Abstract
The water-borne herbicides are involved in the toxicity of aquatic animals resulting in impaired health status and low productivity. Dietary medicinal herbs present a practical solution to relieve the impacts of herbicides toxicity on the performances of aquatic animals. Herein, we investigated the toxicity of commercial glyphosate-induced oxidative stress, immunosuppression, liver and kidney dysfunction, and the protective role of ginger or ginger nanoparticles in Nile tilapia. Fish were allocated into four groups: the first group presented the control without glyphosate toxicity and ginger feeding, the second group intoxicated with glyphosate at 0.6 mg/L and fed ginger free diet, the third group intoxicated with glyphosate and fed ginger at 2.5 g/kg, and the fourth group intoxicated with glyphosate and fed ginger nanoparticles at 2.5 g/kg. Fish were kept under the experimental conditions for four weeks, and the samples of blood and tissues were collected after 2 and 4 weeks. Markedly, fish exposed to glyphosate showed the highest ALT and AST activities, glucose and cortisol levels, and malondialdehyde levels (MDA) in gills and tissues. While fish in the control and fish intoxicated with glyphosate and fed ginger nanoparticles had the lowest ALT and AST activities, glucose and cortisol levels, and MDA levels after 2 and 4 weeks (P < 0.05). Fish fed dietary ginger had lower ALT and AST activities, glucose and cortisol levels, and MDA levels than the glyphosate intoxicated group after 2 and 4 weeks (P < 0.05). Interestingly, fish-fed ginger nanoparticles showed lower urea and creatinine levels and higher total protein, albumin, and globulin than the glyphosate intoxicated group (P < 0.05) and similar to the control (P > 0.05). Further, fish intoxicated with glyphosate and fed ginger nanoparticles had the highest GSH, lysozyme activity, and immunoglobulin levels after 2 and 4 weeks (P < 0.05). In conclusion, ginger nanoparticles are superior to the standard ginger form in enhancing the antioxidative and immune responses of Nile tilapia exposed to glyphosate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a close relationship between aquaculture and agriculture activities where both are depending mainly on the drainage water for cultivation (Gewaily et al. 2021). Nevertheless, the heavy usage of herbicides and insecticides in agriculture to protect from herbals and insects is a limiting factor for the aquaculture sector (Bojarski and Witeska 2020). Usually, aquaculture activity depends on the reuse of the drainage water from the agriculture sector due to the limitation of water resources in some countries (Rossi et al. 2020). The waterborne derivatives of herbicides and pesticides negatively affect aquatic animals' health status and productivity (Dar et al. 2022; Naiel et al. 2020). Glyphosate is a very toxic herbicide involved in the protection against common herbs grown on crop farms and is known for its carcinogenic effects (Van Bruggen et al. 2018). Exposure to subchronic glyphosate led to oxidative stress, immunosuppression, inflammation, and apoptosis in various fish species (Ma et al. 2019; Mohapatra et al. 2021; Yalsuyi et al. 2021). The accumulation of herbicides, pesticides, and insecticides in the farming water affects the gills, skin, and intestines tissues of fish as the first mucosal lines directly contact the surrounding water (Banaee et al. 2020; Saha et al. 2021; Yang et al. 2020). More importantly, herbicide toxicity induces an imbalance in producing and removing reactive oxygen metabolites (ROS), leading to high lipid peroxidation and oxidative stress (Yang et al. 2020). Consequently, the toxic derivatives led to impairment in the entire body of fish's metabolic, physiological, and immunological conditions (Dawood et al. 2020a; Sutili et al. 2020).
Sustainable clean approaches are recently applied to ensure aquatic animals' well-being and high productivity under biotic and abiotic stressors (Paray et al. 2021). Nowadays, the focus is on applying eco-friendly functional additives in aquafeed to enhance aquatic animals' metabolic, physiological, and immunological responses (Elumalai et al. 2021). In this regard, medicinal herbs got close attention associated with their rich bioactive components and high functionality (Brum et al. 2018; Cardoso et al. 2021). The high pharmacological potential of medicinal herbs as natural antioxidative and anti-inflammation agents suggests using these additives to relieve the impacts of herbicides, insecticides, and pesticides on aquatic animals (Sinha et al. 2021; Yousefi et al. 2021). Zingiber officinale or ginger is a natural medicinal plant with high bioactive properties (Morvaridzadeh et al. 2020, 2021). The dietary inclusion of ginger led to marked enhancement in the growth performance, intestinal health, digestion capacity, antioxidative, and immune responses of several fish species (Chung et al. 2021b; Fazelan et al. 2020; Korni and Khalil 2017). Further, high resistance against bacterial infection was seen in infected aquatic animals due to the high antibacterial effect of ginger (Chung et al. 2021a; Korni et al. 2021). The production of ginger nanoparticles was also illustrated as an innovative approach for maximizing the functionality of ginger (Korni et al. 2021). In aquaculture, ginger nanoparticle usage resulted in high immunity and resistance to infection with pathogenic bacteria (Korni et al. 2021). Recently, it has been stated that ginger supplementation relieved the negative impacts of dimethoate exposure on the antioxidative response and histological features of gills, liver, and kidneys in Nile tilapia (Oreochromis niloticus) (Soror et al. 2021).
Nile tilapia is a freshwater fish species with high commercial value and can be cultured in the drainage water resulting from the agriculture sector (El-Sayed 2019). In Nile tilapia, sublethal toxicity of glyphosate induced negative histopathological impacts and inflammation features in the kidney (Hassan et al. 2022), gills (Jiraungkoorskul et al. 2003), liver (Abdelmagid et al. 2021), and spleen (Zheng et al. 2021) tissues. Further, immunosuppression (Abdelmagid et al. 2022), oxidative stress (Acar et al. 2021), and pro-inflammation responses were seen in Nile tilapia exposed to glyphosate herbicide. Therefore, it can be hypothesized that using ginger or its nanoparticles could relieve the negative impacts of glyphosate toxicity in Nile tilapia. This study aimed to evaluate the toxicity of glyphosate-induced oxidative stress, immunosuppression, liver and kidney dysfunction, and the protective role of ginger or ginger nanoparticles in Nile tilapia.
Material and methods
Ethical approval
All experimental techniques and fish care protocols used in the current study were followed by the Guidelines of Animal Care Use and were approved by the Institutional Animal Care Use Committee Research Ethics Board, Faculty of Veterinary Medicine, Benha University, Egypt.
Ginger nanoparticles and test diets preparation
Ginger was obtained from the local market as a fine powder and stored in glass containers in the refrigerator until further use. Ginger nanoparticles were prepared by following Yadav et al. (2012) using a planetary ball mill (Retsch PM 400, Germany) at 550 rpm for 4 h till they reached size 100 nm. Commercial basal diets (30% crude protein, Uccam Food, Egypt) were crushed to a fine powder and split into three parts, with the first diet serving as the control. At a 2.5 g/kg diet dosage, the second and third diets were properly incorporated with ginger and ginger nanoparticles, respectively (Soror et al. 2021). Ginger and ginger nanoparticles were diluted in distilled water and mixed with a crushed diet to produce dough. The soft dough was re-pelleted using a meat mincer. The prepared pellets (2–3 mm) were kept at room temperature for 48 h to dry out, sealed in clean, dry plastic bags, and stored at 4 °C until needed.
Fish and experimental procedure
A total of 250 healthy all males Nile tilapia (25.71 ± 0.5 g) have been obtained from a private fish farm at Kafr El-Sheikh Governorate, Egypt. Then, they were allocated in two fiberglass tanks (750 L capacity) provided with continuous aeration for two weeks acclimation period. During this period, fish were hand-fed daily with the control diet at a 3% body weight twice daily (8:00 and 16:00). Fish were stocked in twelve glass aquaria (89 × 30 × 29 cm) at 15 fish per aquarium. The aquaria present four groups in triplicates, and each aquarium is supplied with continuous aeration. The first group was fed a basal diet and kept in glyphosate-free water (control). The second group was intoxicated with glyphosate at 0.6 mg/L and fed the basal diet. The third group was intoxicated with glyphosate and fed ginger at 2.5 g/kg. The fourth group was intoxicated with glyphosate and fed ginger nanoparticles at 2.5 g/kg. The water from groups two to four was partially exchanged three times weekly, and the dose of glyphosate (Roundup 48%, Agrochem, Alwatneia Co., Alex., Egypt) was maintained within the required concentration (0.6 mg/L) in each aquarium. Meanwhile, the water was renewed with dechlorinated tap water in the control group. Glyphosate lethal concentration (LC50; 12 mg/L) was calculated earlier by Abdelmagid et al. (2021), and fish was exposed to 1/20 of LC50 (0.6 mg/L) following Abdelmagid et al. (2022). All fish have received the experimental diets twice daily at a feeding rate of 3% of the total body weight for four weeks under 12 h day:12 h night photoperiod regime. Water quality was maintained at 25 ± 1 °C, 5.1 ± 0.2 mg/L, 0.23 ± 0.07 mg/L, and 7.2 ± 0.2 for the temperature, dissolved oxygen, ammonia concentration, and pH, respectively. Water quality parameters (temperature, pH, and dissolved oxygen) were measured using Jenway, 370 pH meter, UK, and Crison OXI 45 P, EU, while total ammonia by following APHA (2017). The water exchange was done daily to eliminate fecal matter and uneaten food to maintain water quality parameters. The particle size distribution and zeta potential of ginger nanoparticles were determined using Zetasizer MS2000 (Malvern, United Kingdom). Additionally, the microstructure of ginger nanoparticles was examined by Ultraviolet spectrophotometer (V-750, Jasco Inc) by exposing the sample to visible light at rang 200-800 nm. The ginger nanoparticle morphology was observed under transmission electron microscopy (JEM2100, Japan) at an accelerating voltage of 200 K.V.
Blood and tissue collection
Blood and tissue samples were collected from all treated groups after 2 weeks of exposure and at the end of the experiment (4 weeks). Fish were anesthetized with MS-222 (100 μg/mL), then blood samples from nine fish per treatment (3 fish/ replicate) were collected without anticoagulant. To separate serum, these samples were left in a slant position for 30 min and centrifuged at 3000 r.p.m for 15 min. The serum was collected and kept at -20 ˚C for immunological and biochemical assays.
For evaluation of antioxidant enzyme activity, liver and gills specimens were taken from nine fish per treatment (3 fish/ replicate), homogenized in 9 volumes of ice-cold 0.05 Mm potassium phosphate buffer saline PH 7.4 using a glass homogenizer. The homogenates were centrifuged at 6000 r.p.m for 15 min at 4˚C. The resultant supernatant was used to determine malondialdehyde (MDA) and reduced glutathione (GSH).
Serum biochemical and immune assays
Serum glucose level was estimated according to the method reported by Trinder (1969). Cortisol was determined following the method described by Munro, and Lasley (1988), based on the competitive binding technique where cortisol competes with horseradish peroxidase (HRP)-labeled cortisol.
Serum alanine aminotransferase activity (ALT) was determined according to the method described by Huang et al. (2006). Aspartate aminotransferase activity (AST) was assayed according to Schumann et al. (2011). The urea level in serum was measured using commercial kits (Biomed, Germany) based on Levinson (1978) method. Creatinine in the serum was assayed following the protocol described by Moss et al. (1975). Serum total proteins and albumin were determined according to the method described by Doumas et al. (1981). The globulins concentration was calculated by subtracting the albumin values from the total protein values.
Serum lysozyme activity was determined according to the method described by Ellis (1990), depending on the lysis of Micrococcus lysodeikticus suspension. The activity was calculated from a standard curve prepared with lysozyme from chicken egg white. The concentration of serum total immunoglobulins was evaluated at 450 nm according to the method described by Siwicki (1993).
Liver and gills antioxidant assay
The concentration of MDA in liver and gills tissues was determined based on the protocol of Esterbauer, and Cheeseman (1990), which depends on the determination of thiobarbituric acid reactive substance content (TBARs) at 530 nm. Reduced GSH assay was based on GSH reaction with DTNB (5, 5`-dithiobis nitro benzoic acid) produces a yellow product of nitromercaptobenzoic acid that absorbs at 412 nm (Beutler 1963).
Statistical analysis
All data were tested for normality and homogeneity by Shapiro–Wilk's and Levene's tests, respectively. Then, a one-way analysis of variance was used to determine the statistical significance of differences among groups, followed by Duncan's test as post hoc for making a multiple comparison using SPSS software (Version 25, SPSS Inc., Chicago, IL, USA). The values were expressed as the mean ± standard error. Mean values were considered significant at a P-value < 0.05 probability level.
Results
Characterization of ginger nanoparticles
As shown in Fig. 1, UV/VIS spectroscopy showed one peak of ginger nanoparticles at maximum absorbance 57.08 obtained at wavelength 340 nm. The size distribution of ginger nanoparticles analyzed by Zetasizer for size determination showed an average diameter of 906.9 d.nm. TEM image showed that ginger nanoparticles were circular in shape.
Growth performance
The obtained results indicated that Nile tilapia-fed ginger or ginger nanoparticles after 4-week exposure to sublethal concentration of glyphosate had no significant differences in the case of final weight and specific growth rate (P > 0.05) (Fig. 2).
Liver and kidney-related metabolites
Liver and kidney indicators in Nile tilapia fed ginger or its nanoparticles after 2- and 4-weeks exposure to glyphosate are shown in Table 1. Markedly, fish exposed to glyphosate showed the highest ALT and AST activities, while fish in the control group had the lowest ALT and AST activities after 2 and 4 weeks (P < 0.05). Fish fed dietary ginger had higher ALT, AST, urea, and creatinine levels than the control and lowered ALT, AST, urea, and creatinine levels than the glyphosate intoxicated group after 2 and 4 weeks (P < 0.05). Interestingly, fish-fed ginger nanoparticles showed lower ALT and AST activities than the glyphosate intoxicated group (P < 0.05) without significant differences from the control (P > 0.05). Further, urea and creatinine levels showed the lowest values meaningfully in fish fed the control diet without glyphosate toxicity, and fish fed ginger nanoparticles with glyphosate toxicity after 2 and 4 weeks (P < 0.05).
Blood proteins
The blood total protein, albumin, and globulin levels were meaningfully increased (P < 0.05) in the control group and severely reduced in fish exposed to glyphosate toxicity after 2 and 4 weeks (Table 2). No significant differences were seen between the control and fish intoxicated with glyphosate and fed ginger nanoparticles (P > 0.05). Moreover, fish fed dietary ginger and exposed to glyphosate had higher total protein, albumin, and globulin levels (P < 0.05) than the glyphosate intoxicated group without ginger feeding after 2 and 4 weeks.
Stress-related markers
Fish exposed to glyphosate showed the highest glucose and cortisol levels, while fish in the control group had the lowest glucose and cortisol levels after 2 and 4 weeks (P < 0.05) (Table 3). Fish fed dietary ginger had higher glucose and cortisol levels than the control and lower glucose and cortisol levels than the glyphosate intoxicated group after 2 and 4 weeks (P < 0.05). Interestingly, fish-fed ginger nanoparticles showed non-significant differences from the control after 2 and 4 weeks (P > 0.05).
Liver and gills antioxidant status
In the gills and liver tissues, fish exposed to glyphosate showed the lowest glutathione (GSH) levels (Fig. 3A and B), while fish in the control group and fish intoxicated with glyphosate and fed ginger nanoparticles had the highest GSH after 2 and 4 weeks (P < 0.05). Further, fish-fed dietary ginger had higher GSH than the glyphosate intoxicated group after 2 and 4 weeks (P < 0.05). Interestingly, fish-fed ginger nanoparticles showed non-significant differences from the control after 2 and 4 weeks (P > 0.05).
Antioxidant activity and lipid peroxidation level in Nile tilapia fed ginger or its nanoparticles (ginger-NPs) after 2- and 4-weeks exposure to sub-lethal concentration of glyphosate (Gly). Bars present means ± S.E. (n = 3) with different letters, differ significantly (P < 0.05). Reduced glutathione (GSH) and malondialdehyde (MDA) level
In the gills and liver tissues, fish exposed to glyphosate showed the highest malondialdehyde (MDA) levels (Fig. 3C and D), while fish in the control group and fish intoxicated with glyphosate and fed ginger nanoparticles had the lowest MDA levels after 2 and 4 weeks (P < 0.05). Further, fish-fed dietary ginger had a lower MDA level than the glyphosate intoxicated group after 2 and 4 weeks (P < 0.05).
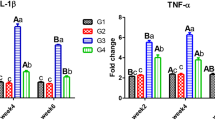
Immune response
Markedly, fish exposed to glyphosate showed the lysozyme activity (Fig. 4A) and total immunoglobulin level (Fig. 4B), while fish in the control group and fish intoxicated with glyphosate and fed ginger nanoparticles had the highest lysozyme activity and total immunoglobulin level (P < 0.05). Further, fish-fed dietary ginger had higher lysozyme activity and total immunoglobulin levels than the glyphosate intoxicated group after 2 and 4 weeks (P < 0.05). Interestingly, fish-fed ginger nanoparticles showed non-significant differences from the control after 2 and 4 weeks (P > 0.05).
Discussion
Water contamination with herbicides derivatives is a significant concern that threatens the viability and quality of the ecosystem and aquatic animals (Blahova et al. 2020; Bojarski and Witeska 2020; Stara et al. 2021). The integrated agriculture–aquaculture systems are a direct reason for pollution with herbicides that can interrupt the health of aquatic animals (Soror et al. 2021; Yousefi et al. 2021). This study showed the negative impacts of waterborne glyphosate and ginger nanoparticles' protective roles. The results indicated severe impacts of glyphosate toxicity on the hepato-renal function, stress-related biomarkers, and immune and antioxidative responses of Nile tilapia. Interestingly, dietary ginger and ginger nanoparticles ameliorated the impacts of glyphosate on Nile tilapia health.
This study showed that negative waterborne herbicides impacts are involved in the impairment of aquatic animals' metabolic and biochemical functions (Abdel-Warith et al. 2021; Samanta et al. 2014). Blood biochemical metabolites associated with liver (ALT and AST) and kidney functions (urea and creatinine) as well as the stress-related markers (cortisol and glucose) are usually detected in the blood to understand the impact of herbicides on fish health (Bacchetta et al. 2014; Ramaiah 2007). Besides, the level of blood proteins (total protein, albumin, and globulin) is influenced by the nutrient metabolism, hormones, enzymes, antibodies, and general immunity of fish, which can be disrupted by herbicide toxicity (Al-Ghanim et al. 2020; Gholami-Seyedkolaei et al. 2013). The results showed that Nile tilapia exposed to glyphosate have high ALT, AST, urea, and creatinine levels in blood samples after 2 and 4 weeks. The results agree with Yousefi et al. (2021), who indicated high levels of ALT and AST in common carp (Cyprinus carpio) exposed to glyphosate. Further, Dawood et al. (2020b) claimed high urea and creatinine levels in tilapia exposed to deltamethrin. The rise in ALT and AST was most likely due to cytolysis and enzyme leakage into the bloodstream, suggesting liver and kidney injury (Bacchetta et al. 2014). While increased urea level is associated with gills damage and high creatinine is associated with muscular dysfunction (Soror et al. 2021). The levels of blood proteins, albumin, and globulin were declined by glyphosate toxicity after 2 and 4 weeks, referring to reducing total protein resulting from liver tissue damage due to oxidative stress (Brum et al. 2018). In addition, the study showed high glucose and cortisol levels in the blood of tilapia exposed to glyphosate after 2 and 4 weeks. The results are similar to Yousefi et al. (2021), who reported high cortisol and glucose levels in common carp intoxicated with glyphosate. The cortisol hormone, which manages the organism's reaction to stresses, regulates the blood glucose level (Polakof et al. 2012). Cortisol is a sign of primary stress response, while an increase in glucose levels in serum indicates a secondary stress response in case of glyphosate toxicity (Langiano and Martinez 2008). The increased cortisol levels indicate that glyphosate generated stress in Nile tilapia which can be related to the high content of surfactants like polyoxyethylene amine (POEA) (Glusczak et al. 2007). Furthermore, glyphosate toxicity causes endocrine-disrupting effects by disrupting the function of the hypothalamus–pituitary–gonadal axis (Soso et al. 2007).
The disruption of ALT, AST, urea, and creatinine can also be related to increased glucose and cortisol levels (Soror et al. 2021; Yousefi et al. 2021). On the other hand, dietary ginger or ginger nanoparticles regulated the levels of ALT, AST, urea, creatinine, cortisol, and glucose, indicating healthy fish status. In a similar sense, Soror et al. (2021) reported that dietary ginger regulated the levels of ALT, AST, urea, creatinine, cortisol, and glucose and increased blood total protein, albumin, and globulin in Nile tilapia exposed to dimethoate. The reduction of ALT, AST, urea, and creatinine and the increased total protein, albumin, and globulin levels are probably attributed to ginger's antioxidative role, which balances the lipid peroxidation leading to inhibition of oxidative stress and thereby regular liver and kidney function (Ali et al. 2008).
The toxicity of glyphosate is responsible for generating reactive oxygen metabolites (ROS) that induce oxidative stress (Muhammad et al. 2021; Zheng et al. 2021). ROS production can interact with cellular lipid membranes leading to lipid peroxidation and damage to DNA and cellular function (Kavitha and Venkateswara Rao 2007). It has been reported that glyphosate could impair cellular function through cytoplasmic membrane toxicity and the production of oxidative stress (Yang et al. 2020; Yousefi et al. 2021). The concentration of malondialdehyde (MDA) is an indicator of lipid peroxidation (Zhang et al. 2020), while glutathione (GSH) is an antioxidant molecule (Forman et al. 2009), and an imbalance of ROS production and removal leads to the activation of GSH to counteract with high MDA concentration (Lackner 1998). This study detected the GSH activity and MDA levels in the gills and liver tissues. Gills in the first organ directly impacted by glyphosate toxicity result in the dysfunction of respiration and osmoregulation (Dawood et al. 2021). At the same time, liver tissue is responsible for detoxifying toxicants, xenobiotics, and secretion of pathogenic invaders (Tanaka et al. 1999). Thus, it is necessary to correlate the impact of glyphosate and the antioxidative capacity of gills and liver tissues that may explain the disrupted hepato-renal function and immune and antioxidative responses of Nile tilapia under the current trial conditions. The results showed high MDA levels and low GSH in fish exposed to glyphosate; however, dietary ginger regulated MDA and GSH after 2 and 4 weeks. Nile tilapia-fed dietary ginger or ginger nanoparticles displayed low MDA levels and high GSH, indicating high antioxidative capacity to cope with the impacts of glyphosate toxicity after 2 and 4 weeks. The results are in line with Yousefi et al. (2021) and Yang et al. (2019), who reported high MDA levels in common carp and Chinese mitten crab, respectively. Besides, Modesto and Martinez (2010), and Ma et al. (2019) reported a reduction in the antioxidative capacity of Prochilodus lineatus and common carp exposed to glyphosate. On the other hand, similar reports indicated that dietary ginger resulted in high antioxidative capacity in Nile tilapia (Soror et al. 2021) and common carp (Fazelan et al. 2020). Ginger contains polyphenols, gingerols, and shogaols with anti-inflammatory and antioxidative capacity, which can degenerate the ROS and protect the cell membrane from oxidation (Ali et al. 2008). In this context, dietary ginger enhanced the antioxidative capacity of Nile tilapia exposed to dimethoate (Soror et al. 2021).
There is a close connection between the antioxidative and immune responses, which can also be impaired by toxicity with glyphosate (Peillex and Pelletier 2020). In earlier reports, glyphosate toxicity resulted in immunosuppression of Nile tilapia (Zheng et al. 2021), Chinese mitten crab (Yang et al. 2019), and common carp (Yousefi et al. 2021), and silver catfish (Sutili et al. 2020). This study detected the lysozyme and total immunoglobulins after two and four weeks of glyphosate toxicity in Nile tilapia fed with or without ginger and its nanoparticles. Lysozyme activity and total immunoglobulins are innate immune responses that protect against infection with pathogenic bacteria through the damage of bacterial cell walls (Whyte 2007) and secretion of antibodies (Tellez-Bañuelos et al. 2010), respectively. The results showed impaired lysozyme and total immunoglobulin level in fish exposed to glyphosate, but fish treated with ginger had enhanced lysozyme activity and total immunoglobulin levels after 2 and 4 weeks. Yousefi et al. (2021) reported that common carp exposed to glyphosate had reduced lysozyme activity and total immunoglobulin levels. Herbicides toxicity induces immunotoxicity through a complex network of inflammatory cytokines release, immunoglobulins regulation, immune cell proliferation inhibition, and lysozyme activity changes (Yang et al. 2021). Exposure to glyphosate induces lipid peroxidation and oxidative stress causing deterioration of immune cells (B type) function. Thus, lowering the immunoglobulin production and inflammatory cytokines release and thereby immunosuppression (Chen et al. 2005; Mela et al. 2007). Interestingly, the inclusion of dietary ginger relieved the impacts of glyphosate and increased the lysozyme activity and total immunoglobulins under the current trial conditions. Similarly, the inclusion of ginger and its nanoparticles enhanced the lysozyme activity and total immunoglobulins in common carp (Fazelan et al. 2020; Mohammadi et al. 2020) and Nile tilapia (Brum et al. 2018). More research is needed to determine the specific mechanism of ginger's immunomodulatory effect. The presence of bioactive metabolites in ginger, including terpenes, zingiberol, zingiberene, zingiberene, zingerone, oleoresin, gingerol, shogaol, and paradol, is the most likely cause (Jesudoss et al. 2017). These components have an antibacterial and immunomodulatory role, which begins with the initiation of local intestinal immunity and, thereby, the entire body's immunity (Dawood 2021).
It is more likely that the nanoparticles of ginger are more effective than normal ginger particles in the protection against glyphosate-induced hepato-renal dysfunction, immunosuppression, and oxidative stress under the current trial conditions. Indeed, nano-engineered substances are illustrated as more effective and functional than the standard form (Korni and Khalil 2017). Nanoparticles have a small surface with high activity making them accessible during absorption and more functional inside the fish body (Singh et al. 2021). In this context, the inclusion of ginger nanoparticles is illustrated to be more effective than standard ginger form in Nile tilapia.
Conclusion
In conclusion, glyphosate toxicity represents environmental agriculture- aquaculture risk resulting in the dysfunction of the hepato-renal tissues and impairment of the antioxidative and immune responses of Nile tilapia. However, dietary ginger and its nanoparticles markedly relived the toxic impacts of glyphosate, resulting in regulated hepato-renal tissues, antioxidative, and immune responses. Interestingly, ginger nanoparticles are superior to the standard ginger form in enhancing the antioxidative and immune responses of Nile tilapia exposed to glyphosate.
Data availability
Data and materials are available upon request.
References
Abdelmagid AD, El Asely AM, Said AM (2021) Evaluation of Foeniculum vulgare impact on glyphosate hepato-toxicity in Nile tilapia: biochemical, molecular and histopathological study. Aquac Res 52:5397–5406
Abdelmagid AD, Said AM, Gawad EAA, Shalaby SA, Dawood MAO (2022) Propolis nanoparticles relieved the impacts of glyphosate-induced oxidative stress and immunosuppression in Nile tilapia. Environ Sci Pollut Res 29:19778–19789
Abdel-Warith A-WA, Younis EM, Al-Asgah NA, Gewaily MS, El-Tonoby SM, Dawood MAO (2021) Role of Fucoidan on the growth behavior and blood metabolites and toxic effects of atrazine in Nile Tilapia Oreochromis niloticus (Linnaeus, 1758). Animals 11:1448
Acar Ü, İnanan BE, Navruz FZ, Yılmaz S (2021) Alterations in blood parameters, DNA damage, oxidative stress and antioxidant enzymes and immune-related genes expression in Nile Tilapia (Oreochromis niloticus) exposed to glyphosate-based herbicide. Comp Biochem Physiol c Toxicol Pharmacol 249:109147
Al-Ghanim KA, Mahboob S, Vijayaraghavan P, Al-Misned FA, Kim YO, Kim H-J (2020) Sub-lethal effect of synthetic pyrethroid pesticide on metabolic enzymes and protein profile of non-target Zebrafish, Danio rerio. Saudi Journal of Biological Sciences 27:441–447
Ali BH, Blunden G, Tanira MO, Nemmar A (2008) Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol 46:409–420
APHA, A (2017) WEF, Standard Methods for the Examination of Water and Wastewater, a joint publication of the American Public Health Association (APHA), the American Water Works Association (AWWA), and the Water Environment Federation (WEF). American Public Health Association, Washington
Bacchetta C, Rossi A, Ale A, Campana M, Parma MJ, Cazenave J (2014) Combined toxicological effects of pesticides: a fish multi-biomarker approach. Ecol Ind 36:532–538
Banaee M, Akhlaghi M, Soltanian S, Sureda A, Gholamhosseini A, Rakhshaninejad M (2020) Combined effects of exposure to sub-lethal concentration of the insecticide chlorpyrifos and the herbicide glyphosate on the biochemical changes in the freshwater crayfish Pontastacus Leptodactylus. Ecotoxicology 29:1500–1515
Beutler E (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Blahova J, Cocilovo C, Plhalova L, Svobodova Z, Faggio C (2020) Embryotoxicity of atrazine and its degradation products to early life stages of zebrafish (Danio rerio). Environ Toxicol Pharmacol 77:103370
Bojarski B, Witeska M (2020) Blood biomarkers of herbicide, insecticide, and fungicide toxicity to fish—a review. Environ Sci Pollut Res 27:19236–19250
Brum A, Cardoso L, Chagas EC, Chaves FCM, Mouriño JLP, Martins ML (2018) Histological changes in Nile tilapia fed essential oils of clove basil and ginger after challenge with Streptococcus agalactiae. Aquaculture 490:98–107
Cardoso AJdS, dos Santos WV, Gomes JR, Martins MTS, Coura RR, Oliveira MGdA, Salaro AL, Ferreira PdMF, Carneiro APS, Zuanon JAS (2021) Ginger oil, Zingiber officinale, improve palatability, growth and nutrient utilisation efficiency in Nile tilapia fed with excess of starch. Anim Feed Sci Technol 272:114756
Chen X, Yao G, Hou Y (2005) Pentachlorophenol reduces B lymphocyte function through proinflammatory cytokines in Carassius auratus. Food Chem Toxicol 43:239–245
Chung S, Ribeiro K, Teixeira DV, Copatti CE (2021a) Inclusion of essential oil from ginger in the diet improves physiological parameters of tambaqui juveniles (Colossoma macropomum). Aquaculture 543:736934
Chung S, Ribeiro K, Melo JFB, Teixeira DV, Vidal LVO, Copatti CE (2021b) Essential oil from ginger influences the growth, haematological and biochemical variables and histomorphometry of intestine and liver of Nile tilapia juveniles. Aquaculture 534:736325
Dar OI, Aslam R, Pan D, Sharma S, Andotra M, Kaur A, Jia A-Q, Faggio C (2022) Source, bioaccumulation, degradability and toxicity of triclosan in aquatic environments: a review. Environ Technol Innov 25:102122
Dawood MAO (2021) Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev Aquac 13:642–663
Dawood MAO, Abdel-Tawwab M, Abdel-Latif HMR (2020a) Lycopene reduces the impacts of aquatic environmental pollutants and physical stressors in fish. Rev Aquac 12:2511–2526
Dawood MAO, AbdEl-kader MF, Moustafa EM, Gewaily MS, Abdo SE (2020b) Growth performance and hemato-immunological responses of Nile tilapia (Oreochromis niloticus) exposed to deltamethrin and fed immunobiotics. Environ Sci Pollut Res 27:11608–11617
Dawood MAO, Noreldin AE, Sewilam H (2021) Long term salinity disrupts the hepatic function, intestinal health, and gills antioxidative status in Nile tilapia stressed with hypoxia. Ecotoxicol Environ Saf 220:112412
Doumas BT, Bayse DD, Carter RJ, Peters T, Schaffer R (1981) A candidate reference method for determination of total protein in serum. I. Development and validation. Clin Chem 27:1642–1650
Ellis A (1990) Lysozyme activity. Technique in Fish Immunology, 101–103
El-Sayed A-FM (2019) Tilapia culture, 2nd edn. Elsevier/Academic Press, Londonand Oxford, UK and San Diego, p. 348. Academic Press
Elumalai P, Kurian A, Lakshmi S, Faggio C, Esteban MA, Ringø E (2021) Herbal immunomodulators in aquaculture. Rev Fish Sci Aquac 29:33–57
Esterbauer H, Cheeseman KH (1990) [42] Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal, Methods in Enzymology. Academic Press, pp. 407–421
Fazelan Z, Vatnikov YA, Kulikov EV, Plushikov VG, Yousefi M (2020) Effects of dietary ginger (Zingiber officinale) administration on growth performance and stress, immunological, and antioxidant responses of common carp (Cyprinus carpio) reared under high stocking density. Aquaculture 518:734833
Forman HJ, Zhang H, Rinna A (2009) Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med 30:1–12
Gewaily MS, Shukry M, Abdel-Kader MF, Alkafafy M, Farrag FA, Moustafa EM, Doan HV, Abd-Elghany MF, Abdelhamid AF, Dawood MA (2021) Dietary Lactobacillus plantarum relieves Nile tilapia (Oreochromis niloticus) juvenile from oxidative stress, immunosuppression and inflammation induced by deltamethrin and Aeromonas hydrophila. Front Mar Sci 8:203
Gholami-Seyedkolaei SJ, Mirvaghefi A, Farahmand H, Kosari AA (2013) Effect of a glyphosate-based herbicide in Cyprinus carpio: assessment of acetylcholinesterase activity, hematological responses and serum biochemical parameters. Ecotoxicol Environ Saf 98:135–141
Glusczak L, Miron DdS, Moraes BS, Simões RR, Schetinger MRC, Morsch VM, Loro VL (2007) Acute effects of glyphosate herbicide on metabolic and enzymatic parameters of silver catfish (Rhamdia quelen). Comp Biochem Physiol C Toxicol Pharmacol 146:519–524
Hassan MA, Hozien ST, Abdel Wahab MM, Hassan AM (2022) Ameliorative effect of selenium yeast supplementation on the physio-pathological impacts of chronic exposure to glyphosate and or malathion in Oreochromis niloticus. BMC Vet Res 18:159
Huang X-J, Choi Y-K, Im H-S, Yarimaga O, Yoon E, Kim H-S (2006) Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors 6:756–782
Jesudoss VAS, Victor Antony Santiago S, Venkatachalam K, Subramanian P (2017) Chapter 21 - Zingerone (Ginger Extract): Antioxidant Potential for Efficacy in Gastrointestinal and Liver Disease. In: Gracia-Sancho J, Salvadó J (eds) Gastrointestinal Tissue. Academic Press, pp 289–297
Jiraungkoorskul W, Upatham ES, Kruatrachue M, Sahaphong S, Vichasri-Grams S, Pokethitiyook P (2003) Biochemical and histopathological effects of glyphosate herbicide on Nile tilapia (Oreochromis niloticus). Environ Toxicol 18:260–267
Kavitha P, Venkateswara Rao J (2007) Oxidative stress and locomotor behaviour response as biomarkers for assessing recovery status of mosquitofish, Gambusia affinis after lethal effect of an organophosphate pesticide, monocrotophos. Pestic Biochem Physiol 87:182–188
Korni FMM, Khalil F (2017) Effect of ginger and its nanoparticles on growth performance, cognition capability, immunity and prevention of motile Aeromonas septicaemia in Cyprinus carpio fingerlings. Aquac Nutr 23:1492–1499
Korni FMM, Abo El-Ela FI, Moawad UK, Mahmoud RK, Gadelhak YM (2021) Prevention of Edwardsiellosis in Clarias gariepinus using ginger and its nanoparticles with a reference to histopathological alterations. Aquaculture 539:736603
Lackner R (1998) “Oxidative stress” in fish by environmental pollutants. In: Braunbeck T, Hinton DE, Streit B (eds) Fish Ecotoxicology. Birkhäuser Basel, Basel, pp. 203–224
Langiano VdC, Martinez CBR (2008) Toxicity and effects of a glyphosate-based herbicide on the Neotropical fish Prochilodus lineatus. Comp Biochem Physiol C Toxicol Pharmacol 147:222–231
Levinson SS (1978) Kinetic centrifugal analyzer and manual determination of serum urea nitrogen, with use of o-phthaldialdehyde reagent. Clin Chem 24:2199–2202
Ma J, Zhu J, Wang W, Ruan P, Rajeshkumar S, Li X (2019) Biochemical and molecular impacts of glyphosate-based herbicide on the gills of common carp. Environ Pollut 252:1288–1300
Mela M, Randi MAF, Ventura DF, Carvalho CEV, Pelletier E, Oliveira Ribeiro CA (2007) Effects of dietary methylmercury on liver and kidney histology in the neotropical fish Hoplias malabaricus. Ecotoxicol Environ Saf 68:426–435
Modesto KA, Martinez CBR (2010) Roundup® causes oxidative stress in the liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 78:294–299
Mohammadi G, Rashidian G, Hoseinifar SH, Naserabad SS, Doan HV (2020) Ginger (Zingiber officinale) extract affects growth performance, body composition, haematology, serum and mucosal immune parameters in common carp (Cyprinus carpio). Fish Shellfish Immunol 99:267–273
Mohapatra S, Kumar R, Sundaray JK, Patnaik ST, Mishra CSK, Rather MA (2021) Structural damage in liver, gonads, and reduction in spawning performance and alteration in the haematological parameter of Anabas testudineus by glyphosate- a herbicide. Aquac Res 52:1150–1159
Morvaridzadeh M, Fazelian S, Agah S, Khazdouz M, Rahimlou M, Agh F, Potter E, Heshmati S, Heshmati J (2020) Effect of ginger (Zingiber officinale) on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Cytokine 135:155224
Morvaridzadeh M, Sadeghi E, Agah S, Fazelian S, Rahimlou M, Kern FG, Heshmati S, Omidi A, Persad E, Heshmati J (2021) Effect of ginger (Zingiber officinale) supplementation on oxidative stress parameters: A systematic review and meta-analysis. J Food Biochem 45:e13612
Moss GA, Bondar RJ, Buzzelli DM (1975) Kinetic enzymatic method for determining serum creatinine. Clin Chem 21:1422–1426
Muhammad UA, Yasid NA, Daud HM, Shukor MY (2021) Glyphosate herbicide induces changes in the growth pattern and somatic indices of crossbred red Tilapia (O. niloticus × O. mossambicus). Animals 11(5):1209
Munro C, Lasley B (1988) Non-radiometric methods for immunoassay of steroid hormones. Prog Clin Biol Res 285:289–329
Naiel MAE, Shehata AM, Negm SS, Abd El-Hack ME, Amer MS, Khafaga AF, Bin-Jumah M, Allam AA (2020) The new aspects of using some safe feed additives on alleviated imidacloprid toxicity in farmed fish: a review. Rev Aquac 12:2250–2267
Paray BA, El-Basuini MF, Alagawany M, Albeshr MF, Farah MA, Dawood MAO (2021) Yucca schidigera usage for healthy aquatic animals: potential roles for sustainability. Animals 11:93
Peillex C, Pelletier M (2020) The impact and toxicity of glyphosate and glyphosate-based herbicides on health and immunity. J Immunotoxicol 17:163–174
Polakof S, Panserat S, Soengas JL, Moon TW (2012) Glucose metabolism in fish: a review. J Comp Physiol B 182:1015–1045
Ramaiah SK (2007) A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol 45:1551–1557
Rossi AS, Fantón N, Michlig MP, Repetti MR, Cazenave J (2020) Fish inhabiting rice fields: bioaccumulation, oxidative stress and neurotoxic effects after pesticides application. Ecol Ind 113:106186
Saha S, Chukwuka AV, Mukherjee D, Patnaik L, Nayak S, Dhara K, Saha NC, Faggio C (2021) Chronic effects of Diazinon® exposures using integrated biomarker responses in freshwater walking catfish, Clarias batrachus. Appl Sci 11(22):10902
Samanta P, Pal S, Mukherjee AK, Ghosh AR (2014) Evaluation of metabolic enzymes in response to excel mera 71, a glyphosate-based herbicide, and recovery pattern in freshwater teleostean fishes. Biomed Res Int 2014:425159
Schumann G, Klauke R, Canalias F, Bossert-Reuther S, Franck PF, Gella F-J, Jørgensen PJ, Kang D, Lessinger J-M, Panteghini M (2011) IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 C. Part 9: reference procedure for the measurement of catalytic concentration of alkaline phosphatase. Clin Chem Lab Med 49:1439–1446
Singh AR, Desu PK, Nakkala RK, Kondi V, Devi S, Alam MS, Hamid H, Athawale RB, Kesharwani P (2022) Nanotechnology-based approaches applied to nutraceuticals. Drug Deliv Transl Res 12:485–499
Sinha R, Jindal R, Faggio C (2021) Protective effect of Emblica officinalis in Cyprinus carpio against hepatotoxicity induced by malachite green: ultrastructural and molecular analysis. Appl Sci 11(8):3507
Siwicki A (1993) Nonspecific defense mechanisms assay in fish. II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin (Ig) level in serum. Fish Diseases Diagnosis and Preventions Methods
Soror EI, El Asely AM, Abdel Gawad EA, Radwan HA, Abbass AA (2021) Recuperative effects of honey bee pollen, ginger (Zingiber officinale), and Moringa oleifera in Nile tilapia (Oreochromis niloticus L.) after sub-lethal exposure to dimethoate. Aquaculture 530:735886
Soso AB, Barcellos LJG, Ranzani-Paiva MJ, Kreutz LC, Quevedo RM, Anziliero D, Lima M, Silva LBd, Ritter F, Bedin AC, Finco JA (2007) Chronic exposure to sub-lethal concentration of a glyphosate-based herbicide alters hormone profiles and affects the reproduction of female Jundiá (Rhamdia quelen). Environ Toxicol Pharmacol 23:308–313
Stara A, Pagano M, Albano M, Savoca S, Di Bella G, Albergamo A, Koutkova Z, Sandova M, Velisek J, Fabrello J, Matozzo V, Faggio C (2021) Effects of long-term exposure of Mytilus galloprovincialis to thiacloprid: a multi-biomarker approach. Environ Pollut 289:117892
Sutili FJ, Golombieski JI, Schneider SI, Battisti EK, Braz PH, Gressler LT, Zanella R (2020) Effects of chlorantraniliprole insecticide on innate immune response of silver catfish (Rhamdia quelen) naturally infected with Aeromonas hydrophila. Microb Pathog 149:104584
Tanaka R, Higo Y, Murata H, Nakamura T (1999) Accumulation of hydroxy lipids in live fish with oxidative stress. Fish Sci 65:796–797
Tellez-Bañuelos MC, Santerre A, Casas-Solis J, Zaitseva G (2010) Endosulfan increases seric interleukin-2 like (IL-2L) factor and immunoglobulin M (IgM) of Nile tilapia (Oreochromis niloticus) challenged with Aeromonas hydrophila. Fish Shellfish Immunol 28:401–405
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6:24–27
Van Bruggen AHC, He MM, Shin K, Mai V, Jeong KC, Finckh MR, Morris JG (2018) Environmental and health effects of the herbicide glyphosate. Sci Total Environ 616–617:255–268
Whyte SK (2007) The innate immune response of finfish – a review of current knowledge. Fish Shellfish Immunol 23:1127–1151
Yadav TP, Yadav RM, Singh DP (2012) Mechanical milling: a top down approach for the synthesis of nanomaterials and nanocomposites. Nanosci Nanotechnol 2:22–48
Yalsuyi AM, Vajargah MF, Hajimoradloo A, Galangash MM, Prokić MD, Faggio C (2021) Evaluation of behavioral changes and tissue damages in Common carp (Cyprinus carpio) after exposure to the herbicide glyphosate. Vet Sci 8(10):218
Yang X, Song Y, Zhang C, Pang Y, Song X, Wu M, Cheng Y (2019) Effects of the glyphosate-based herbicide roundup on the survival, immune response, digestive activities and gut microbiota of the Chinese mitten crab, Eriocheir Sinensis. Aquatic Toxicol 214:105243
Yang C, Lim W, Song G (2020) Mediation of oxidative stress toxicity induced by pyrethroid pesticides in fish. Comp Biochem Physiol C Toxicol Pharmacol 234:108758
Yang C, Lim W, Song G (2021) Immunotoxicological effects of insecticides in exposed fishes. Comp Biochem Physiol C Toxicol Pharmacol 247:109064
Yousefi M, Adineh H, Reverter M, Khademi Hamidi M, Vatnikov YA, Kulikov EV, Hoseinifar SH, Van Doan H (2021) Protective effects of black seed (Nigella sativa) diet supplementation in common carp (Cyprinus carpio) against immune depression, oxidative stress and metabolism dysfunction induced by glyphosate. Fish Shellfish Immunol 109:12–19
Zhang M, Yin X, Li M, Wang R, Qian Y, Hong M (2020) Effect of nitrite exposure on haematological status, oxidative stress, immune response and apoptosis in yellow catfish (Pelteobagrus fulvidraco). Comp Biochem Physiol C Toxicol Pharmacol 238:108867
Zheng T, Jia R, Cao L, Du J, Gu Z, He Q, Xu P, Yin G (2021) Effects of chronic glyphosate exposure on antioxidative status, metabolism and immune response in tilapia (GIFT, Oreochromis niloticus). Comp Biochem Physiol C Toxicol Pharmacol 239:108878
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, Afaf D. Abdelmagid, Alshaimaa M. Said, Eman A. Abd El-Gawad, Sara A. Shalaby, Mahmoud A.O. Dawood; Data curation, Afaf D. Abdelmagid, Alshaimaa M. Said, Eman A. Abd El-Gawad, Sara A. Shalaby, Mahmoud A.O. Dawood; Funding acquisition, Afaf D. Abdelmagid, Alshaimaa M. Said, Eman A. Abd El-Gawad, Sara A. Shalaby; Investigation, Afaf D. Abdelmagid, Alshaimaa M. Said, Eman A. Abd El-Gawad, Sara A. Shalaby, Mahmoud A.O. Dawood; Project administration, Afaf D. Abdelmagid, Alshaimaa M. Said, Eman A. Abd El-Gawad, Sara A. Shalaby, Mahmoud A.O. Dawood; Resources, Afaf D. Abdelmagid, Alshaimaa M. Said, Eman A. Abd El-Gawad, Sara A. Shalaby, Mahmoud A.O. Dawood; Writing – original draft, Eman A. Abd El-Gawad, Mahmoud A.O. Dawood; Writing – review & editing, Mahmoud A.O. Dawood.
Corresponding author
Ethics declarations
Consent to participate
The authors are informed and agree with the study.
Consent to publish
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelmagid, A.D., Said, A.M., Abd El-Gawad, E.A. et al. Glyphosate-induced liver and kidney dysfunction, oxidative stress, immunosuppression in Nile tilapia, but ginger showed a protection role. Vet Res Commun 47, 445–455 (2023). https://doi.org/10.1007/s11259-022-09961-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-022-09961-0