, Corbeil RR, Corbeil LB (1993) Immunization of virgin cows with surface antigen TF1.17 of Tritrichomonas foetus. Infect Immun 61:1385–1394
Bratton DL, Henson PM (2011) Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol 32:350–357
Clark CG, Diamond LS (2002) Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev 15:329–341
Collántes-Fernández E, Fort MC, Ortega-Mora LM, Schares G (2018) Trichomonas. In: Florin-Christensen M, Schnittger L (eds) Parasitic protozoa of farm animals and pets. Springer International Publishing, Cham, pp 313–388
Corbeil LB, Anderson ML, Corbeil RR, Eddow JM, BonDurant RH (1998) Female reproductive tract immunity in bovine trichomoniasis. Am J Reprod Immunol 39:189–198
Corbeil LB, Munson L, Campero C, BonDurant RH (2001) Bovine trichomoniasis as a model for development of vaccines against sexually-transmitted disease. Am J Reprod Immunol 45:310–319
Corbeil LB, Campero CM, Rhyan JC, BonDurant RH (2003) Vaccines against sexually transmitted diseases. Reprod Biol Endocrinol 1:118
Czuprynski CJ, Hamilton HL (1985) Bovine neutrophils ingest but do not kill Haemophilus somnus in vitro. Infect Immun 50:431–436
Dabrowska J, Karamon J, Kochanowski M, Sroka J, Zdybel J, Cencek T (2019) Tritrichomonas foetus as a causative agent of tritrichomonosis in different animal hosts. J Vet Res 63:533–541
Daubeuf S, Aucher A, Bordier C, Salles A, Serre L, Gaibelet G, Faye JC, Favre G, Joly E, Hudrisier D (2010) Preferential transfer of certain plasma membrane proteins onto T and B cells by trogocytosis. PLoS ONE 5:e8716
De Azevedo NL, De Souza W (1993) Fine structure and cytochemistry of Tritrichomonas foetus and rat neutrophil interaction. J Eukaryot Microbiol 40:636–642
Deniset JF, Kubes P (2016) Recent advances in understanding neutrophils. F1000Res 5:2912
Edmondson MA, Joiner KS, Spencer JA, Riddell KP, Rodning SP, Gard JA, Givens MD (2017) Impact of a killed Tritrichomonas foetus vaccine on clearance of the organism and subsequent fertility of heifers following experimental inoculation. Theriogenology 90:245–251
Etchevers L, Stassi AF, Belotti EM, Diaz PU, Durante LI, Notaro US, Chiaraviglio JA, Rey F, Salvetti NR, Ortega HH et al (2023) Exogenous ACTH stimulus during the preovulatory period alters patterns of leukocyte recruitment in the ovary of dairy cows. Theriogenology 195:176–186
Fingerhut L, Dolz G, de Buhr N (2020) What is the evolutionary fingerprint in neutrophil granulocytes? Int J Mol Sci 21(12):4523
Gilmartin AA, Ralston KS, Petri WA Jr (2017) Inhibition of amebic lysosomal acidification blocks amebic trogocytosis and cell killing. MBio 8(4):e01187–17. https://doi.org/10.1128/mBio.01187-17
Herbst C, Harshyne LA, Igyarto BZ (2022) Intracellular monitoring by dendritic cells - a new way to stay informed - from a simple scavenger to an active gatherer. Front Immunol 13:1053582
Ikeda JS, BonDurant RH, Corbeil LB (1995) Bovine vaginal antibody responses to immunoaffinity-purified surface antigen of Tritrichomonas foetus. J Clin Microbiol 33:1158–1163
Joly E, Hudrisier D (2003) What is trogocytosis and what is its purpose? Nat Immunol 4:815
Kettritz R (2016) Neutral serine proteases of neutrophils. Immunol Rev 273:232–248
Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13:159–175
Kvasnicka WG, Hanks D, Huang JC, Hall MR, Sandblom D, Chu HJ, Chavez L, Acree WM (1992) Clinical evaluation of the efficacy of inoculating cattle with a vaccine containing Tritrichomonas foetus. Am J Vet Res 53:2023–2027
Lietaer L, Demeyere K, Heirbaut S, Meyer E, Opsomer G, BogadoPascottini O (2021) Flow cytometric assessment of the viability and functionality of uterine polymorphonuclear leukocytes in postpartum dairy cows. Animals (Basel) 11(4):1081
Liu X, Hoene M, Wang X, Yin P, Häring H-U, Xu G, Lehmann R (2018) Serum or plasma, what is the difference? Investigations to facilitate the sample material selection decision making process for metabolomics studies and beyond. Anal Chim Acta 1037:293–300. https://doi.org/10.1016/j.aca.2018.03.009
Lopes MG, Alharthi AS, Lopreiato V, Abdel-Hamied E, Liang Y, Coleman DN, Dai H, Correa MN, Socha MT, Ballou MA et al (2021) Maternal supplementation with cobalt sources, folic acid, and rumen-protected methionine and its effects on molecular and functional correlates of the immune system in neonatal Holstein calves. J Dairy Sci 104:9340–9354
Love D, Fajt VR, Hairgrove T, Jones M, Thompson JA (2017) Metronidazole for the treatment of Tritrichomonas foetus in bulls. BMC Vet Res 13:107
Martin CJ, Peters KN, Behar SM (2014) Macrophages clean up: efferocytosis and microbial control. Curr Opin Microbiol 17:17–23
Martin KA, Henderson J, Brewer MT (2021) Bovine Trichomonosis cases in the United States 2015–2019. Front Vet Sci 8:692199
Matlung HL, Babes L, Zhao XW, van Houdt M, Treffers LW, van Rees DJ, Franke K, Schornagel K, Verkuijlen P, Janssen H et al (2018) Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep 23(3946–3959):e3946
Mercer F, Johnson PJ (2018) Trichomonas vaginalis: pathogenesis, symbiont interactions, and host cell immune responses. Trends Parasitol 34:683–693
Mercer F, Ng SH, Brown TM, Boatman G, Johnson PJ (2018) Neutrophils kill the parasite Trichomonas vaginalis using trogocytosis. PLoS Biol 16:e2003885
Ochs J, Hausser-Kinzel S, Weber MS (2023) Trogocytosis challenges the cellular specificity of lineage markers and monoclonal antibodies. Nat Rev Immunol 23(9):539–540. https://doi.org/10.1038/s41577-023-00920-7
Oliveira BM, Pinto A, Correia A, Ferreira PG, Vilanova M, Teixeira L (2020) Characterization of myeloid cellular populations in mesenteric and subcutaneous adipose tissue of Holstein-Friesian cows. Sci Rep 10:1771
Olivera-Valle I, Latorre MC, Calvo M, Gaspar B, Gomez-Oro C, Collazos A, Breton A, Caballero-Campo P, Ardoy M, Asensio F et al (2020) Vaginal neutrophils eliminate sperm by trogocytosis. Hum Reprod 35:2567–2578
Ortega-Mora LM, Sánchez-Sánchez R, Rojo-Montejo S, Román-Trufero A, Montenegro-Gregorio D, Puentes-Colorado E, Parra-Romero A, Regidor-Cerrillo J, Osoro K, Collantes-Fernández E (2022) A new inactivated Tritrichomonas foetus vaccine that improves genital clearance of the infection and calving intervals in cattle. Front Vet Sci 6(9):1005556. https://doi.org/10.3389/fvets.2022.1005556
Parsonson IM, Clark BL, Dufty JH (1976) Early pathogenesis and pathology of Tritrichomonas foetus infection in virgin heifers. J Comp Pathol 86:59–66
Pham CT (2006) Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 6:541–550
Pham T, Mero P, Booth JW (2011) Dynamics of macrophage trogocytosis of rituximab-coated B cells. PLoS ONE 6:e14498
Rae DO, Crews JE (2006) Tritrichomonas foetus. Vet Clin North Am Food Anim Pract 22:595–611
Ralston KS, Solga MD, Mackey-Lawrence NM, Somlata, Bhattacharya A, Petri WA Jr (2014) Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature 508:526–530
Ramirez-Ledesma MG, Romero-Contreras YJ, Rodriguez MC, Reyes-Cortes R, Cuellar-Mata P, Avila EE (2022) Trichomonas vaginalis triggers neutrophil extracellular traps reducing parasite integrity and growth. Parasitol Res 121:1355–1367
Rhyan JC, Wilson KL, Burgess DE, Stackhouse LL, Quinn WJ (1995) Immunohistochemical detection of Tritrichomonas foetus in formalin-fixed, paraffin-embedded sections of bovine placenta and fetal lung. J Vet Diagn Invest 7(1):98–101. https://doi.org/10.1177/104063879500700116
Roth JA, Kaeberle ML (1981) Evaluation of bovine polymorphonuclear leukocyte function. Vet Immunol Immunopathol 2:157–174
Samer S, Chowdhury A, Wiche Salinas TR, Estrada P, Reuter M, Tharp G, Bosinger S, Cervasi B, Auger J, Gill K et al (2023) Lymph-Node-Based CD3(+) CD20(+) cells emerge from membrane exchange between T follicular helper cells and B cells and increase their frequency following simian immunodeficiency virus infection. J Virol 97:e0176022
Schriek P, Villadangos JA (2023) Trogocytosis and cross-dressing in antigen presentation. Curr Opin Immunol 83:102331
Shim HB, Deniset JF, Kubes P (2022) Neutrophils in homeostasis and tissue repair. Int Immunol 34(8):399–407
Shin JH, Jeong J, Maher SE, Lee HW, Lim J, Bothwell ALM (2021) Colon cancer cells acquire immune regulatory molecules from tumor-infiltrating lymphocytes by trogocytosis. Proc Natl Acad Sci U S A 118(48):e2110241118. https://doi.org/10.1073/pnas.2110241118
Singh BN, Lucas JJ, Hayes GR, Kumar I, Beach DH, Frajblat M, Gilbert RO, Sommer U, Costello CE (2004) Tritrichomonas foetus induces apoptotic cell death in bovine vaginal epithelial cells. Infect Immun 72(7):4151–4158. https://doi.org/10.1128/IAI.72.7.4151-4158.2004
Steele S, Radlinski L, Taft-Benz S, Brunton J, Kawula TH (2016) Trogocytosis-associated cell to cell spread of intracellular bacterial pathogens. Elife 5:e10625. https://doi.org/10.7554/eLife.10625
Steele SP, Chamberlain Z, Park J, Kawula TH (2019) Francisella tularensis enters a double membraned compartment following cell-cell transfer. Elife 8:e45252. https://doi.org/10.7554/eLife.45252
Uribe-Querol E, Rosales C (2021) The multiple roles of trogocytosis in immunity, the nervous system, and development. Biomed Res Int 2021:1601565
Vilela RC, Benchimol M (2013) IL-10 release by bovine epithelial cells cultured with Trichomonas vaginalis and Tritrichomonas foetus. Mem Inst Oswaldo Cruz 108:110–112
Voyich JM, Ansotegui R, Swenson C, Bailey J, Burgess DE (2001) Antibody responses of cattle immunized with the Tf190 adhesin of Tritrichomonas foetus. Clin Diagn Lab Immunol 8:1120–1125
Acknowledgements
We would like to thank the Biological Sciences Department, and the College of Science at Cal Poly Pomona for providing a space and intellectual environment for these studies, as well as a MACS Quant Flow cytometer. We would like to thank the Provost’s Teacher-Scholar program at Cal Poly Pomona for protected time for this research, and the California Faculty Association union for advocacy of workload protections in support of scholarly pursuits. We would like to thank Dr. Craig LaMunyon for use of the Nikon Confocal Microscope. We would like to thank the Animal Care and Use Committee (IACUC) at Cal Poly Pomona for review of these studies and the Cattle Unit at Cal Poly Pomona, especially Holly Greene, Efrain Loera, and Javier Ramirez for care of the animals, training, and facilitating blood draws. We would like to thank the Agricultural Research Initiative at Cal Poly Pomona for funding of the work, and Dr. Wei Bidlack for advice on the project and logistical assistance with grant administration. We would also like to thank Dr. Patricia Johnson for proofreading the manuscript. We would also like to thank Dr. Hyungchul Han for scientific input on the project and Dr. Yi-Pei Chen for technical advice. We would also like to thank the McNair Scholars program, the California State University Grant scholarship, and the Bridges-to-PhD program at Cal Poly Pomona for support of student researchers. We would also like to thank Drs. Lynette Corbeil, Roberto Palomares, Charles J Czuprynski, and Kirkwood Land for advice on culture of Tritrichomonas foetus and bovine cells.
Funding
This work was funded by the California State University Agricultural Research Initiative (CSU-ARI) at Cal Poly Pomona grant #s 22-04-104, 21-04-112, and 23-04-105.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jonathan Najera, Michael M Berry, Ashley D Ramirez, Bryan Ramirez Reyes, Arielle Angel, Juanita K Jellyman and Frances Mercer. The first draft of the manuscript was written by Frances Mercer and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Statement on animal ethics
The protocols used in this study was approved by the Cal Poly Pomona Institutional Animal Care and Use Committee (protocol 20.031).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Najera, J., Berry, M.M., Ramirez, A.D. et al. Bovine neutrophils kill the sexually-transmitted parasite Tritrichomonas foetus using trogocytosis. Vet Res Commun 48, 865–875 (2024). https://doi.org/10.1007/s11259-023-10260-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-023-10260-5