Abstract
In this study, the prophylactic and therapeutic activities of thyme extract at different concentrations against experimental Cryptosporidium parvum infection in immunosuppressed rats were investigated. Thyme extract was prepared at four different concentrations (10%, 30%, 50%, and 100%) and administered as a single oral dose of 1 mL for evaluation of its prophylactic efficacy. Five consecutive days after infection was detected in all rats, therapeutic evaluations were also performed. According to the results obtained by daily counting of oocysts in stools, the prophylactic and therapeutic effects of thyme extract administration were significant in comparison to the control group (P˂0.01). Oocyst shedding continued in the control group at high numbers from the beginning to the end of the study, while oocyst counts in the prophylaxis groups remained low throughout the study. On the other hand, oocyst excretion rates were high in the therapeutic groups and decreased rapidly after thyme extract administration. At the end of the study, oocyst excretion had completely stopped for some rats administered thyme extract. There was no group in which oocyst shedding ceased for all rats. No significant differences were observed in the therapeutic or prophylaxis groups regarding the doses administered (P > 0.01). Renal and hepatic functions were monitored by measuring urea, creatinine, alanine transaminase, and aspartate transaminase levels before and after thyme extract administration. As a result, it was concluded that oral thyme extract administration at the doses applied in this study is effective and safe in the prophylactic and therapeutic treatment of experimental cryptosporidiosis in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryptosporidium parvum may be the most common disease-causing species worldwide due to its extensive host network and zoonotic potential (Chalmers and Katzer 2013). While cryptosporidiosis was initially reported only in immunocompromised individuals, it is now seen as one of the main causes of childhood diarrhoea and it is becoming a serious global public health problem (Sparks et al. 2015). The causative agent is typically transmitted from person to person, but direct animal contact and water sources contaminated with animal faeces are also important transmission routes (Ghenghesh et al. 2012). Although pets have more contact with humans, they are not seen as zoonotic risk factors; on the contrary, direct contact with farm animals and visits to infected farms are considered as risk factors for C. parvum infections (Hunter et al. 2004; Smith et al. 2009). Ruminants have been reported as the main source of C. parvum transmission to humans in most reported outbreaks in the Americas, Europe, and Asia (Xiao et al. 2004; Caccio 2005). The spread of C. parvum, one of the most common causative agents of zoonotic infections, via the faeces of infected calves is particularly important (Fayer et al. 2000; Santin et al. 2004).
Considering that an infected calf can produce 1 × 109 oocysts and only 10 oocysts are required to infect a healthy calf, a single infected calf can produce enough oocysts to infect 100 million calves (Wells and Thomson 2014). Besides their zoonotic importance, Cryptosporidium species are of great importance in cases of neonatal diarrhoea among farm animals and are responsible for 75–95% of neonatal calf diarrhoea cases together with rotavirus, coronavirus, and enterotoxigenic Escherichia coli (Constable et al. 2016). Due to the very high morbidity rates of C. parvum infections on cattle farms, they cause serious economic losses as a result of prevention and treatment costs, growth and development retardation of the animals, and calf losses (Thomson et al. 2017). For the prevention of economic losses, especially in terms of calf deaths and workforce losses, and to contribute to national livestock productivity, controlled experimental studies should be performed with different species that are clinically affected by C. parvum (Şenel 2021).
Although more than 200 active substances have been tried in the treatment of Cryptosporidium spp., a complete parasitological cure has not been achieved (O’Donoghue 1995; Manjunatha et al. 2017) evaluated the in vitro anticryptosporidial activity of 6220 compounds with known antiprotozoal activity including nitazoxanide, an active substance approved by the FDA as an anticryptosporidial for humans (Miyamoto and Eckmann 2015). However, the anticryptosporidial efficacy of nitazoxanide is limited in immunocompromised individuals such as malnourished children and HIV patients (Amadi et al. 2009). There are a few other active substances with moderate anticryptosporidial effects, such as paromomycin, paromomycin/azithromycin combination, and roxithromycin (Gargala 2008). In animals, azithromycin, nitazoxanide, paromomycin, halofuginone lactate, tylosin, and decoquinate are some active substances that may be partially effective (Shahiduzzaman and Daugschies 2012; Yasa Duru et al. 2013; Yagci et al. 2017). Considering the limited efficacy of the agents used for both humans and animals, their failure in eradication, and their toxic effects, there is an urgent need for new, effective, and safe anticryptosporidial agents (Nguyen-Ho-Bao et al. 2022; Su et al. 2022).
Thyme (Thymus vulgaris) has strong antibacterial, antiviral, antifungal, antiparasitic, and antioxidant effects thanks to the thymol and carvacrol that it contains (Burt 2004). Although the antibacterial activity of thyme essential oil has been the subject of studies for many years, more recent studies have also focused on its antiparasitic activity (Burt 2004; Malatyalı et al. 2009; Remmal et al. 2011; Arczewska-Wlosek and Swiatkiewicz 2012; Hafez et al. 2019). The results of in vitro studies of thyme essential oil and carvacrol against C. parvum are particularly promising (Gaur et al. 2018).
This study was undertaken with the aim of systematically investigating the prophylactic and therapeutic efficacy of oral thyme extract at different concentrations against experimental cryptosporidiosis induced by C. parvum in immunosuppressed rats.
Materials and methods
Collection of Cryptosporidium parvum oocysts and preparation for inoculation
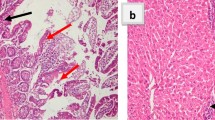
The oocysts used in this study were obtained from the faeces of naturally infected calves aged 5–15 days, which were brought to the Kırıkkale University Faculty of Veterinary Medicine’s Animal Hospital with complaints of acute diarrhoea. The aetiology of diarrhoea was determined using rapid diagnostic kits (Rainbow Calf Scours BIO K 306, BioX, Belgium), and the faeces of calves monoinfected with C. parvum were classified according to their density using the carbol-fuchsin staining method. Only faeces monoinfected with C. parvum and with high oocyst density were used for stock enrichment. The collection, counting, and purification of oocysts were done by modifying the method used by O’Brien and Jenkins (2007); the centrifuge volume was modified proportionally with 50-mL conical centrifuge tubes. A solution of 10 µL from the enriched oocyst stock was dropped onto Thoma slides and all oocysts were counted (Fig. 1). Collected oocysts were stored at 4 °C until inoculation.
Preparation and analysis of thyme extract: The thyme extract was prepared by Ankara University Technopark Company BIOART R&D. The thyme extraction solvent was prepared from glycerine and water at a ratio of 1:3, respectively, and the drug-to-extract ratio was 1:14 (EMA 2021). An Agilent Technologies 1200 Series high-performance liquid chromatography (HPLC) device was used for the analysis of the thyme extract. The HPLC analysis was conducted according to the method of Hajimehdipoor et al. (2010) with minor modifications. The HPLC device and a diode array detector were used and UV absorbances were measured at 274 nm for both thymol and carvacrol. The column used was an ACE C18 (4.6 × 250 mm, 5 μm). The mobile phase was an isocratic combination of acetonitrile and H2O at a ratio of 50:50 with a flow rate of 1 mL/min. The injection volume for all samples and the standard solutions was 5 µL. The components of the thyme extract used in this research are given in Table 1.
Animal material
Sixty-six female Sprague Dawley rats with age of 8–10 weeks and live weight of 230–260 g were used. The animals were divided into the 3 main groups of prophylaxis, treatment, and control. The prophylaxis and treatment groups were further divided into 4 sub-groups and administered 10%, 30%, 50%, or 100% thyme extract. The control group contained 10 rats while all experimental sub-groups had 7 rats (Table 2).
Rats were provided grain-based standard pellet chow and filtered tap water ad libitum. Before starting the experiment, the rats were kept in the laboratory for 14 days in order to ensure their adaptation to the environment and the study population. Stool samples were collected from each animal and checked for Cryptosporidium spp. and other gastrointestinal parasites before the experiment began, and all stool samples were negative.
Experimental infection and administration of thyme extracts
An immunosuppression protocol was applied to all rats before inoculation of the C. parvum oocysts. For this purpose, 0.25 mg/kg dexamethasone (Dexacure®, Provet Veterinary Products San. Tic. A.Ş., Turkey) was added to the drinking water of the rats for 7 days before the oocyst inoculation and the administration was continued until the end of the study (Rehg et al. 1988). All rats were inoculated intragastrically with 1 × 105 oocysts in 100 µL of inoculate on the 9th day after immunosuppression using a 16-gauge, 76-mm ball-tipped stainless steel oral gavage. The detailed working protocol is illustrated in Fig. 2.
To examine the prophylactic and therapeutic effects of oral thyme extract in cases of C. parvum infections, four different concentrations of the utilized thyme extract were applied to prophylaxis and treatment sub-groups such that 1 mL of thyme extract was administered orally once a day at concentrations of 10%, 30%, 50%, and 100%, representing four different doses.
The rats in the prophylaxis group were given thyme extract at the indicated concentrations as a single administration 2 h before C. parvum oocyst inoculation. After inoculation, thyme extract was administered to the treatment groups at the concentrations indicated for 5 consecutive days, starting from the 9th day after inoculation, which was the first day when infection was detected in all groups. In the control group, thyme was not administered before or after the oocyst inoculation, but a placebo (filtered tap water) was administered during the treatment period.
Infection follow-up (oocyst count) and blood analysis
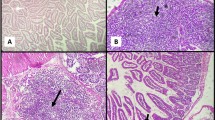
From the 4th day after the oocyst inoculation until the 16th day, when the study was terminated, daily stool samples were collected and oocyst counts were performed with the carbol-fuchsin staining method (Heine 1982) (Fig. 3). For this purpose, using a pipette, 50 µL of mixed and homogenized stool sample was placed onto a slide that had been previously cleaned with an ether-alcohol mixture and degreased, and the same amount of carbol-fuchsin stain was added to the solution. A fine stool smear was prepared using the edge of the coverslip. Immediately after the prepared smear was air-dried, immersion oil was dropped onto the slide and C. parvum oocysts were examined under the microscope at 100× magnification. Oocyst counts were performed by scanning 20 random microscope fields at 100× magnification (Sungur et al. 2008).
To evaluate renal and hepatic functions, blood samples were taken twice (before thyme extract administration and on the last day of the study) from the control and experimental groups. Urea and creatinine levels were measured to evaluate renal functions, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured to evaluate hepatic functions. The blood samples were collected from the lateral tail vein. Samples were centrifuged at 2000 × g for 10 min and the serum was separated, and then measurements were made using a Multiskan GO microplate spectrophotometer (Thermo Scientific, USA).
Statistical analysis
All data were examined for parametric test assumptions with the Shapiro-Wilk test for normality and the Levene test for homogeneity of variances before performing statistical analysis. Descriptive statistics were presented as arithmetic mean ± standard error of the mean (SEM). The effects of group, time, and their interactions were analysed using a mixed model for repeated measures. In this model, animals were included as random effects and group, time, and their interactions were included as fixed effects. Bonferroni-corrected simple effects analysis was applied for cases where the interaction term was found to be significant. The statistical significance level was set at P < 0.05. All analyses were performed using Stata 12/MP4 statistical software.
Results
Clinical examination
It was observed that the rats were clinically healthy during the quarantine period. Live weights of the rats decreased during the immunosuppression period; body condition scores were low during immunosuppression; the fur structure deteriorated, becoming dull and disordered; and the movements of the rats decreased. No deaths, drug toxicity symptoms, or changes in clinical findings were observed in any of the groups during or after thyme administration.
Blood parameters
Statistical evaluations of ALT, AST, urea, and creatinine values measured from serum samples collected before and after treatment for all groups are presented in Table 3.
Statistically, the difference between pre- and post-treatment ALT and AST values in the control group (C) and all treatment groups (T1, T2, T3, T4) was significant (P˂0.05) (Table 3). However, the differences between the treatment sub-groups that received thyme at four different doses and the control group not treated with thyme were found to be insignificant. This result showed that the increase in ALT and AST was due to the dexamethasone used for immunosuppression. It was determined that the ALT and AST values, which were above the normal physiological ranges in all groups before thyme administration, did not show additional increases after thyme administration, and the changes in the values of these treatment groups were parallel to those of the control group.
Urea and creatinine values were found to be within normal physiological ranges in the treatment groups (T1, T2, T3, T4) and the control group. The differences between the urea and creatinine values of the treatment groups and the control group before and after treatment and also those between the treatment groups were statistically insignificant (P > 0.05). Among all groups, interactions among the evaluated variables were not found to be statistically significant (P > 0.05) (Table 3).
Daily variation of oocyst numbers
After oocyst inoculation, oocyst examinations were performed daily for all rats using carbol-fuchsin staining. On the 8th day after inoculation, all rats were confirmed to be infected with C. parvum, excreting oocysts, and oocyst counts were performed on the treatment day (9th day after inoculation). Daily oocyst counts were performed for all rats for 8 days in total, including 5 days of treatment and 3 days of control after the treatment period. Comparative oocyst counts are given in Tables 4 and 5.
Upon analysis of the prophylaxis and control groups, the interactions of time and group × time were not statistically significant. The differences between the prophylaxis groups (P1, P2, P3, P4) and the control group were statistically significant (P < 0.05) (Table 4). This showed that administration of thyme extract for prophylaxis at four different doses had a protective effect (Table 4). The number of oocysts excreted in all sub-groups in which different doses of thyme were administered for prophylaxis decreased gradually within days and remained at low levels compared to the control group. In the control group, oocyst counts remained quite high. Although the number of oocysts decreased in the P1 sub-group compared to the pre-treatment values, there was no complete elimination. The number of oocysts in the faeces of 5 out of 21 rats in the P2, P3, and P4 sub-groups was zero.
As a result of stool examination, differences in oocyst numbers between the treatment and control groups and the interactions of group, time, and group × time were found to be significant (P < 0.05) (Table 5). When the oocyst values were examined, the number of counted oocysts was 0 for 3 rats in the T1 and T2 sub-groups, and 1 or 2 oocysts could be counted in 20 fields for the remaining 4 rats in each group. The number of oocysts was 0 for 4 rats in the T3 and T4 sub-groups, and 1 oocyst was counted for each of the remaining rats in that sub-group.
The number of counted oocysts was 0 for 3 rats in the T1 and T2 sub-groups and 4 rats each in the T3 and T4 sub-groups, and 1 oocyst was detected in the stool examinations of the remaining rats. During the infection period, the number of oocysts in the stools was consistently high, and at the end of the study, 8–20 oocysts (average: 12.05) were detected per rat in the control group.
Discussion
Cryptosporidiosis is an infection that requires the simultaneous administration of aetiological treatment in addition to symptomatic treatment (Göbel 1987). Despite the fact that more than 200 active substances have been tried in the treatment of Cryptosporidium spp., researchers are now focusing on new and alternative treatments because those currently administered cannot completely eliminate the infection and have various toxic effects (Dinler and Ulutas 2017).
Research on natural products that can be used as food supplements alternatively to chemical substances in the fight against Cryptosporidium spp. has become very popular in recent years. Some of the phytotherapeutics were used against cryptosporidiosis; Gingerbread (Artemisia spicigera), pine bark (Pinus maritima), olive (Oliva europaea), mango leaf (Magnifera indica), pomegranate peel (Punica grabatum), garlic (Allium sativum) and curcumin (Kim and Healey 2001; Perrucci et al. 2006; Al-Mathal and Alsalem 2012; Gaafar 2012; Khater et al. 2017; Asadpour et al. 2018; Shahbazi et al. 2021).
Thyme has been fed to animals directly and its essential oil or extracts have been used in many experimental and clinical parasitological studies (Behnia et al. 2008; Malatyalı et al. 2009; Amin and El-Kabany 2013; Attia et al. 2015; Amin et al. 2016; Luis et al. 2016; Hafez et al. 2019). Thyme has good anticoccidial properties (Remmal et al. 2011; Abbas et al. 2012; Arczewska-Wlosek and Swiatkiewicz 2012).
Considering the possible effects of thymol and carvacrol on C. parvum, Gaur et al. (2018) examined the effects of thyme extract on parasites in vitro and reported successful results. They emphasized that their experiments with the HCT-8 cell culture medium were a good alternative for animal models, but converting the obtained data into an experimental animal model and then controlled clinical studies are necessary next steps (Gaur et al. 2018). It was also reported that bioactive substances such as thymol and carvacrol in thyme can be used to reduce the shedding and infectivity of Cryptosporidium parasites by suppressing their growth, development, and invasion characteristics as a result of affecting Ca+-mediated signal transmission, cytoplasmic metabolic activities such as ATP synthesis, and some enzymatic activities (Santoro et al. 2007; Murphy et al. 2010; Bessoff et al. 2013).
The experimental C. parvum infection model used in the present study was previously described in the literature for rats (Rehg et al. 1988). In order to evaluate its prophylactic and therapeutic efficacy, thyme extract was given to each main group at four different doses and the results were compared with those of the control group. At the end of the study, it was observed that the number of oocysts was quite low in rats of the prophylaxis group, oocyst shedding had decreased rapidly in the treatment group, and the oocyst counts were 0 in 14 of 28 rats in the treatment group. Although there was a decrease in oocyst shedding in the rats of the prophylaxis group administered 10% thyme extract, none of these rats had oocyst counts of 0. For the other considered doses (30%, 50%, 100%), the oocyst count was 0 for 5 rats, with a decrease in the oocyst counts of all other rats in these groups. For this reason, it was concluded that it would be more appropriate to use 30%, 50%, and 100% extracts of thyme for prophylaxis.
While no oocysts were observed in scans of 20 microscopic fields for 3 rats in the 10% and 30% treatment sub-groups, only 1 oocyst was counted in 20 fields for the other 4 rats in these sub-groups. In the 50% and 100% treatment sub-groups, no oocysts were observed in the stools of 4 of 7 rats based upon scans of 20 microscopic fields, while 1 oocyst was observed for each of the remaining 3 rats. Based on this result, it was concluded that 50% and 100% thyme concentrations were more effective than 10% and 30% concentrations, although all thyme concentrations considered in this study were effective in the treatment of C. parvum with no statistical differences.
At the end of the study, oocyst counts were quite high (8–20 oocysts) in rats of the control group compared to the prophylaxis and treatment groups. The difference between the oocyst counts of the prophylaxis and treatment groups and the control group was statistically significant (Tables 4 and 5). All of these findings revealed that thyme can be used for prophylactic and therapeutic purposes against C. parvum. The results obtained by Gaur et al. (2018), who investigated the efficacy of thyme extract against C. parvum in vitro, are compatible with the findings of the present work.
In this study, the prophylaxis and treatment efficacy rates of thyme extract at different concentrations were compared with those of a control (placebo) group. Different animal study models applied by different researchers have been shared in the literature. The efficacies of paromomycin (Verdon et al. 1995), halofuginone lactate (Rehg 1995), and lasalocid sodium (Castro Hermida et al. 2000) in farm animals against C. parvum were confirmed in similar previous studies. Verdon et al. (1995) observed higher oocyst shedding using paromomycin than the rate found in our study. While an oocyst shedding rate of 0 was not achieved in the rats of the previously mentioned paromomycin study, oocyst shedding was completely eliminated in 50% of the rats to which we administered thyme treatment in our study. In contrast to the results reported by Verdon et al. (1995), the number of oocysts was not increased after treatment was ceased in our study. Thyme was evaluated in terms of both therapeutic and prophylactic activities against C. parvum in the study reported by Rehg (1995). In our study, the prophylactic efficacy of thyme extract administered as a single dose was more successful than the prophylactic efficacy of halofuginone lactate applied for 21 days as summarized in the literature, and the therapeutic efficacy rates were similar. However, in contrast to 11 days of halofuginone lactate administration, 5 days of thyme administration was more practical of a treatment and reduced the average detected oocyst numbers to lower values. Castro Hermida et al. (2000) evaluated the prophylactic efficacy of lasalocid sodium against C. parvum in weanling mice and reported that this agent completely prevented oocyst uptake into the intestines. Oocyst excretion was limited in rats to which we administered a single dose of thyme before inoculation. In terms of prophylactic effectiveness, it can be said that lasalocid sodium is more effective than thyme. However, serious toxic effects of lasalocid sodium have been reported in various studies (Pongs 1989; Luginbühl and Pfister 1996). For this reason, its use in the European Union is prohibited (Şahal et al. 2005). No toxic effects of thyme were reported in our experimental study with rats or in pre-clinical studies that administered thyme to research animals, and the dose of thyme required to cause acute toxicity is much higher than the amount that can be consumed (Gwynn 2014; HSDB 2015; Baker and Grant 2018).
In the present study, the rats’ body condition scores were low and the fur had an unhealthy appearance before and after thyme administration. In healthy rats during quarantine, these changes began with immunosuppression and continued due to the ongoing use of dexamethasone until the study was terminated. This finding is in line with the results reported by Malkawi et al. (2018).
To evaluate the effect of thyme administration at different doses on renal functions in the treatment groups, urea and creatinine values were measured before and after treatment and were found to be within normal physiological ranges with statistically insignificant differences (Giknis and Clifford 2006; He et al. 2017). Based on these findings, it was concluded that different doses of thyme administration do not have negative effects on renal functions.
AST and ALT values measured before and after treatment were significantly higher than the reference values. High values were also reported by Jackson et al. (2008), who found that liver values in rats administered dexamethasone could increase up to 10 times above the reference values. The fact that the values were found to be similarly high in the control group could indicate that the high values were related not only to the use of dexamethasone but also the use of thyme. However, the changes in AST and ALT levels in all groups were found to be statistically insignificant, leading to the conclusion that thyme administration at the doses used in this study does not cause hepatocellular damage in rats.
Conclusion
In this study, it was found that thyme extract was effective in treating experimental cryptosporidiosis in rats both prophylactically and therapeutically. For prophylaxis, thyme extract applied in a single dose at different concentrations successfully limited the severity of cryptosporidiosis when compared to the control group (P˂0.01). However, we suggest that prophylactic efficacy be demonstrated more strongly with futures studies conducted over different time intervals. Oral administration of thyme extract for 5 days was also found to be therapeutically effective in rapidly reducing the high levels of oocyst shedding and it completely eliminated shedding for some of the rats (P˂0.01). Furthermore, no signs of toxicity were observed during this study and serum biochemical changes were not significant in comparison to the control group for the measured values of the parameters considered here (P > 0.01). In summary, these data support recent in vitro studies that have suggested thyme to be effective against cryptosporidiosis, indicating that further detailed evaluations of this agent are necessary. Such research will be useful for revealing the therapeutic and prophylactic effects of experimental infection, especially in farm animals of economic importance.
Contributions
EK took an active role in the design of the study and the implementation of the animal experiments; SYD took an active role in the design of the study; SG performed oocyst enrichment and daily oocyst counting; ÖD performed biochemical analyses of blood sera; SS carried out the preparation and content analysis of thyme extract; YŞ took an active role in the design of the study and the implementation of the animal experiments; UK performed the statistical analysis. All authors contributed to the interpretation of the data obtained from the study, the writing of the manuscript, and final approval of the manuscript.
Data Availability
All data generated or analysed during this study are included in this published article (and supplementary information files).
References
Abbas RZ, Iqbal Z, Khan A, Sindhu ZUD, Khan JA, Khan MN, Raza A (2012) Options for Integrated Strategies for the Control of Avian Coccidiosis. Int J Agric Biol 14:1014–1020
Al-Mathal EM, Alsalem AM (2012) Pomegranate (Punica granatum) peel is effective in a murine model of experimental Cryptosporidium parvum. Exp Parasitol 131(3):350–357. https://doi.org/10.1016/j.exppara.2012.04.021
Amadi B, Mwiya M, Sianongo S, Payne L, Watuka A, Katubulushi M, Kelly P (2009) High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: a randomised controlled trial. BMC Infect Dis 9: 195 BMC. https://doi.org/10.1186/1471-2334-9-195
Amin MM, El-Kabany H (2013) Evaluation of protective and treatment of Thyme (Thymus vulgaris) oil on Toxocara vitulorum infected rats. J Rad Res Appl Sci 6(1):209–232
Amin MM, Hafez EN, Abd Raboo MA (2016) Assesment of Radiation-Attenuated Vaccine or Thyme Oil Treatment on Controlling DNA Damage and Nitric Oxide Synthesis in Brain of Rat Infected with Toxocara canis. Arab J Nucl Sci Appl 49(2):199–210
Arczewska-Wlosek A, Swiatkiewicz S (2012) The effect of a diatary herbal extract bled on the performance of broilers challenged with Eimeria oocysts. J Anim Feed Sci 21:133–142. https://doi.org/10.22358/jafs/66058/2012
Asadpour M, Namazi F, Razavi SM, Nazifi S (2018) Comparative efficacy of curcumin and paromomycin against Cryptosporidium parvum infection in a BALB/c model. Vet Parasitol 250:7–14. https://doi.org/10.1016/j.vetpar.2017.12.008
Attia RAH, Mahmoud AE, Farrag HMM, Makboul R, Mohammed ME, Ibraheim Z (2015) Effect of myrrh and thyme on Trichinella spiralis enteral and paranteral phases with inducible nitric oxide expression in mice. Mem Inst Oswaldo Cruz Rio de Janeiro 110(8):1035–1041. https://doi.org/10.1590/0074-02760150295
Baker BP, Grant JA (2018) Thyme & Thyme Oil Profile. https://hdl.handle.net/1813/56143 Accessed 3 August 2022
Behnia M, Haghighi A, Komeylizadeh H, Tabaei SJS, Abadi A (2008) Inhibitory Effects of Iranian Thymus vulgaris Extracs on in Vitro Growth of Entamoeba histolytica. Korean J Parasitol 46(3):153–156. https://doi.org/10.3347/kjp.2008.46.3.153
Bessoff K, Sateriale A, Lee KK, Huston CD (2013) Drug repurposing screen reveals FDAapproved inhibitors of human HMG-CoA reductase and isoprenoid synthesis that block Cryptosporidium parvum growth. Antimicrob Agents Chemother 57:1804–1814
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022
Caccio SM (2005) Molecular epidemiology of human cryptosporidiosis. Parassitologia 47(2):185–192
Castro Hermida JA, Freir Santos F, Oteiza López AM, Vergara Castiblanco CA, Ares-Mazás ME (2000) In vitro and in vivo efficacy of lasalocid for treatment of experimental cryptosporidiosis. Vet Parasitol 90(4):265–270. https://doi.org/10.1016/S0304-4017(00)00243-0
Chalmers RM, Katzer F (2013) Looking for Cryptosporidium: the application of advances in detection and diagnosis. Trends Parasitol 29:237–251. https://doi.org/10.1016/j.pt.2013.03.001
Constable P, Hinchcliff K, Done S, Gruenberg W (2016) Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. Elsevier, Amsterdam
Dinler C, Ulutas B (2017) Cryptosporidiosis in ruminants: update and current therapeutic approaches. Am J Anim Vet Sci 12(3):96–103. https://doi.org/10.3844/ajavsp.2017.96.103
Ema (2021) Guideline on the quality of combination herbal medicinal products/traditional herbal medicinal products (EMEA/HMPC/CHMP/CVMP/214869/2006). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003286.pdf Accessed 31 August 2021
Fayer R, Morgan U, Upton SJ (2000) Epidemiology of Cryptosporidium: transmission, detection and identification. Int J Parasitol 30:1305–1322. https://doi.org/10.1016/S0020-7519(00)00135-1
Gaafar MR (2012) Efficacy of Allium sativum (garlic) against experimental cryptosporidiosis. Alexandria J Med 48:59–66. https://doi.org/10.1016/j.ajme.2011.12.003
Gargala G (2008) Drug treatment and novel drug target against Cryptosporidium. Parasite 15:275–281. https://doi.org/10.1051/parasite/2008153275
Gaur S, Kuhlenschmidt TB, Kuhlenschmidt MS, Andrade JE (2018) Effect of oregano essential oil and carvacrol on Cryptosporidium parvum infectivity in HCT-8 cells. Parasitol Int 67:170–175. https://doi.org/10.1016/j.parint.2017.11.001
Ghenghesh KS, Ghanghish K, El-Mohammady H, Franka E (2012) Cryptosporidium in countries of the Arab world: the past decade (2002–2011). Libyan J Med 7. https://doi.org/10.3402/ljm.v7i0.19852
Giknis MLA, Clifford CB (2006) Clinical laboratory parameters for Crl: CD (SD) rats. Charles River Lab 1–14. https://www.criver.com/sites/default/files/resources/rm_rm_r_clinical_parameters_cd_rat_06.pdf Accessed 14 June 2022
Göbel E (1987) Diagnose und therapie der akuten kryptosporidiose beim kalb. Tierärztl Umsch 42:863–869
Gwynn RL (2014) Manual of Biocontrol Agents, 5th edn. British Crop Protection Council, Alton
Hafez EN, Hafez MN, Amin MM (2019) Effect of vaccination with irradiated Toxocara canis larvae or thyme oil treatment on testicular histochemical and immunohistochemical changes of rats. Trop Biomed 36(2):430–442
Hajimehdipoor H, Shekarchi M, Khanavi M, Adib N, Amri M (2010) A validated highperformance liquid chromatography method for the analysis of thymol and carvacrol in Thymus vulgaris L. volatile oil. Pharmacognosy Mag 6(23):154. https://doi.org/10.4103/0973-1296.66927
He Q, Su G, Liu K, Zhang F, Jiang Y, Gao J, Liu L, Jiang Z, Jin M, Xie H (2017) Sex-specific reference intervals of hematologic and biochemical analytes in Sprague-Dawley rats using the nonparametric rank percentile method. PLoS ONE 12:e0189837. https://doi.org/10.1371/journal.pone.0189837
Heine J (1982) Eine einfache nachweismethode für kryptosporidien im kot. Zentralblatt für Veterinärmedizin R B 29:324–327. https://doi.org/10.1111/j.1439-0450.1982.tb01233.x
HSDB (2015) National Library of Medicine Hazardous Substances Data Bank (HSDB). http://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
Hunter PR, Hughes S, Woodhouse S, Syed Q, Verlander NQ, Chalmers RM, Morgan K, Nichols G, Beeching N, Osborn K(2004) Sporadic cryptosporidiosis case-control study with genotyping. Emerg Infect Dis 10:1241–1249. https://doi.org/10.3201/eid1007.030582
Jackson ER, Kilroy C, Joslin DL, Schomaker SJ, Pruimboom-Brees I, Amacher DE (2008) The early effects of short-term dexamethasone administration on hepatic and serum alanine aminotransferase in the rat. Drug Chem Toxicol 31:427–445. https://doi.org/10.1080/01480540802390247
Khater MM, El-Sayed SH, Yousof HAS, Mahmoud SS, El-Dib N, El-Badry AA(2017) Anti-Cryptosporidium efficacy of Olea europaea and Actinidia deliciosa in a neonatal mouse model. Kasr Al Ainy Med J 23:32–37 https://doi.org/10.4103/1687-4625.207190
Kim CH, Healey JM (2001) Effects of pine bark extract administered to immunosuppressed adult mice infected with Cryptosporidium parvum. Am J Chin Med 29(3–4):469–475. https://doi.org/10.1142/S0192415X01000484
Luis E, Ferreira BI, Benincasa AL, Fachin SC, França SSHT, Contini ACS, Chagas ROB (2016) Thymus vulgaris L. essential oil and its main component thymol: Anthelmintic effects against Haemonchus contortus from sheep. Vet Parasitol 228:70–76. https://doi.org/10.1016/j.vetpar.2016.08.011
Luginbühl A, Pfister K (1996) Die kryptosporidiose des kalbes als schwerwiegendes bestandesproblem. Schweiz Arch Tierheilkd 138:195–200. https://doi.org/10.5169/seals-590794
Malatyalı E, Özçelik S, Gürsoy N (2009) Kekik (Thymus vulgaris), Kimyon (Cuminum cyminum) ve Mersin (Myrtus communis) Bitkilerinden Elde Edilen Yağların İn Vitro Antileishmanial Etkileri. Turk Hij Den Biyol Dergi 66(1):7–13
Malkawi AK, Alzoubi KH, Jacob M, Matic G, Ali A, Al Faraj A, Almuhanna F, Dasouki M, Rahman AMA (2018) Metabolomics based profiling of dexamethasone side effects in rats. Front Pharmacol 9:46. https://doi.org/10.3389/fphar.2018.00046
Manjunatha UH, Vinayak S, Zambriski JA, Chao AT, Sy T, Noble CG, Bonamy GMC, Kondreddi RR, Zou B, Gedeck P, Brooks CF, Herbert GT, Sateriale A, Tandel J, Noh S, Lakshminarayana SB, Lim SH, Goodman LB, Bodenreider C, Feng G, Zhang L, Blasco F, Wagner J, Leong FJ, Striepen B, Diagana TT (2017) A Cryptosporidium PI (4) K inhibitor is a drug candidate for cryptosporidiosis. Nature 546:376–380. https://doi.org/10.1038/nature22337
Miyamoto Y, Eckmann L (2015) Drug development against the major diarrhea-causing parasites of the small intestine. Cryptosporidium and Giardia Front Microbiol 6:1208. https://doi.org/10.3389/fmicb.2015.01208
Murphy RC, Ojo KK, Larson ET, Castellanos-Gonzalez A, Perera BGK, Keyloun KR, Kim JE, Bhandari JG, Muller NR, Verlinde CLMJ, White AC, Merritt EA, Van Voorhis WC, Maly DJ (2010) Discovery of potent and selective inhibitors of CDPK1 from C. parvum and T. gondii. ACS Med Chem Lett 1:331–335. https://doi.org/10.1021/ml100096t
Nguyen-Ho-Bao T, Ambe LA, Berberich M, Hermosilla C, Taubert A, Daugschies A, Kamena F (2022) Octaarginine Improves the Efficacy of Nitazoxanide against Cryptosporidium parvum. Pathogens 11:653. https://doi.org/10.3390/pathogens11060653
O’Brien CN, Jenkins MC (2007) A rapid method for producing highly purified Cryptosporidium parvum oocysts. J Parasitol 93:434–436. https://doi.org/10.1645/GE-1020R.1
O’Donoghue PJ (1995) Cryposporidium and Cryptosporidiosis in man and animals. Int J Parasitol 25:139–195. https://doi.org/10.1016/0020-7519(94)E0059-V
Perrucci S, Fichi G, Buggiani C, Rossi G, Flamini G(2006) Efficacy of mangiferin against Cryptosporidium parvum in a neonatal mouse model. Parasitol Res 99(2):184-8. https://doi.org/10.1007/s00436-006-0165-4
Pongs P (1989) Kryptosporidien-infektion beim kalb. Behandlungsversuch mit lasalocid-na unter praxisbedingungen. Tieraerztl Umsch 44:100–101
Rehg JE, Hancock ML, Woodmansee DB (1988) Characterization of a dexamethasone-treated rat model of cryptosporidial infection. J Infect Dis 158(6):1406–1407. https://doi.org/10.1093/infdis/158.6.1406
Rehg JE (1995) The activity of halofuginone in immunosuppressed rats infected with Cryptosporidium parvum. J Antimicrob Chemother 35:391–397. https://doi.org/10.1093/jac/35.3.391
Remmal A, Achahbar S, Bouddine L, Chami N, Chami F (2011) In vitro destruction of Eimeria oocysts by essential oils. Vet Parasitol 182:121–126. https://doi.org/10.1016/j.vetpar.2011.06.002
Santin M, Trout JM, Xiao L, Zhou L, Greiner E, Fayer R (2004) Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet Parasitol 122:103–117. https://doi.org/10.1016/j.vetpar.2004.03.020
Santoro GF, das Graças Cardoso M, Guimarães LGL, Salgado APSP, Menna-Barreto RFS, Soares MJ(2007) Effect of oregano (Origanum vulgare L.) and thyme (Thymus vulgaris L.) essential oils on Trypanosoma cruzi (Protozoa: Kinetoplastida) growth and ultrastructure. Parasitol Res 100:783–790. https://doi.org/10.1007/s00436-006-0326-5
Shahbazi P, Nematollahi A, Arshadi S, Farhang HH, Shahbazfar AA (2021) The Protective Effect of Artemisia spicigera Ethanolic Extract against Cryptosporidium parvum Infection in Immunosuppressed Mice. Iran J Parasitol 16(2):279–288. https://doi.org/10.18502/ijpa.v16i2.6318
Shahiduzzaman M, Daugschies A (2012) Therapy and prevention of cryptosporidiosis in animals. Vet Parasitol 188:203–214. https://doi.org/10.1016/j.vetpar.2012.03.052
Smith RP, Chalmers RM, Elwin K, Clifton-Hadley FA, Mueller Doblies D, Watkins J, Paiba GA, Giles M (2009) Investigation of the role of companion animals in the zoonotic transmission of cryptosporidiosis. Zoonoses Public Health 56:24–33. https://doi.org/10.1111/j.1863-2378.2008.01178.x
Sparks H, Nair G, Castellanos-Gonzalez A, White ACJ(2015) Treatment of Cryptosporidium: What We Know, Gaps, and the Way Forward. Curr Trop Med Reports 2:181–187 https://doi.10.1007/s40475-015-0056-9
Su J, Shen Y, Li N, Li Y, Zhang Z, Xiao L, Guo Y, Feng Y (2022) Comparative Characterization of CpCDPK1 and CpCDPK9, Two Potential Drug Targets against Cryptosporidiosis. Microorganisms 10:333. https://doi.org/10.3390/microoorganisms10020333
Sungur T, Kar S, Güven E, Aktaş M, Karaer Z, Vatansever Z (2008) Cryptosporidium spp’nin Dışkıdan Nested PCR ve Carbol Fuchsin Boyama Yöntemi ile Teşhis Edilmesi. Türkiye Parazitol Dergi 32(4):305–308
Şahal M, Karaer Z, Yasa Duru S, Çizmeci S, Tanyel B (2005) Cryptosporidien-Infektion bei neugeborenen Kälbern aus der Umgebung von Ankara: Klinische und hämatologische Untersuchungen sowie Behandlung mit Lasalocid-Na. Dtsch tierärztl Wschr 112:201–240
Şenel Y(2021) Cryptosporidium parvum ile deneysel enfekte sıçanlarda oral heparin uygulamasının terapötik etkinliğinin lasalosid sodyum ve halofuginon laktat ile karşılaştırmalı değerlendirilmesi. Doktora Tezi, Ankara Üniversitesi Sağlık Bilimleri Enstitüsü İç Hastalıkları (Veteriner) Anabilim Dalı, Dissertation Ankara
Thomson S, Hamilton CA, Hope JC, Katzer F, Mabbott NA, Morrison LJ, Innes EA(2017) Bovine cryptosporidiosis: impact, host-parasite interaction and control strategies. Vet Res 48:42. https://doi.org/10.1186/s13567-017-0447-0
Verdon R, Polianski J, Gaudebout C, Marche C, Garry L, Carbon C, Pocidalo JJ (1995) Evaluation of High-Dose Regimen of Paromomycin against Cryptosporidiosis in the Dexamethasone- Treated Rat Model. Antimicrob Agent Chemother 39(9):2155–2157. https://doi.org/10.1128/AAC.39.9.2155
Wells B, Thomson S(2014) Cryptosporidiosis in cattle. Moredun Foundation, UK. News Sheet Vol. 6, No. 1. https://www.moredun.org.uk/sites/default/files/member-docs/pdf/Mfns%206.1.pdf Accessed 29 December 2021
Xiao L, Fayer R, Ryan U, Upton SJ (2004) Cryptosporidium taxonomy: recent advances and ımplications for public health. Clin Microbiol Rev 17:72–97. https://doi.org/10.1128/CMR.17.1.72-97.2004
Yagci B, Ocal N, Yasa Duru S, Akyol M(2017) The efficacy of a combination of azithromycin and toltrazuril for the treatment of calves naturally infected with cryptosporidiosis: A randomised, double-blind, placebo-controlled comparative clinical trial. Veterinární Medicína 62:308–314. https://doi.org/10.17221/125/2015-VETMED
Yasa Duru S, Öcal N, Yağci B, Gazyağcı S, Duru Ö, Yıldız K(2013) Die therapheutische wirksamkeit von tylosin bei der kälberkryptosporidiose. Kafkas Univ Vet Fak Derg 19:175–180. https://doi.org/10.9775/kvfd.2013.8671
Acknowledgements
We would like to thank Ankara University Technopark Company BIOART R&D for help in preparing the thyme extract.
Funding
This work was supported by the Scientific Research Projects Coordination Unit of Kırıkkale University (Project number 2020/039).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval:
Approval was granted by the Ethics Committee of Kırıkkale University (19 December 2019, No. 64/2019).
Authorship contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Consent for publication
All authors gave their consent for publication of the study.
Conflict of interest
The authors declared that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kara, E., Yasa Duru, S., Gökpinar, S. et al. Investigation of the prophylactic and therapeutic effectiveness of oral thyme extract in rats experimentally infected with cryptosporidium parvum. Vet Res Commun 47, 663–673 (2023). https://doi.org/10.1007/s11259-022-10025-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-022-10025-6