Abstract
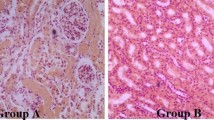
Rabbit haemorrhagic disease (RHD) is caused by a calicivirus infection that kills most adult rabbits 24–72 h after viral inoculation. Two liver enzymes (AST, aspartate aminotransferase, and ALT, alanine aminotransferase) were monitored in blood samples of calicivirus-infected rabbits during the short course of RHD. Values of AST were used to differentiate three stages of hepatocellular degeneration in RHD: mild (up to 20-fold increase in AST), moderate (150–200-fold elevation of AST) and severe (more than 1000-fold elevation in AST). Liver samples of rabbits from these three biochemical stages of hepatocellular degeneration of RHD were studied by transmission electron microscopy to define the fine structure of the hepatocytes. In the mild hepatocellular degeneration there was proliferation (microvesiculation) of the smooth endoplasmic reticulum and swelling of mitochondria into spheroid bodies with loss of cristae. In moderate hepatocellular degeneration, vacuolization of cytoplasm and mitochondrial damage continued to be present, and there was also formation of autophagic vesicles. In the severe hepatocellular degeneration of RHD, the altered mitochondria also showed loss of density of their matrix; rupture of cytoplasmic vacuoles led to the formation of large vesicles. Marked depletion of liver glycogen was also found in this late stage of RHD. These data offer a correlation between biochemical and cytological features of the liver during the hepatocellular degeneration of RHD.
Similar content being viewed by others
Abbreviations
- ALT:
-
alanine aminotransferase
- AST:
-
aspartate aminotransferase
- RHD:
-
rabbit haemorrhagic disease
- SER:
-
smooth endoplasmic reticulum
- TEM:
-
transmission electron microscopy
References
Águas, A.P., 1982. The use of the osmium teroxide-potassium ferrocyanide as an extracellular marker in electron microscopy. Stain Technology, 57, 69–73
Águas, A.P., Grande N.R. and Carvalho, E., 1991. Inflammatory macrophages contain high amounts of intravesicular ferritin and are associated with pouches of connective tissue. American Journal of Anatomy, 189, 89–96
Alonso, C., Oviedo, J.M., Martin-Alonso J.M., Diaz, E., Boga, J.A. and Parra, F., 1998. Programmed cell death in the pathogenesis of rabbit hemorrhagic disease. Archives of Virology, 143, 321–332
Boobis, A.R., Fawthrop, D.J. and Davies, D.S., 1992. Oxford Textbook of Pathology, (Oxford University Press, Oxford)
Capucci, L., Fallacara, F., Grazioli, S., Lavazza, A., Pacciarini, M.L. and Brocchi, E., 1998. A further step in the evolution of rabbit hemorrhagic disease virus: the appearance of the first consistent antigenic variant. Virus Research, 58, 115–126
Chasey, D., 1997. Rabbit haemorrhagic disease: the new scourge of Oryctolagus cuniculus. Laboratory Animals, 31, 33–44
Ciminale, V., Zotti, L., D'Agostino, D.M., Ferro, T., Casareto, L., Franchini, G., Bernardi, P. and Chieco-Bianchi, L., 1999. Mitochondrial targeting of the p13II protein coded by the x-II ORF of human T-cell leukemia/lymphotropic virus type I (HTLV-I). Oncogene, 18, 4505–4514
Coen, M., Lenz, E.M., Nicholson, J.K., Wilson, I.D., Pognan, F. and Lindon, J.C., 2003. An integrated metabonomic investigation of acetaminophen toxicity in the mouse using NMR spectroscopy. Chemical Research in Toxicology, 16, 295–303
Cooke, B.D. and Fenner, F., 2002. Rabbit haemorrhagic disease and the biological control of wild Oryctolagus cuniculus, in Australia and New Zealand, Wildlife Research, 29, 689–706
Cornelius, C.E., 1989. Clinical Biochemistry of Domestic Animals, (Academic Press, New York)
D'Agostino, D.M., Zotti, L., Ferro, T., Franchini, G., Chieco-Bianchi, L. and Ciminale, V., 2000. The p13II protein of HTLV type 1: comparasion with mitochondrial proteins coded by other human viruses. AIDS Research and Human Retroviruses, 16, 1765–1770.
Ferreira, P.G., Costa-e-Silva, A., Monteiro, E., Oliveira, M.J.R. and Aguas, A.P., 2004. Transient decrease in blood heterophils and sustained liver damage caused by calicivirus infection of young rabbits that are naturally resistant to rabbit haemorrhagic disease. Research in Veterinary Science, 76, 83–94
Ferreira, P.G., Costa-e-Silva, A., Monteiro, E., and Águas, A.P., 2005. Leukocyte hepatocyte interaction in calicivirus infection: differences between rabbits that are resistant or susceptible to rabbit haemorrhagic disease (RHD). Veterinary Immunology and Immunopathology, 10, 217–221
Gelmetti, D., Grieco, V., Rossi, C., Capucci, L. and Lavazza, A., 1998. Detection of rabbit haemorrhagic disease virus (RHDV) by in situ hybridisation with a digoxigenin labelled RNA probe. Journal of Virological Methods, 72, 219–226
Jaeschke, H., Gores, G.J., Cederbaum, A.I., Hinson, J.A., Pessayre, D. and Lemasters, J.J., 2002. Mechanisms of hepatotoxicity. Toxicological Sciences, 65, 166–176
Jones, T.C., Hunt, R.C. and King, N.W., 1997. Veterinary Pathology, (Williams & Wilkins, Baltimore)
Jung, J.Y., Lee, B.J., Tai, J.H., Park, J.H. and Lee, Y.S., 2000. Apoptosis in rabbit haemorrhagic disease. Journal of Comparative Pathology, 123, 135–140
Kanno, T., Fujita, H., Muranaka, S., Yano, H., Utsumi, T., Yoshioka, T., Inoue, M. and Utsumi, K., 2002. Mitochondrial swelling and cytochrome c release: sensitivity to cyclosporine A and calcium. Physiological Chemistry and Physics and Medical NMR, 34, 91–102
Kimura, T., Mitsui, I., Okada, Y., Furuya, T., Ochiai, K., Umemura, T. and Itakura, C., 2001. Distribution of rabbit haemorrhagic disease virus RNA in experimentally infected rabbits. Journal of Comparative Pathology, 124, 134–141
Meyer, D.J., Coles, E.H. and Rich, L.J., 1992. Veterinary Laboratory Medicine: Interpretation and Diagnosis, (W.B. Saunders, Philadelphia)
Mikami, O., Park, J.H., Kimura, T., Ochiai, K. and Itakura, C., 1999. Hepatic lesions in young rabbits experimentally infected with rabbit haemorrhagic disease virus. Research in Veterinary Science, 66, 237–242
Nepomnyashchikh, L.M., Lushnikova, E.L. and Semenov, D.E., 2001. Ultrastructural changes in cardiomyocyte mitochondria during regenerative and plastic insufficiency of the myocardium. Bulletin of Experimental Biology and Medicine, 131, 181–185
Parra, F. and Prieto, M., 1990. Purification and characterization of a calicivirus as the causative agent of a lethal hemorrhagic disease in rabbits. Journal of Virology, 64, 4013–4015
Pessayre, D., Mansouri, A., Haouzi, D. and Fromenty, B., 1999. Hepatotoxicity due to mitochondrial dysfunction. Cell Biology and Toxicology, 15, 367–373
Prieto, J.M., Fernandez, F., Alvarez, V., Espi, A., Garcia Marín, J.F., Alvarez, M., Martin, J.M. and Parra, F., 2000. Immunohistochemical localisation of rabbit haemorrhagic disease virus VP-60 antigen in early infection of young and adult rabbits. Research in Veterinary Science, 68, 181–187
Rej, R., 1983. Immunochemical quantification of isoenzymes of aspartate aminotransferase and lactate dehydrogenase. Clinical Biochemistry, 16, 17–19
Shien, J.H., Shien, H.K. and Lee, L.H., 2000. Experimental infections of rabbits with rabbit haemorrhagic disease virus monitored by polymerase chain reaction. Research in Veterinary Science, 68, 255–259
Teifke, J.P., Reimann, I. and Schirrmeier, H., 2002. Subacute liver necrosis after experimental infection with rabbit haemorrhagic disease virus (RHDV). Journal of Comparative Pathology, 126, 231–234
Ulrich, R.G., Bacon, J.A., Brass, E.P., Cramer, C.T., Petrella, D.K. and Sun, E.L., 2001. Metabolic, idiosyncratic toxicity of drugs: overview of the hepatic toxicity induced by the anxiolytic, panadiplon. Chemico-Biological Interactions, 134, 251–270
Valicek, L., Smid, B. and Rodak, L., 1992. Immunoelectron microscopy of rabbit haemorrhagic disease virus using monoclonal antibodies. Acta Virologica, 36, 589–591
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferreira, P.G., Costa-e-Silva, A., Monteiro, E. et al. Liver Enzymes and Ultrastructure in Rabbit Haemorrhagic Disease (RHD). Vet Res Commun 30, 393–401 (2006). https://doi.org/10.1007/s11259-006-3297-1
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11259-006-3297-1