Abstract
Canine Soft Tissue Sarcoma (STS) cell line A-72 has been largely employed for antiviral and antiproliferative studies. However, there are few information on their characteristics. Our aim was to evaluate A-72 expression level of genes and proteins involved in the innate immune response and cell cycle, their ability to respond to infective stressors and their possible use as a cellular model for anti-cancer studies in human and animal medicine. For this purpose, we evaluated the basal expression of immune-related, cell cycle and DNA repair genes on this cell line and tumoral tissues. A-72 ability to respond to a wild-type strain of Salmonella typhimurium was assessed. S. typhimurium showed ability to penetrate A-72 causing pro-inflammatory response accompanied by a decrease of cell viability. IL10 and IL18 genes were not expressed in A-72 while CXCL8, NOS2, CXCR4 and PTEN were highly expressed in all samples and TP53 was slightly expressed, as shown in human STS. Our results outline the ability of A-72 to respond to a bacterial agent by modifying the expression of important genes involved in innate immune response and provide a useful model for in vitro evaluation of new therapeutic approaches that could be translated into the human oncology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soft tissue sarcoma (STS) is a term used both in human and animal oncology to describe different types of cancer that can arise from many anatomical sites. Despite their widespread dissemination into the organism, these tumors have a common mesenchymal origin and have similar biological and histological features (Dennis et al. 2011). Tumors that are included in the STS group are: perivascular wall tumors, liposarcoma, malignant fibrous histiocytoma, mesenchymoma, myxosarcoma, non-plexus derived peripheral nerve sheath tumors, undifferentiated sarcoma and fibrosarcoma (Bray 2016). These cancers are usually described as pseudo-encapsulated masses with poorly defined margins and malignant tumor cells have the ability to exit the cancer core penetrating through the pseudo-capsule. Consequently, after the surgical resection, a microscopic tissue residue in situ can remain, resulting in tumor recurrence (Bray 2017). In humans, through rare (about 1% in adults), STS are highly debilitating malignancies and are often associated with significant morbidity and mortality; the standard of care for localized disease in adults has been wide surgical resection (Dodd et al. 2010; Gamboa et al. 2020). The rarity and heterogeneity of patient samples (about 100 different histologic and molecular sub-types of STS are recognized) complicate clinical investigations into STS biology (Dodd et al. 2010; Gamboa et al. 2020). Sarcomas research relied on human cell lines and immunocompromised mice. Some human cell lines derived from STS are available and have also been used as xenografts in immunocompromised mice in order to study anticancer drug development, drug sensitivity and treatments response (Dodd et al. 2010). In dogs, if a complete tumor surgery resection is possible, prognosis is good (Bray et al. 2014). Today it is accepted that radical excision margins (about 2–3 cm) will provide good local cancer control (Bray 2016); however, no diagnostic tests are currently available to predict the correct dimension of surgical margin required for each tumor, so relapse can develop between 17 and 75% of dogs and is associated with increased risk of death (Bray 2017).
Despite many advances in elucidating progression, invasiveness and metastatic spread of STSs, the use of in vitro sarcomas models has led to relatively few new drug treatments and according to current literature, surgical resection is still the treatment of choice to achieve long-term disease-free survival or a cure both in humans and dogs (Ettinger 2003; Selting et al. 2005; Hager et al. 2017). Dogs and humans STSs display similar histological and immunohistochemical features (Milovancev et al. 2015), therefore, there is great interest in animal models of primary tumors to elucidate STS biology underlying their development, progression, and treatment (Dodd et al. 2010). From this point of view, the availability of STS animal cell cultures can be useful in order to verify the efficacy of treatments to be transferred first to the veterinary and then to human research against cancer.
In dogs, radiation therapy plays an important role in the management of STSs, but it has little role as a single treatment modality. Radiation therapy is appropriate for incompletely excised tumors or for preoperative treatment. Due to the generally poor response of the STS to chemotherapy, it has a role as additional treatment to treat incompletely resected tumors, high-grade tumors, and metastatic disease or as palliative therapy in unresectable tumors (Ettinger 2003). Doxorubicin-containing protocols and mitoxantrone are the most effective drugs against canine STS, with reported response rates of approximately 20% (Ettinger 2003; Selting et al. 2005). In humans, radiotherapy in addition to surgery has shown to enhance local control and resectability in some kind of STSs (Hager et al. 2017). Several agents have been investigated for chemotherapy in human and, to date, doxorubicin and ifosfamide remain the most effective chemotherapy drugs available for the treatment of majority of these tumors (Liu et al. 2018; Ratan and Patel 2016).
Under this point of view, multimodal therapies based on the association of surgery with radiotherapy/chemotherapy/immunotherapy/electrochemotherapy could represent an alternative therapeutic strategy (Spugnini et al. 2019; Torrigiani et al 2019); this could determine a reduction in surgical excision and STS recurrence. A recent study suggests that chemotherapy causes a decrease of tumor growth by immune-modulatory effects (Bajpai and Susan 2016). A promising strategy could be to develop new anti-STS therapy based on immune response modulation using attenuated bacteria (Chirullo et al. 2015a, b).
In this respect, cell lines could be helpful to test in vitro immunomodulant anticancer therapies, allowing to reduce the use of laboratory animals in the preliminary tests and to achieve results in shorter time. In this context, it is pivotal to develop and evaluate in vitro models of dog-STS, furthermore, the evaluation of the suitability of the canine spontaneous STS as a model for the study of the human STS is of great interest. However, few and recent STS cell lines are available in dog (Snyder et al. 2011; Son et al. 2019). Moreover, to the authors’ knowledge, these cells lines have not been characterized in terms of gene expression and their ability to interact with infective stressors is unknown. In this framework, the canine tumor cell line A-72 was obtained by Binn and co-workers in 1980 (Binn et al. 1980) from a tumor of 1 cm diameter taken from the left thigh of an eight years old female Golden Retriever. After the explant, cells were serially passaged, and they maintained a fibroblastic appearance (Binn et al. 1980). Since then, many researchers have used A-72 as cell line for in vitro test on viruses (Martin-Calvo et al. 1994; Pratelli and Moschidou 2012; Parthiban et al. 2014), vaccines (Barros et al. 2017), antiviral and anti-proliferative compounds (Croci et al. 2011; Fan et al. 2014). However, little is known about the A-72 cells in terms of the origin phenotype, expression of genes involved in the innate immune response, DNA repairs and cell cycle regulation. The aim of our study was to characterize A-72 cells first evaluating the basal expression level of genes and proteins involved in the innate immune response and in the cell cycle; secondly assessing their modulation induced by a bacterial agent (specifically S. Typhimurium), in order to evaluate the ability of cells to respond to this stressor. S. Typhimurium is the second most frequent serotype of Salmonella isolated in Europe from both humans and veterinary matrices and represents the most prevalent and disseminated serovar worldwide (Ferrari et al. 2019)and its attenuated form was deeply investigated for its antitumor activity in human and murine cells (Chirullo et al. 2015a).
Materials and methods
Cell culture
A-72 cells line (canine tumor, IZSLER biobank OIE codex BS TCL 1) at 98th passage were purchased and used for different experiments. A-72 cells were grown in a mixture of Eagle’s Minimum Essential Medium Eagle (Merck, Milan, Italy, cat M5650) enriched with Sodium Pyruvate 1% (Euroclone, Milan, Italy, cat ECM0542D), 10% (v/v) of Fetal Bovine Serum (FBS, GIBCO™, Thermofisher Scientific, Milan, Italy, cat 10,437–036) and a mixture of Antibiotics (penicillin and streptomycin, 1% v/v, Carlo Erba Reagents, cat FA30WL0022100). For the experiments, 12-well culture plates (2.5 × 105 cells/ml, 2 ml per well) were incubated at 37 °C in 5% CO2 until confluence (16–22 h). Cells were tested at the 104th, 107th and 109th passages as described in Sect. "Gene Expression"; to evaluate basal gene expression. Moreover, cells, at the 109th passage cells were incubated for 24 ± 1 h (hrs) after confluence to evaluate the effects of monolayer aging. Each experiment was repeated six times.
Gene expression
Gene expression was evaluated in A-72 cell line at 104th, 107th and 109th passages and in one neoplastic sample (hemangiosarcoma-HE) obtained during the routinely surgery and stored in RNAlater (Machery-Nagel GmbH & Co KG, Durren, Germany, cat 740,400,500). Surgery samples were obtained by the University of Perugia, Department of Veterinary Medicine.
Gene selection been made based on their role in innate immune response to microorganisms. Moreover, we considered their involvement in STS development and metastasis (Chirullo et al. 2015b; Razzuoli et al. 2017; Li et al. 2017; Gustafson et al. 2018). The following genes were tested: Interleukins (IL) (IL6, CXCL8, IL10, IL15, IL18), Nitric oxide synthase 2 (NOS2), Cluster of Differentiation 14 and 44 (CD14 and CD44), C-X-C chemokine receptor type 4 (CXCR4), Erb-B2 Receptor Tyrosine Kinase 2 (ERBB2), Lymphocyte antigen 96 (LY96), Myeloid differentiation primary response 88 (MYD88), Transforming growth factor beta (TGFB), Nuclear factor kappa-light-chain-enhancer of activated B cells (NFKB/p65), Phosphatase and tensin homolog (PTEN), Signal transducer and activator of transcription 5 (STAT5), Toll-like receptor 4 and 5 (TLR4 and TLR5), onco-suppressor TP53 and DNA repair RAD51; Ribosomal protein S5 (RPS5) was used as reference gene. To this purpose, we used primers sets described in previous works (Table 1). Total RNA extraction from A-72 cells and tumors was carried out by the RNeasy Mini Kit (Qiagen s.r.l., Milan Italy, cat 74,104) on the QiaCube instruments (Qiagen s.r.l.) according to the manufacturer’s instructions, reverse transcription (RT) was performed with the OneScript® cDNA Syntesis Kit (Applied Biological Materials Inc. Richmond, BC, Canada, cat G234) using 250 ng of total RNA. The gene expression was assessed by RT-qPCR using primers reported in Table 1 and the Sybr Green chemical approach (Applied Biological Materials Inc., cat 4,367,659) using our protocols as previously described (Razzuoli et al. 2014). RT and Real-Time PCR amplification were performed on a CFX96™ Real-Time System (Bio-Rad, Milan, Italy). To evaluate the basal expression level in each sample, the relative expression of the target genes was calculated using the formula 2−ΔΔCq. The mean of three test replicates ± 1 standard deviation was considered. To identify PCR-negative samples was chosen a Cq value of 39.
Immunocytochemical assay
The following antibodies were used for the Immunocytochemical analysis: Polyclonal Rabbit CXCR4 (Sigma Aldrich, cat C3116) and S100 (Dako Denmark A/S, cat Z0311); Monoclonal Mouse Actin (ACT, Clone 1A4, Dako Denmark A/S, cat M0851), Calponin (CALP, Clone CALP, Dako Cytomation, cat M3556), CD44 (Clone DF1485, Dako Denmark A/S, cat M7082), Cytokeratin (CK, Clones AE1/AE3, Dako North America, Inc., cat M3515), Desmin (DESM, Clone D33, Dako Denmark A/S, cat M0760), and Vimentin (VIM, Clone V9, Dako Denmark A/S, cat M0725). 1% of Bovine Serum Albumin (BSA, Merck, Milano Italy, cat A3418) in Phosphate Buffered Saline was used for dilution (PBS, Merck, Milano Italy, cat. D8537). The dilutions were performed with 1% BSA (Applichem GmbH, Darmstadt, cat A1391) in PBS.
A-72 cells (2.5 × 105 cells/ml) were treated with the primary antibody diluted as follow: CK 1:50 for 10 min (mins), VIM 1:200 for 30 min, ACT and DESM 1:100 for 30 min, CALP and S100 1:400 for 30 min, CD44 1:7 and CXCR4 1:500 overnight (Modesto et al. 2020). Afterward, samples were washed three times for 5 min with PBS and treated with the secondary antibody (Dako EnVision+ Dual Link System-HRP, Dako North America, Inc. cat K4061): ACT and DESM for 1 h, CXCR4 for 50 min, CALP, CD44, CK, and VIM for 30 min and S100 for 20 min. Then, cells were washed three times for 5 min with PBS and the chromogenic fixation was carried out with 3,3’-diaminobenzidine (DAB, Dako Liquid DAB + Substrate Chromogen System, Dako North America, Inc. cat K3468); the reaction was blocked in distilled water for 5 min. Cells incubated with the immunoglobulin fraction of the mouse non-immune serum, instead of the primary antibody, were used as negative control. Finally, the slides were controstained with Mayer’s hematoxylin (Sigma Aldrich, cat MHS1) and observed under a light microscope.
Response to infective stressor
To evaluate the ability of this cell line to respond to infective stressor we used a wild-type strain of S. Typhimurium (ST) according to our previous study (Modesto et al. 2020). ST was grown to obtain mid-log phase culture. ST was re-suspended at 108 colony forming unit (CFU)/ml, and A-72 cells were treated with 1 ml of this bacterial suspension (MOI 100:1 CFU/cells) for 1 h at 37 °C in 5% CO2. Cells treated were used to assess the ST stimulation effects through the evaluation of 1) immunomodulation, 2) cells vitality and 3) invasiveness.
Experiment 1: Modulation of innate immune response
After A-72 treatment with ST and incubation at 37 °C in 5% CO2 for 1 h, cells were washed three times with medium only and incubated at 37 °C in 5% CO2 for 3 h with fresh completed medium. The experiment was repeated three times and cells treated exclusively with medium without bacteria suspension were used as negative control. The gene expression analysis was carried out as described in 2.2 section.
Experiment 2: Cell vitality after treatment
After 1 h of ST exposure cells were washed, detached with trypsin (Sigma Aldrich, Saint Louis, Missouri, USA, cat T4049) and blocked with complete medium; after this, 10 μl of Trypan Blue 0.4% (Logos Biosystems, Inc. Korea, cat. T13001) were added to 10 μl of cell suspension to evaluate cell viability using the LUNA II Automated Cell Counter (LUNA™ Logos Biosystems, Inc. Korea). As negative control cells treated with medium only were used. Experiment was repeated thrice.
Experiment 3: Bacterial invasion
Cells at confluence were infected with 1 ml of bacterial suspension (see Sect. "Response to Infective Stressor") at 108 CFU/ml and incubated at 37 °C in 5% CO2 for 1 h in agreement with the protocols described by Razzuoli et al. (Razzuoli et al. 2017). Briefly, after ST exposure, cells were washed with medium and treated with a solution of PBS containing 300 μg/ml colistin sulphate (Microbiol & C. s.n.c., Cagliari, Italy, cat 74,016) at 37 °C in 5% CO2 for 2 h to remove all extracellular bacteria. Absence of toxic side effects on A-72 had been confirmed in preliminary assays. Then, cells were lysed adding 1% Triton X-100 (Sigma Aldrich, Saint Louis, Missouri, USA, cat 108,603) in PBS at room temperature for 5 min. Afterwards, PBS was added to each well; the resulting cell suspension was vortexed, serially diluted and seeded on Xylose Lysine Deoxycholate (XLD; Sigma Aldrich, cat 146,073) agar plates and incubated at 37 °C for 24–48 h. Cells treated with medium only were used as negative control; the experiment was performed thrice.
Sequencing of the key gene CXCR4 and TP53
The presence of mutations that can modify CXCR4 and TP53 expression has been investigated by direct sequencing in A-72 cancer cell line and in canine healthy tissue samples and we compared the obtained sequences with those deposited in the GeneBank database. Tests were performed on A-72 (at 98th and 109th passages) and on 5 healthy canine tissues (2 lymphonodes, 2 kidney and 1 liver), three replications for each experiment have been carried out. DNA was extracted from 1 × 106 cells and 25 mg of each healthy tissue using QIAmp DNA Mini kit (Qiagen, Milan, Italy, cat 51,306) following the manufacturer’s protocol.
Sequencing of TP53 was carried out using primer pair (dogP53F2 5'-CTC CTC AGC ATC TCA TCC G-3' and dog P53R2 5'- ATG GCG AGA GGT AGA TTG C -3') specifically designed to amplify a 2000 pb fragment of the TP53 (reference sequence ID KJ511265.1). Sequencing of CXCR4 was performed as previously described (Modesto et al. 2020). Specific primers pair spanning a 902 bp of the coding region have been designed on the reference sequence (NM_001048026.1) (Table 2).
All primer pairs were designed using Primer3 software version 0.4.0 (https://bioinfo.ut.ee/primer3-0.4.0). PCR reaction mix has been setted as follows: 1X PCR Buffer, 1 mM MgCl2, 1.25 U hot start Taq polymerase, dNTPs mix 10 mM each, 0.25 μl of each primer (Thermo Scientific, Monza, Italy). Thermal profile was 94 °C for 15 min, 35 cycles of 94 °C for 30 s (s), 60 °C for 30 s (for the CXCR4 amplification) or 58 °C for 45 s (for TP53 amplification) and 72 °C for 60 s, a final step at 72 °C for 10 min was included. A negative control was added to each run. The amplification runs were performed on a GeneAmp9700 (Applied Biosystems, Monza, Italy). The products of amplification were purified using High Pure PCR Product Purification Kit (Roche Diagnostics, Monza, Italy, cat 11,732,668,001). Sequencing reaction products were purified using the DyeEx 2.0 Spin Kit (Qiagen, Milan, Italy, cat 63,204) and a capillary electrophoresis was run on the Applied Biosystems 3500 genetic Analyzer (Thermo Scientific, Monza, Italy). The consensus sequence of the entire 902 bp segment has been obtained by the forward and reverse overlapping sequences aligned with BioEdit Sequence Alignment Editor version 7.2.5. Consensus sequences were aligned with reference sequences using the ClustalW multiple alignment function.
Statistical analyses
Data sets were submitted to a Kolmogorov–Smirnov test to check Gaussian distributions; significant differences within normal distributions were checked by one-way ANOVA or Student’s t test. The significance threshold was set at P < 0.05 (Prism 5, GraphPad Software).
Results
Basal gene expression
All target genes under study have been found expressed in A-72 with the exception of IL10 and IL18 (Table 3). In particular, CD44, CXCR4, ERBB2, IL6, CXCL8, NOS2, LY96, MYD88, NFKB/p65, PTEN, TP53, RAD51, STAT5, TGFB and TLR5 expression was observed in all tested samples. Concerning the other genes, CD14 was expressed in 72.2% of samples, TLR4 in 38.9% and IL15 in 5.5%. Moreover, same analysis were performed on sarcoma samples provided by the Department of Veterinary Medicine of University of Perugia. These tumoral tissue showed the expression of all the genes under study with the exception of PTEN (Table 3).
Modulation of gene expression at different cell passages and effect of monolayer aging
After basal gene expression analysis, we focused on the modulation of the gene expression at three different passages. Our results showed modulation of gene expression after 107th and 109th passages (Table 4) with respect to the 104th passage. At passage 107th we observed a significant up-regulation of CXCR4 (P < 0.0001), LY96 (P < 0.0001), MYD88 (P = 0.0040), TLR5 (P = 0.0098) and TGFβ (P = 0.00111) and significant down-regulation (defined by a value less than 1) of IL6 (P = 0.00047) and CXCL8 (P < 0.0001). Regarding the 109th passage, we observed a significant gene expression decrease of CD44 (P = 0.0401), IL6 (P = 0.035), CXCL8 (P = 0.0002), NFKB/p65 (P = 0.00017), TP53 (P = 0.00023), RAD51 (P = 0.0159) and TLR5 (P = 0.0076) (Table 4).
After 24 h of monolayer achievement (109th passage), cells showed a decrease of CD14 (P = 0.0069), and the up-regulation of CD44 (P = 0.0005), ERBB2 (P < 0.0001), NFKB/p65 (P < 0.0001), PTEN (P = 0.046), TP53 (P = 0.00023), STAT5 and TLR5 (P = 0.0076), while the other genes were not modulated (Fig. 1).
Aging effect on A-72 gene expression. Data are expressed as 2−ΔΔCq where ΔCq = Cq (target gene)—Cq (reference gene); values are the mean of three test replicates ± 1 standard deviation and ΔΔCq = ΔCq (109th passage at confluence plus 24 h of incubation at 37 °C)—ΔCq (control 109.th passage at confluence). Negative samples were given a Cq 39 fictitious value. Asterisks indicate significant differences between control and aging cells evaluated by Student's t-test: *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001
Immunocytochemical assay
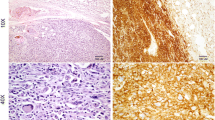
To characterize A-72 cells, immunocytochemical analyses were performed using CXCR4, VIM, CALP, DESM, S100 and CK cellular markers. Cells, at 109th passages, resulted strongly positive for CXCR4, VIM and CALP (Fig. 2a, b and c); moreover, we obtained weak positivity for CD44 and ACTIN (Fig. 2d and e) while DESM, S100 and CK were negative.
Response to infective stressor: interaction between A-72 and ST
The second aim of our study was to investigate the effect A-72 cells exposure to ST. ST showed ability to penetrate A-72 (Log 10 4.14 ± 0.82) causing a significant (P = 0.001) reduction of cell viability (- Log 10 0.2;—12.5% Fig. 3). Moreover, ST’s infection (MOI 100:1 CFU/cells) caused the up-regulation of IL6 (P < 0.000 1), CXCL8 (P < 0.0001) and IL15 (P < 0.0001) expression 1 h post-infection, while MYD88 (P = 0.0242), NFKB/p65 (P = 0.0414), RAD51 (P = 0.0002) and TP53 (P = 0.0012) were down-regulated (Fig. 4). Other genes under study were not significantly modulated.
Response to Infective Stressor. Modulation of Gene expression caused by A-72 and ST interaction. Data are expressed as 2−.ΔΔCq. Negative samples were given a Cq 39 fictitious value. Asterisks indicate significant differences between controls and different passages cells: *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001
Sequencing of the key gene CXCR4 and TP53
The comparison of the sequences obtained by sequencing of CXCR4 and TP53 in A-72 cell line and in canine healthy and neoplastic tissue samples with sequences available in GeneBank highlighted the absence of mutations in the amplified fragments. Moreover, no differences were found in CXCR4 and TP53 sequences obatined by different passages of the cell line.
Discussion
To date, sarcoma biology studies have been conducted in human cell lines and xenograft tumors, and there has been extremely limited progress in the non-surgical or post-surgical treatment options available to patients compared to other cancers (Dodd et al. 2010; Post 2012; Gamboa et al. 2020). In STS, however, there is a limited number of tumor cell lines available for functional testing and target validation. Data from large-scale cancer cell line studies such as the Cancer Cell Line Encyclopedia and Sanger Cancer Cell Line Project showed that < 2% of the commercially available cell lines studied are derived from STS and the majority of these belong to only one of the recognized subgroups (Salawu et al. 2016).
Therefore, animal models are indispensable tools for the study of STS because a scarcity of clinical samples makes difficult a large-scale analysis of human samples, and on the other hand, there is a growing need to establish a wider range of STS cell lines for functional testing (Dodd et al. 2010; Salawu et al. 2016).
A-72 is a continuous cell line isolated in 1980; since then, A-72 has undergone numerous passages in different laboratories in the world (Croci et al. 2011; Pratelli and Moschidou 2012). In this respect, the use of cell lines for research studies requires detailed knowledge of the purity, phenotype, species/tissues of origin, protein and gene basal expression (Geraghty et al. 2014). Therefore, the monitoring of cell lines contamination and their characterization is extremely important in order to achieve accurate conclusions. In our study, we characterized A-72 cell line focusing on the basal expression of genes involved in the innate immune response and in the cell cycle regulation; moreover, we evaluated the effect of cellular passages and monolayer aging on gene expression in order to verify the maintenance of the original phenotype characteristics. The second aim of our study was the evaluation of the A-72 ability to interact with an infectious stressor, whose the attenuated form was already explored as a novel anti-cancer therapy (Chirullo et al. 2015b; Bolhassani et al. 2017). In order to reproduce ordinary laboratory activity, cells at three different passages (104th, 107th and 109th) were used.
Our results showed in A-72 the expression of all tested genes under study with the exception of IL10 and IL18, in agreement with previous studies that reported no expression of those genes in this type of cells (Rutz and Ouyang 2016; Yasuda et al. 2019). However, these interleukins were expressed in the cancerous tissues included in the study; this difference could be attributed to the sample nature, being A-72 a continuous cell line while the tumor tissue is constituted by different cell types (Cancer Associated Fibroblast, T lymphocytes Macrophages e.g.).
The CXCR4 expression tested by RT-qPCR agrees with the strong positivity at the immunocytochemical assay (Table 3 and Fig. 2). The high expression of this protein in A-72 suggests that the cell line originated from an aggressive cancer, indeed CXCR4 is known to be abundantly expressed in STS and is an independent predictor of poor prognosis and metastatic disease (Kim et al. 2011).
CD44 is a glycoprotein involved in cell–cell signalling, migration and adhesion as well as in malignant tumor initiation; moreover, it is involved in several tyrosine kinases activation such as ERBB2 and TGFβ, that have been implicated in key oncogenesis pathways, this marker is also associated with stem cancer cells in human STS (Henderson et al. 2018) and predicts worse oncologic outcomes. In our study, CD44 showed moderate level of gene expression but low protein presence in A-72 (Table 3 and Fig. 2), suggesting the stem potential maintenance of these cells. Other cell cycle regulators were investigated in terms of transcripts including TP53, ERBB2 and PTEN. TP53 plays an important role in the regulation of cell response to stress and damage (Levine 2020); ERBB2 is a member of the epidermal growth factor receptor family, acting as tyrosine kinase receptors and it is considered a potent mediator of cell growth and cancer development (Wolfson et al. 2016); while PTEN is involved in many cellular functions including cell survival, proliferation, migration and adhesion (Yan et al. 2021). Our data showed a low PTEN gene expression in A-72 while ERBB2 presented high expression level (Table 3), in agreement with a previous study conducted on human STS (Trabelsi et al. 2015). HE sample showed no expression of PTEN, this result may be explained by technical and/or biological reasons. The presence of nucleotide variants, in the PTEN sequence of the tissue, may be responsible for a mismatch between primer and template that prevents the detection of PTEN expression. Instead, taking into account a biological explanation, several sarcomas carry genetic abnormalities (deletions and mutations) in well-known tumor suppressor genes (i.e. TP53 and PTEN) which cause deregulation and loss of function of these genes. The loss of PTEN function affects important pathways implicated in cell proliferation, survival, migration, and genomic stability. A recent study on human sarcomas and STSs reported that the loss of PTEN expression is present in 38.6% of tumors and the reduction or loss of PTEN has been reported in a subset of MPNSTs, both in human and animal models (Stefano and Scambia 2019). The A-72 showed a low expression of TP53 which could be due to an altered pathway in a similar way to what has already occurred in humans (Post 2012). Concerning TGFβ and RAD51 a moderate expression was assessed, while for STAT5 a high expression was detected. This is an important finding since TGFβ is a multifunctional cell regulatory cytokine, able to modify proliferation, differentiation, tissue repair, and extracellular matrix formation. Although this protein is usually involved in cell growth suppression, it may play an important role in promoting cancer development (Massagué 2008). The expression of STAT5 could be of clinical importance considering its possible role as target of immunotherapy (Verdeil et al. 2019). RAD51 plays a pivotal regulatory role in meiotic and homologous recombination DNA repair. The over-expression of this gene has been observed in a variety of cancer and has been found associated with chemoresistance in human STS (Brown et al. 2009; Nagaraj et al. 2011; Tennstedt et al. 2013). A TP53 mutation seems to be the cause of RAD51 over-expression (Hannay et al. 2007), nevertheless in our study no TP53 mutations were detected.
Cells showed a fibroblast-like morphology confirmed by the molecular findings and the immunocytochemical results (expression of vimentin and negativity for CK), indicating the cell line mesenchymal origin supposedly referable to a perivascular wall tumor for his positivity to calponin and actin (Pérez et al. 1996; Avallone et al. 2007).
Furthermore, the involvement of cell markers essential for the interaction with bacteria was investigated. Indeed, the basal expression level of TLRs 4 and 5, belonging to the family of Pattern Recognition Receptors (PRRs), as well as LY96 and CD14, expressed by many cell types, in order to recognize bacteria and to induce the innate immune response were investigated (Velloso et al. 2015). The binding of microbial PAMPs to host cell TLRs leads to an inflammatory response, including the secretion of cytokines and chemokines. The expression in all samples of NFKB/p65, one of the major members of the NF-kB protein family, along with NOS2, MYD88, IL6 and CXCL8, implies the ability of this cell line to mount an inflammatory response. In our experiment, the treatment of A-72 with ST determined the up-regulation of some important proinflammatory cytokines and chemokines (IL-6 and CXCL8), and the modulation of transcription factor genes (NF-kB1, NFKB/p65, MYD88) demonstrating the activation of an inflammatory response through the NF-kB pathway (Fig. 4). The expression in A-72 of these molecules is in line with our previous results (Razzuoli et al. 2014, 2017, 2018) showing that the interaction between ST and A-72, as previously reported, induces CXCL8 expression, which allow ST to penetrate into the cells (Gewirtz et al. 2000; Stecher et al.2007; Chirullo et al. 2015a, b).
In this study, we also demonstrated the effect of several cell passages on A-72; this is a very important issue that must be evaluated not only during gene expression studies but also in host/pathogen interaction experiments and evaluation of anticancer therapy evaluation. Indeed, both passages and aging seem to alter the gene expression of several important genes: NFKB/p65, TP53, TLR5 (Table 4 and Fig. 1). In particular, we observed a decrease of CD14, a molecule associated with LY96 and TLR4 in response to Lipopolysaccharides (LPS); suggesting a possible alteration in cell sensitivity to bacteria or LPS (Begni et al. 2005; Amadori & Razzuoli et al. 2014) after 24 h of monolayer achievement.
The variations of gene expression outlined by our results are in agreement with studies on other cell lines that demonstrated changes in basal gene expression related to cell passages (Begni et al. 2005; Amadori & Razzuoli et al. 2014). In our opinion, these variations should be considered when drawing up the experimental design.
Our results demonstrate the validity of A-72 cell line as in vitro animal model for the study of specific subtypes of human STSs (namely malignant peripheral nerve sheath tumor) and support the hypothesis of using primary STS canine spontaneous tumors, which develop in the native microenvironment of an animal with an intact immune system, for the preclinical study of new treatments.
Conclusions
Our data highlighted the maintenance by the A-72 cell line of supposed tumor of origin (STS) phenotypical characteristics and gene expression parameters. In addition, A-72 showed the ability to respond to an infectious stressor such as ST. This characteristic can be exploited using the A-72 as a possible in vitro model for the preliminary assessment of novel anticancer therapeutic approaches founded on the use of the already established attenuated form of these bacteria.
References
Amadori & Razzuoli (2014) Immune Control of PRRS: Lessons to be Learned and Possible Ways Forward - PubMed. https://pubmed.ncbi.nlm.nih.gov/26664910/. Accessed 1 Jun 2022
Avallone G, Helmbold P, Caniatti M et al (2007) The spectrum of canine cutaneous perivascular wall tumors: morphologic, phenotypic and clinical characterization. Vet Pathol 44:607–620. https://doi.org/10.1354/vp.44-5-607
Bajpai J, Susan D (2016) Adjuvant chemotherapy in soft tissue sarcomas…Conflicts, consensus, and controversies. South Asian J Cancer 5:15–19. https://doi.org/10.4103/2278-330X.179687
Begni B, Amadori M, Ritelli M, Podavini D (2005) Effects of IFN-alpha on the inflammatory response of swine leukocytes to bacterial endotoxin. J Interferon Cytokine Res off J Int Soc Interferon Cytokine Res 25:202–208. https://doi.org/10.1089/jir.2005.25.202
Binn LN, Marchwicki RH, Stephenson EH (1980) Establishment of a canine cell line: derivation, characterization, and viral spectrum. Am J Vet Res 41:855–860
Bolhassani A, Naderi N, Soleymani S (2017) Prospects and progress of Listeria-based cancer vaccines. Expert Opin Biol Ther 17:1389–1400. https://doi.org/10.1080/14712598.2017.1366446
Bray JP (2016) Soft tissue sarcoma in the dog – part 1: a current review. J Small Anim Pract 57:510–519. https://doi.org/10.1111/jsap.12556
Bray JP (2017) Soft tissue sarcoma in the dog – Part 2: surgical margins, controversies and a comparative review. J Small Anim Pract 58:63–72. https://doi.org/10.1111/jsap.12629
Bray JP, Polton GA, McSporran KD et al (2014) Canine soft tissue sarcoma managed in first opinion practice: outcome in 350 cases. Vet Surg VS 43:774–782. https://doi.org/10.1111/j.1532-950X.2014.12185.x
Brown ET, Holt JT (2009) Rad51 overexpression rescues radiation resistance in BRCA2-defective cancer cells. Mol Carcinog 48:105–109. https://doi.org/10.1002/mc.20463
Capellini FM, Vencia W, Amadori M et al (2020) Characterization of MDCK cells and evaluation of their ability to respond to infectious and non-infectious stressors. Cytotechnology 72:97–109. https://doi.org/10.1007/s10616-019-00360-z
Cavalcanti AS, Ribeiro-Alves M, de Pereira LOR et al (2015) Parasite load induces progressive spleen architecture breakage and impairs cytokine mRNA expression in Leishmania infantum-naturally infected dogs. PloS One 10:e0123009. https://doi.org/10.1371/journal.pone.0123009
Chirullo B, Ammendola S, Leonardi L et al (2015) Attenuated mutant strain of Salmonella Typhimurium lacking the ZnuABC transporter contrasts tumor growth promoting anti-cancer immune response. Oncotarget 6:17648–17660. https://doi.org/10.18632/oncotarget.3893
Chirullo B, Pesciaroli M, Drumo R et al (2015b) Salmonella Typhimurium exploits inflammation to its own advantage in piglets. Front Microbiol 6:985. https://doi.org/10.3389/fmicb.2015.00985
Choi E-W, Shin I-S, Bhang D-H et al (2006) Hormonal change and cytokine mRNA expression in peripheral blood mononuclear cells during the development of canine autoimmune thyroiditis. Clin Exp Immunol 146:101–108. https://doi.org/10.1111/j.1365-2249.2006.03187.x
Croci S, Bruni L, Bussolati S et al (2011) Potassium bicarbonate and D-ribose effects on A72 canine and HTB-126 human cancer cell line proliferation in vitro. Cancer Cell Int 11:30. https://doi.org/10.1186/1475-2867-11-30
da Costa A, Oliveira JT, Gärtner F et al (1997) (2011) Potential markers for detection of circulating canine mammary tumor cells in the peripheral blood. Vet J Lond Engl 190:165–168. https://doi.org/10.1016/j.tvjl.2010.09.027
de Barros IN, de Silva SOS, Tanikawi SA, Brandão PE (2017) Molecular stability of a vaccine strain of Canine coronavirus after serial passages in A72 cells. Braz J Vet Res Anim Sci 54:48–53. https://doi.org/10.11606/issn.1678-4456.bjvras.2017.111310
Dennis MM, McSporran KD, Bacon NJ et al (2011) Prognostic Factors for Cutaneous and Subcutaneous Soft Tissue Sarcomas in Dogs. Vet Pathol 48:73–84. https://doi.org/10.1177/0300985810388820
Dodd RD, Mito JK, Kirsch DG (2010) Animal models of soft-tissue sarcoma. Dis Model Mech 3:557–566. https://doi.org/10.1242/dmm.005223
Ettinger SN (2003) Principles of treatment for soft-tissue sarcomas in the dog. Clin Tech Small Anim Pract. 18(2):118–22. https://doi.org/10.1053/svms.2003.36628
Fan W, Xu L, Ren L et al (2014) Functional characterization of canine interferon-lambda. J Interferon Cytokine Res off J Int Soc Interferon Cytokine Res 34:848–857. https://doi.org/10.1089/jir.2014.0009
Ferrari RG, Rosario DKA, Cunha-Neto A, Mano SB, Figueiredo EES, Conte-Junior CA (2019) Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: a Meta-analysis. Appl Environ Microbiol. 85(14):e00591-19. https://doi.org/10.1128/AEM.00591-19
Gamboa AC, Gronchi A, Cardona K (2020) Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin 70:200–229. https://doi.org/10.3322/caac.21605
Geraghty RJ, Capes-Davis A, Davis JM, Downward J, Freshney RI, Knezevic I, Lovell-Badge R, Masters JR, Meredith J, Stacey GN, Thraves P, Vias M, Cancer Research UK (2014) Guidelines for the use of cell lines in biomedical research. Br J Cancer 111(6):1021–46. https://doi.org/10.1038/bjc.2014.166
Gewirtz AT, Rao AS, Simon PO Jr, Merlin D, Carnes D, Madara JL (2000) Neish AS (2000) Salmonella typhimurium induces epithelial IL-8 expression via Ca(2+)-mediated activation of the NF-kappaB pathway. J Clin Invest 105(1):79–92. https://doi.org/10.1172/JCI8066
Gustafson DL, Duval DL, Regan DP, Thamm DH (2018) Canine sarcomas as a surrogate for the human disease. Pharmacol Ther 188:80–96. https://doi.org/10.1016/j.pharmthera.2018.01.012
Hager S, Makowiec F, Henne K et al (2017) Significant benefits in survival by the use of surgery combined with radiotherapy for retroperitoneal soft tissue sarcoma. Radiat Oncol 12:29. https://doi.org/10.1186/s13014-017-0769-0
Hannay JAF, Liu J, Zhu Q-S et al (2007) Rad51 overexpression contributes to chemoresistance in human soft tissue sarcoma cells: a role for p53/activator protein 2 transcriptional regulation. Mol Cancer Ther 6:1650–1660. https://doi.org/10.1158/1535-7163.MCT-06-0636
Henderson T, Chen M, Darrow MA et al (2018) Alterations in cancer stem-cell marker CD44 expression predict oncologic outcome in soft-tissue sarcomas. J Surg Res 223:207–214. https://doi.org/10.1016/j.jss.2017.11.016
Ishikawa S, Takemitsu H, Li G et al (2015) Short communication: molecular characterization of dog and cat p65 subunits of NF-kappaB. Res Vet Sci 99:145–148. https://doi.org/10.1016/j.rvsc.2015.02.009
Kaim U, Moritz A, Failing K, Baumgärtner W (2006) The regression of a canine Langerhans cell tumour is associated with increased expression of IL-2, TNF-alpha, IFN-gamma and iNOS mRNA. Immunology 118:472–482. https://doi.org/10.1111/j.1365-2567.2006.02394.x
Kanae Y, Endoh D, Yokota H et al (2006) Expression of the PTEN tumor suppressor gene in malignant mammary gland tumors of dogs. Am J Vet Res 67:127–133. https://doi.org/10.2460/ajvr.67.1.127
Kim RH, Li BDL, Chu QD (2011) The role of chemokine receptor CXCR4 in the biologic behavior of human soft tissue sarcoma. Sarcoma 2011:593708. https://doi.org/10.1155/2011/593708
Klopfleish R et al. (2009) Derlin-1 and Stanniocalcin-1 are Differentially Regulated in Metastasizing Canine Mammary Adenocarcinomas - ScienceDirect. https://www.sciencedirect.com/science/article/abs/pii/S0021997508001187?via%3Dihub. Accessed 1 Jun 2022
Kurata K, Iwata A, Masuda K et al (2004) Identification of CpG oligodeoxynucleotide sequences that induce IFN-gamma production in canine peripheral blood mononuclear cells. Vet Immunol Immunopathol 102(4):441–50. https://doi.org/10.1016/j.vetimm.2004.08.004
Levine AJ. (2020) p53: 800 million years of evolution and 40 years of discovery (2020) Nat Rev Cancer. Aug;20(8):471-480. https://doi.org/10.1038/s41568-020-0262-1
Li YJ, Day YL, Zhang WB et al (2017) Clinicopathological and prognostic significance of chemokine receptor CXCR4 in patients with bone and soft tissue sarcoma: a meta-analysis. Clin Exp Med 17(1):59–69. https://doi.org/10.1007/s10238-015-0405-y
Liu W, Jiang Q, Zhou Y (2018) Advances of systemic treatment for adult soft-tissue sarcoma. Chin Clin Oncol 7(4):42. https://doi.org/10.21037/cco.2018.08.02
Maissen-Villiger CA, Schweighauser A, van Dorland HA et al (2016) Expression Profile of Cytokines and Enzymes mRNA in Blood Leukocytes of Dogs with Leptospirosis and Its Associated Pulmonary Hemorrhage Syndrome. PloS One 11:e0148029. https://doi.org/10.1371/journal.pone.0148029
Martin-Calvo M, Marcotegui MA, Simarro I (1994) Canine-coronavirus (CCV) characterization in Spain. Epidemiological aspects. Zentralblatt Vet Reihe B J Vet Med Ser B 41:249–256. https://doi.org/10.1111/j.1439-0450.1994.tb00225.x
Massagué J (2008) TGFbeta in Cancer. Cell 134:215–230. https://doi.org/10.1016/j.cell.2008.07.001
Milovancev M, Hauck M, Keller C, Stranahan LW, Mansoor A (2015) Malarkey DE (2015) Comparative pathology of canine soft tissue sarcomas: possible models of human non-rhabdomyosarcoma soft tissue sarcomas. J Comp Pathol 152(1):22–27. https://doi.org/10.1016/j.jcpa.2014.09.005
Modesto P, Fernandez JLC, Martini I et al (2020) Characterization of D-17 Canine Osteosarcoma Cell Line and Evaluation of Its Ability to Response to Infective Stressor Used as Alternative Anticancer Therapy. Anim Open Access J MDPI 10:E1981. https://doi.org/10.3390/ani10111981
Nagaraj S, Nagathihalli, Ganesh Nagaraju (2011) RAD51 as a potential biomarker and therapeutic target for pancreatic cancer. Biochimica et Biophysica Acta BBA - Reviews on Cancer 1816(2):209–218. https://doi.org/10.1016/j.bbcan.2011.07.004
Parthiban M, Aarthi KS, Balagangatharathilagar M, Kumanan K (2014) Evidence of feline panleukopenia infection in cats in India. Virusdisease 25:497–499. https://doi.org/10.1007/s13337-014-0231-y
Peeters D, Peters IR, Farnir F et al (2005) Real-time RT-PCR quantification of mRNA encoding cytokines and chemokines in histologically normal canine nasal, bronchial and pulmonary tissue. Vet Immunol Immunopathol 104:195–204. https://doi.org/10.1016/j.vetimm.2004.11.007
Pérez J, Bautista MJ, Rollón E et al (1996) Immunohistochemical characterization of hemangiopericytomas and other spindle cell tumors in the dog. Vet Pathol 33:391–397. https://doi.org/10.1177/030098589603300404
Post SM (2012) Mouse models of sarcomas: critical tools in our understanding of the pathobiology. Clin Sarcoma Res 2:20. https://doi.org/10.1186/2045-3329-2-20
Pratelli A, Moschidou P (2012) Host range of Canine minute virus in cell culture. J Vet Diagn Investig Off Publ Am Assoc Vet Lab Diagn Inc 24:981–985. https://doi.org/10.1177/1040638712453579
Ratan R, Patel SR (2016) Chemotherapy for soft tissue sarcoma. Cancer. 122(19):2952–60. https://doi.org/10.1002/cncr.30191
Razzuoli E, Amadori M, Lazzara F et al (2017) Salmonella serovar-specific interaction with jejunal epithelial cells. Vet Microbiol 207:219–225. https://doi.org/10.1016/j.vetmic.2017.07.002
Razzuoli E, Mignone G, Lazzara F et al (2018) Impact of cadmium exposure on swine enterocytes. Toxicol Lett 287:92–99. https://doi.org/10.1016/j.toxlet.2018.02.005
Razzuoli E, Villa R, Ferrari A, Amadori M (2014) A pig tonsil cell culture model for evaluating oral, low-dose IFN-α treatments. Vet Immunol Immunopathol 160:244–254. https://doi.org/10.1016/j.vetimm.2014.05.011
Rutz S, Ouyang W (2016) Regulation of Interleukin-10 Expression. Adv Exp Med Biol 941:89–116. https://doi.org/10.1007/978-94-024-0921-5_5
Salawu A, Fernando M, Hughes D et al (2016) Establishment and molecular characterisation of seven novel soft-tissue sarcoma cell lines. Br J Cancer 115:1058–1068. https://doi.org/10.1038/bjc.2016.259
Selting KA, Powers BE, Thompson LJ, Mittleman E, Tyler JW, Lafferty MH, Withrow SJ (2005) Outcome of dogs with high-grade soft tissue sarcomas treated with and without adjuvant doxorubicin chemotherapy: 39 cases (1996-2004). J Am Vet Med Assoc. 227(9):1442–8. https://doi.org/10.2460/javma.2005.227.1442
Selvarajah GT, Bonestroo FAS, Timmermans Sprang EPM et al (2017) Reference gene validation for gene expression normalization in canine osteosarcoma: a geNorm algorithm approach. BMC Vet Res 13:354. https://doi.org/10.1186/s12917-017-1281-3
Silva E, Leitão S, Henriques S et al (2010) Gene transcription of TLR2, TLR4, LPS ligands and prostaglandin synthesis enzymes are up-regulated in canine uteri with cystic endometrial hyperplasia-pyometra complex. J Reprod Immunol 84:66–74. https://doi.org/10.1016/j.jri.2009.10.004
Snyder SA, Linder K, Hedan B, Hauck ML (2011) Establishment and characterization of a canine soft tissue sarcoma cell line. Vet Pathol 48:482–485. https://doi.org/10.1177/0300985810383871
Son NV, Uchida K, Thongtharb A et al (2019) Establishment of cell line and in vivo mouse model of canine Langerhans cell histiocytosis. Vet Comp Oncol 17:345–353. https://doi.org/10.1111/vco.12476
Spugnini EP, Vincenzi B, Amadio B, Baldi A (2019) Adjuvant electrochemotherapy with bleomycin and cisplatin combination for canine soft tissue sarcomas: A study of 30 cases. Open Vet J. 9(1):88–93. https://doi.org/10.4314/ovj.v9i1.15
Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, Dougan G, von Mering C, Hardt WD (2007) Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biology 5(10):e244. https://doi.org/10.1371/journal.pbio.0050244
Tennstedt P, Fresow R, Simon R, Marx A, Terracciano L, Petersen C, Sauter G, Dikomey E, Borgmann K (2013) RAD51 overexpression is a negative prognostic marker for colorectal adenocarcinoma. Int J Cancer 132:2118–2126. https://doi.org/10.1002/ijc.27907
Torrigiani F, Pierini A, Lowe R, Simcic P, Lubas G. (2019) Soft tissue sarcoma in dogs: A treatment review and a novel approach using electrochemotherapy in a case series. Vet Comp Oncol.;1–8 https://doi.org/10.1111/vco.12462
Trabelsi S, Manna N, Chourabi M et al. (2015) Meningeal Hemangiopericytomas and Meningomas: a Comparative Immunohistochemical and Genetic Study - PubMed. https://pubmed.ncbi.nlm.nih.gov/26514459/. Accessed 1 Jun 2022
Turchetti AP, da Costa LF, de Romão EL et al (2015) Transcription of innate immunity genes and cytokine secretion by canine macrophages resistant or susceptible to intracellular survival of Leishmania infantum. Vet Immunol Immunopathol 163:67–76. https://doi.org/10.1016/j.vetimm.2014.11.010
Velloso LA (2015) TLR4 at the Crossroads of Nutrients, Gut Microbiota, and Metabolic Inflammation - PubMed. https://pubmed.ncbi.nlm.nih.gov/25811237/. Accessed 1 Jun 2022
Verdeil G, Lawrence T, Schmitt-Verhulst A-M, Auphan-Anezin N (2019) Targeting STAT3 and STAT5 in Tumor-Associated Immune Cells to Improve Immunotherapy. Cancers 11:E1832. https://doi.org/10.3390/cancers11121832
Wolfson E, Goldenberg M, Solomon S et al (2016) Nucleolin-binding by ErbB2 enhances tumorigenicity of ErbB2-positive breast cancer. Oncotarget 7:65320–65334. https://doi.org/10.18632/oncotarget.11323
Yan L, Tsujita K, Fujita Y, Itoh T (2021) PTEN is required for the migration and invasion of Ras-transformed MDCK cells. FEBS Lett 595:1303–1312. https://doi.org/10.1002/1873-3468.14053
Yasuda K, Nakanishi K, Tstsui H (2019) Interleukin-18 in Health and Disease - PubMed. https://pubmed.ncbi.nlm.nih.gov/30717382/. Accessed 1 Jun 2022
Acknowledgements
The authors want to thank Jordi Leonardo Castrillo Fernández for the technical assistance.
Funding
This research was funded by the Italian Ministry of Health, grant number IZS PLV 08/15 and IZS PLV 11/17 RC. The APC was funded by IZS PLV 11/20 RC.
Author information
Authors and Affiliations
Contributions
Elisabetta Razzuoli (E.R). and Paola Modesto (P.M) contributed to the study conception and design; methodology: E.R., Chiara De Ciucis (C.D.C.), Katia Varello (K.V.), Samanta Mecocci (S.M.), Antonio Di Meo (A.D.M.) and P.M; formal analysis performed by C.D.C, Chiara Campanella (C.C.), Isabella Martini (I.M), Barbara Chirullo (B.C.), Paola Petrucci (P.P.), Roberto Zoccola (R.Z.); investigation carried out by C.D.C., K.V.; resources were provided by Michela Tarantino (M.T.), Maria Goria (M.G.), P.M., Elena Bozzetta (E.B.) and E.R.; the first draft of the manuscript was written by B.C., P.M., E:R., C.D.C. and all authors commented on previous versions of the manuscript; project administration by P.M.; funding acquisition by P.M. All authors have read and agreed to the submitted version of the manuscript.
Corresponding authors
Ethics declarations
Institutional review board statement
This study did not require ethical approval.
Conflicts of interest
“The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results”.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Razzuoli, E., Chirullo, B., De Ciucis, C.G. et al. Animal models of Soft Tissue Sarcoma for alternative anticancer therapy studies: characterization of the A-72 Canine Cell Line. Vet Res Commun 47, 1615–1627 (2023). https://doi.org/10.1007/s11259-023-10115-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-023-10115-z