Abstract
Reynoutria japonica (Japanese knotweed) is a highly invasive female plant that primarily reproduces through vegetative regeneration during secondary distribution. Despite producing a substantial number of viable hybrid seeds, the occurrence of seedlings and young plants in the wild remains rare; the reasons for this rarity are unclear. Environmental intolerance and chromosomal anomalies during hybridisation can impede seedling development. This study evaluates the combined impact of these factors by examining substrate, temperature, and light effects on germination, as well as assessing seedling genome sizes and viability. Seeds were cultivated in both natural and controlled environments to manage external influences. Flow cytometry was employed to evaluate chromosomal arrangements. The final germination was notably high at 99%, and 68% of seedlings thrived under controlled conditions, emphasising hybrid seedling viability regardless of highly polyploid levels ranging from pentaploid to aneuploid (2C DNA genome size from 5.17 to 11.95 pg). Thus, it is evident that seeds can germinate and produce vital seedlings despite various chromosomal sets. Even fluctuating temperatures and type of substrate do not limit seed germination. However, these results were obtained under laboratory conditions, with seeds and seedlings receiving regular irrigation. A significant seedling mortality rate (99%) was observed in the field experiment, and the final germination was also low there (15%). Observations suggest that water stress might be the cause of this mortality. Further research on water stress is necessary because it could be the primary factor limiting the successful generative spread of the knotweeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effective alien species management requires understanding the ecological processes affecting a species’ life cycle and population dynamics (Sakai et al. 2001), as well as knowledge of the intrinsic biological attributes of the species. For instance, understanding the conditions limiting successful germination, seedling establishment, and subsequent plant growth is crucial for predicting their potential spread (Guisan and Thuiller 2005).
This study focuses on seed survival and seedling establishment of the invasive plant Reynoutria japonica (Houtt.) var. japonica (Japanese knotweed) in its secondary distribution range (i.e., in a non-native distribution area). It’s widely known that in secondary distribution, it occurs as san octoploid (2n = 88) (Bailey and Stace 1992; Mandák et al. 2003), a male-sterile (female) clone, spreading mainly through vegetative reproduction via rhizome or stem fragments (Bailey 1994; Beerling et al. 1994). However, it can also produce numerous viable seeds through hybridisation (Online Resource Fig. S1), resulting in seeds with various chromosomal sets (Tiébré et al. 2007; Saad et al. 2011; Mereďa et al. 2023), which may enhance the invasive success of the Reynoutria complex. For example, R. ×bohemica (Chrtek et Chrtková), an interspecific hybrid between R. japonica and R. sachalinensis (F. Schmidt Petrop), is more competitive and vital than its parental taxa (Bímová et al. 2003; Parepa et al. 2014). The seeds of R. japonica exhibit a high germination rate, at least in laboratory conditions (Bailey 1994; Engler et al. 2011; Forman and Kesseli 2003). However, the reason why so few germinated seeds and seedlings are found in the wild remains unclear, underscoring the complexity of knotweed reproductive ecology.
It is known that environmental stress, a consequence of unsuitable conditions (e.g., Pessarakli 2015), plays a pivotal role in germination and seedling establishment. For example, seeds require a specific minimum moisture content to initiate germination, while excessive or insufficient water can hinder the process (Fay and Schultz 2009; Baskin and Baskin 2014). Also, temperature highly influences seed germination and seedling growth. During dry periods characterised by high temperatures and increasing water stress, germination may be interrupted and seedlings may face destruction (Baskin and Baskin 2014). Lighting conditions and soil characteristics can also influence germination and seedling establishment (Bronick and Lal 2005; Chen et al. 2020; Basset et al. 2023). Additionally, the germination of seeds might be influenced by the negative effect of maternal plants, potentially inhibiting the germination process. Several studies suggest that the presence of knotweeds inhibits the germination of other plant species (Vrchotová and Šerá 2008; Murrell et al. 2011; Mikulic-Petkovsek et al. 2022). Thus, it is possible that R. japonica may also inhibit its own seeds, and therefore knotweed seedlings are not found beneath the parent canopy. Another factor influencing seedling establishment is genetic growth constraints. The seeds of R. japonica can have various hybrid origins (Bailey et al. 2009) (Online Resource Fig. S1) with many aneuploid cytotypes (Tiébré et al. 2007; Saad et al. 2011; Mereďa et al. 2023) potentially impeding seedling development. Moreover, several studies tend to emphasise the adverse impact of environmental factors (Bailey 1994; Engler et al. 2011; Forman and Kesseli 2003; Saad et al. 2011), such as cold winter serving as a selection factor of early progeny cohorts surviving regardless of their ploidy levels (Saad et al. 2011). From the existing literature, it is not clear which of these factors is crucial, whether it is an individual one or a combination. The revelation of why seedlings of knotweeds are not observed in nature would clarify many uncertainties regarding the generative reproduction of the whole genus. It would also have a notable effect on the approach to the management of the entire group of invasive knotweed taxa, conservation of natural resources, and predicting the spread of new knotweed taxa in open landscapes (Abbott 1992; Sakai et al. 2001; Saad et al. 2011).
To fill this gap, we combined in-situ and ex-situ experiments to explore how various environmental conditions affect the germination and seedling survival of R. japonica seeds. We assessed the effect of temperature fluctuations during the day and night (i.e., 22/15, 25/10 and 25/5 °C; Day/Night) and the effects of different nutrient and humus substrates (nutrient-rich: universal garden soil, nutrient-poor: sand). We also evaluated germination success and seedling growth in soil collected from beneath maternal plants and the effects of different sunlight conditions (i.e., low, medium and high sunlight). Additionally, we determined which cytological types are successful by examining the genome size of surviving seedlings. These methods can help to identify potential environmental or genetic barriers to successful germination and subsequent seedling growth.
Material and methods
Study species
Reynoutria japonica is an insect-pollinated, gynodioecious, herbaceous perennial plant native to Asia (China, Japan, parts of Korea and Taiwan), originally imported to Europe as an ornamental plant (Siebold 1848; Beerling et al. 1994). It is known as one of the worst European invasive plants (Nentwig et al. 2018), but the problem with their invasive spreading is global (e.g., Clements et al. 2016). R. japonica invades riparian, anthropogenic habitats and many artificial habitats, such as public parks or gardens (Vojík 2023). The adult plant is up to 3 m tall, with leaf blades 5–12 cm long, 5–8 cm wide, broadly ovate, pointed at the apex and truncate at the base, and petioles 1–3 cm long. Flowering occurs in late summer; the flowers are creamy white, regular, tepals 5, and are not differentiated into calyx and corolla. The inflorescence is initially erect but droops at maturity. The stems are green with reddish spots, glaucous stout, erect, branched and flexuous above, arising from strong rhizomes (Beerling et al. 1994).
Seed collection and germination experiments
The population of R. japonica for collecting the seeds was selected in the Czech Republic (Central Bohemia region—Online Resource Fig. S2). The site’s climatic characteristics are as follows (Vašátko 2023): altitude above sea level is 205 m, with a mean annual temperature of 9 °C. During summer, the mean temperature is 18.3 °C, while in winter it is 0.07 °C. The study site receives an average annual precipitation of 583 mm.
For the determination of R. japonica plants, morphological identification was based on leaf and flower characters, including the presence of trichomes, the length and width of the leaves, and the shape of the leaf base and apex as well as existing flowers with small, flat, empty anthers with stigma exceeding the perianth (Beerling et al. 1994).
Seeds were collected from the ten healthiest stems on the fully matured plant. The required number of healthy-looking and fully-sized seeds was selected for germination experiments (i.e., 2880 seeds). The seeds were collected at the end of autumn 2022. Because the longevity of the seeds can be significantly different (e.g., between families or localities, Walters et al. 2005), we used fresh seeds for the experiments, which were first dried at room temperature (ca. 20 °C) for a week. Furthermore, they were dark stratified at 5 °C for six weeks to simulate the winter period in Central Europe (e.g., Kołodziejek 2019; Mandák et al. 2006).
Description of Experiments
Two germination experiments were undertaken, the first (EXP1) assessing the effect of different substrate types and temperature regimes on seed germination (ex-situ) and the second (EXP2) considering differences in germination between different substrate types and light conditions under and away from R. japonica stands (in-situ).
EXP1: Controlled germination study (ex-situ experiment)
The seeds were incubated in dishes (18 × 10 × 4.5 cm, volume: 0.5 l) containing 0.4 l of tested substrate. Universal garden soil was selected as the nutrient-rich humic substrate, while nutrient humus-poor sand was tested in contrast. Additionally, soil taken from beneath the maternal plants was tested, covered with R. japonica leaves (a common occurrence under R. japonica stands) and the same soil without leaves for comparison (Table 1).
The dishes with seeds were kept under a simulated daily light regime of 14 h light and 10 h dark with three different temperature regimes ( Table 1) to see if the fluctuating temperature harms the seed. Seeds were watered regularly with distilled water (i.e., 100 ml of distilled water to each dish every two days, ensuring consistent moisture levels throughout the experiment). Each population sample consisted of 30 seeds in three replicates for each substrate type (i.e., 12 dishes were tested for each temperature regime). To limit any deviation because of the big difference between day and night temperatures in treatments with medium and high fluctuation, the experiment was conducted in two ClimaBoxes; this approach was used to ensure that the results were not affected by the differences between ClimaBoxes. A total of 1800 seeds were tested (360 seeds for 22/15 °C, 720 for 25/10 °C, and 720 for 25/5 °C). All germinated seeds were counted and recorded at two-day intervals for 20 days (seeds were considered germinated when the radicle appeared). The experiments were performed in complete randomised blocks.
For seedling recruitment, three hundred germinated seeds were randomly selected from the abovementioned treatments and cultivated in a greenhouse with a constant temperature of 21 °C. The seedlings were watered regularly, and their subsequent development was observed to determine whether they continued to grow or perished. The seedlings were grown on universal garden soil. After six months, 20 seedlings were randomly selected for cytological analyses.
EXP2: Field germination study (in-situ experiment)
EXP2 followed the methodology of EXP1, but the dishes with seeds were placed at the study site of R. japonica, where the seeds were collected. The dishes were placed in the soil so that their upper edges were level with the soil surface. The dishes had drainage holes on their bottoms to facilitate the outflow of excess water. At the same time, ambient moisture could penetrate them. Therefore, they were isolated from the surrounding soil but not from raising water or rainfall. The location of the particular blocks of dishes was chosen to ensure that 12 dishes were in the shade from the maternal plant (low sunlight), 12 dishes in about 50% of full sunlight (medium), and 12 dishes in full sunlight (high) (the experimental design and the numbers of seed were combined in Table 1). The experiments were performed in randomised blocks. The experiment was covered with a fine mesh to prevent further seeds from falling into the dish.
The germinated seeds were not removed; they were left in the dishes, and their growth or mortality was recorded. The seedlings were not additionally watered; everything was left to the site’s environmental conditions.
The experiment ran for 70 days, starting on the 1st of May and concluding on the 10th of July. The germinated seeds were counted and recorded at one-week intervals. At the end of the experiment, only one seedling survived, which underwent cytological analysis. At the studied site, freely growing seedlings were discovered in a wet terrain depression, and after six months, nine of them were randomly selected for flow cytometry analysis. Flow cytometry was also performed on the maternal stand of R. japonica and four male plants of R. ×bohemica growing in the same locality.
Genome size and ploidy levels
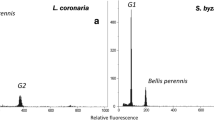
To determine genome size, DNA weight per nucleus (genome size; expressed in picograms [pg]) was analysed using flow cytometry, based on a two-step procedure using Otto I and II buffers (Otto 1990). As its genome size (2C DNA = 3.38 pg) is close to that of the species studied, the common daisy Bellis perennis was used as an internal reference standard (Schönswetter et al. 2007). In each case, 1 cm2 of fresh leaf tissue from the study species and from B. perennis were macerated together with a sharp blade and placed in a Petri dish containing 0.1 ml of ice-cold Otto I buffer (0.1 M citric acid monohydrate, 0.5% v/v Tween 20) for about 90 s, after which the suspension was filtered through a 42 µm nylon mesh. Nuclei within the filtered suspension were then stained with 1 ml of Otto II buffer (0.4 M Na2HPO4 ·12H2O) supplemented with 1 ml of DAPI stock solution (DAPI 10 mg dissolved in 100 ml H2O) + 50 µl β-mercaptoethanol (2 μL/mL). Each sample was then incubated at room temperature for 10 min and analysed using a Sysmex‐Partec CyFlow SL equipped with a green solid-state laser flow cytometer (532 nm, 100 mW output power; Sysmex Partec GmbH, Görlitz, Germany). DNA ploidy / DNA aneuploidy levels refer to nuclear DNA content in the sense of Suda et al. (2006).
Substrate analysis
Soil oxidisable carbon (COX) was selected to assess the quality of the tested substrates as a dominant component of soil organic matter significantly affected the soil structure, water retention, and formation of soil colloids (Wander 2004).
For determination of COX, modified Tjurin’s method was used, i.e., wet oxidation of organic carbon with potassium dichromate (K2Cr2O7) solution in a sulfuric acid (H2SO4) and subsequent potentiometric titration of the unreacted residue of the chrome-sulfur mixture with Mohr’s salt ((NH4)2Fe(SO4)2· 6H2O) (Carter and Gregorich 2007).
Data analysis
Two germination traits were measured for both experiments: (1) final germination (hereinafter FG), calculated as the total number of germinated seeds at the end of the germination period, and (2) germination delay (hereinafter GD), calculated as the number of days until first germination was recorded (Cerabolini et al. 2004; Pepe et al. 2020).
Differences in FG (EXP1) according to different temperatures and substrates were tested by generalised linear models (GLM) with Poisson distribution. The final number of germinated seeds (FG) was transformed using reflection to reduce the negative skewness of the response variable distribution. The effect of substrates and light regime (EXP 2) on FG was tested by GLM for the negative binomial family. Temperature, substrate, and light regime were fixed factors in all tested models, and the number of germinated seeds was the response variable. The same models were used for GD analyses (EXP1, EXP2) using a number of days until the first germination recorded as a response variable and different substrates and light regimes as the predictor (fixed effect). Full models were simplified using posterior comparisons, and the most plausible models were selected based on the Akaike information criterion (Akaike 1978) using backward selection. All models were based on main effects without interactions, as the interactions were not statistically significant (Online Resource Table S1).
All analyses were performed using R software (R Development Core Team 2019) and Statistica 13 (TIBCO 2017), with differences considered significant at P ≤ 0.05.
Results
EXP1: Controlled germination study (ex-situ experiment)
Different temperature regimes significantly influenced FG of R. japonica seeds (GLM; Wald.Stat.2 = 43.671, P < 0.001) (Fig. 1). In particular, the average germination at 22/15 °C was lower (x̄ ± SE; 26.3 ± 0.68; average of germinated seeds per dishes, SE: Standard Error of Mean) and statistically significantly different from 25/10 °C (29.6 ± 0.16) and 25/5 °C (29.5 ± 0.17). Interestingly, the substrate type did not have a statistically significant effect on FG (GLM; Wald.Stat.3 = 2.804, P = 0.423).
Different substrate types significantly affected the GD of R. japonica seeds (GLMM; z3,56 = 4.709, p < 0.001). With a two-day GD, R. japonica seeds demonstrated a faster germination rate at both field substrates. In contrast, a delayed germination pattern was observed in the garden and sand substrates (GD = six days) (Online Resource Fig. S3). There were no notable variations in GD across different temperature regimes (GLMM; z2,54 = 0.471, P = 0.638).
After 6 months in controlled conditions, 68% of the seedlings survived.
EXP2: Field germination study (in-situ experiment)
The type of substrate had a significant impact on the FG of R. japonica seeds (GLMM; z2,30 = 1.600, P < 0.01) (Fig. 2), which is a different outcome compared to EXP1 where the substrate type had no significant influence on FG. Among the different substrates tested, the garden substrate exhibited the highest average germination rate (x̄ ± SE; 7.2 ± 1.04; average of germinated seeds per dishes, SE: Standard Error of Mean), while the rest demonstrated considerably lower germination rates in comparison. The lowest average germination was at the field substrate (3.1 ± 0.91) and sand (2.9 ± 0.66). Different availability of light also had a significant impact on the FG of seeds (GLMM; z2,30 = -4.041, P < 0.001) (Fig. 2). The highest average germination was under low sunlight (7.4 ± 1.35) compared to medium (2.7 ± 1.13) and high sunlight (3.9 ± 1.96).
Final germination of R. japonica seeds of EXP2 according to different substrates and light conditions. Central lines = median of final germinated seeds; boxes = the range between the first (lower) quartile (Q1) and the third (upper) quartile (Q3), whiskers = Q1 or Q3 quartile ± SE, whiskers = min. – max
GD was significantly affected by different light regimes (GLMM; z2,30 = 5.468, P < 0.001) (Online Resource Fig. S4). Seeds germinated faster at low sunlight with GD of 7 days compared to seeds at medium (GD = 21) and high sunlight (GD = 14). There was no significant difference in GD according to different substrate types (GLMM; z3,32 = -1.334, P = 0.182).
Seedling mortality was observed in the dry season without rain. At the end of the experiment (i.e., 70 days), only one seedling survived (grew on garden substrate and shade). Cumulative rainfall, including recorded seedlings and their growth, is depicted in Fig. 3.
The number of seedlings and changes in their count caused by death or germination of new ones (arrows) during EXP2. Rainfall is shown in the columns (Vašátko 2023). The directions of the arrows show a decrease or increase number of seedlings; the number indicates the value of live seedlings (seedlings were counted and recorded at one-week intervals)
Genome size and ploidy levels
Flow cytometric analysis demonstrated large differences in genome size between studied Reynoutria plants; the presence of hexaploid and octoploid plants was confirmed at the study site (Online Resource Fig. S5). All adult potential male pollen donors of R. ×bohemica plants were hexaploid (2n = 6x = 66; 2C DNA = 6.69 ± 0.02 pg), while the R. japonica plants, from which seeds were collected and under which soil samples were obtained for this study, were octoploid (2n = 8x = 88; 2C DNA = 9.10 pg). R. japonica seedlings were highly polyploid, with genome sizes ranging from 5.17 to 11.95 (2C DNA in pg). One seedling corresponding to pentaploid was found; there was no seedling corresponding to the genome size of hexaploid; 13 seedlings matched the octoploid genome size, while the rest of the seedlings (16) did not correspond to any common ploidy level. The genome sizes and DNA ploidy levels of all tested samples are summarised in Table 2.
Substrate analysis
The analysis of COX levels in the tested substrates revealed variations. Field substrates exhibited COX of 4.66%, indicating a relatively low level of COX. In contrast, garden substrate displayed a higher COX level of 31.66%. Sand exhibited the lowest COX at 0.05%.
Discussion
The influence of environmental factors on germination and seedling establishment
The presented study confirmed the production of a substantial quantity of viable and highly germinable hybrid seeds of R. japonica. In controlled conditions (EXP1), final germination (FG) reached 88% (22/15 °C) to 99% (25/10 °C, 25/ 5 °C). This finding is not surprising since previous studies have already reported high germination (Bailey 1994; Engler et al. 2011; Forman and Kesseli 2003). Seeds germinated successfully on all tested substrates, indicating low substrate specificity for successful germination. This is consistent with Grime et al. (1988), which states that R. japonica thrives in various soil types. Even fluctuating temperatures did not affect germination success. The seeds endured a temperature drop of up to 20 °C (i.e., in the 25/5 °C treatment). Thus, R. japonica seeds appear to display favourable readiness for germination across diverse substrates and exhibit resilience to variations in weather conditions. It is essential to highlight that our investigation focused on the impact of late spring to early summer temperature fluctuations, ranging from 25 °C to 5 °C, rather than frost. We acknowledge the potential significance of frost as a factor influencing seed germination and seedling development, which will be the subject of further research. Additionally, no negative effects from maternal plants potentially inhibiting germination were found. Seeds grew on soil taken from beneath R. japonica stands (i.e., field substrates) germinated very quickly (four days before those on garden and sand substrates), with FG = 97% (for comparison: garden and sand substrates showed FG = 96%). Based on these findings, we conclude that R. japonica seeds can successfully germinate in soil from under maternal plants; our results are also supported by the discovery of R. japonica seedlings in the studied locality (Online Resource Fig. S6). Forman and Kesseli (2003) also found that seedlings germinated under well-established knotweed stands. However, they also found that the seedlings were unlikely to survive due to the blocking of sunlight by the knotweed canopy. Thus seedling survival is likely to be more dependent on the availability of adequate resources rather than the allelopathic effects of the mother plant.
Under laboratory conditions, we did not identify any clear environmental explanations limiting seed germination.
The field experiment (EXP2) shows a high decrease in germination compared to laboratory conditions (EXP1). The average FG was 15%, and of all the germinated seeds, only one seedling survived (grew on garden substrate and shade, Online Resource Fig. S7). This result may be attributable to the low tolerance of seeds and seedlings to drought. Periods with low precipitation levels could lead to drying of germinating seeds and increased mortality of seedlings across the substrates (see Fig. 3 and Online Resource Fig. S8). The field experiment revealed the garden substrate as the most suitable for germination, showing an 11% higher germination rate compared to other substrates. This can be linked to the highest organic matter content (COX = 31.66), which may help retain moisture over extended periods. Thus, this substrate could ensure optimal nutrient conditions with sufficient moisture during dry periods to enhance the survival of seeds and seedlings. Another essential factor affecting germination was light. The highest average germination rate (24%) was recorded in shaded conditions, which could protect seedlings from direct sunlight and related desiccation. Moreover, under this light condition, many seedlings were observed growing around the experiment site (Online Resource Fig. S6). Based on our results and related field observations, it seems that environmental factors like substrate moisture and light conditions can strongly influence the ability of seeds to germinate and seedlings to establish in the field. This finding is consistent with the results of Forman and Kesseli (2003). The authors suggest that seedling survival is dependent on the availability of adequate resources (light and water) and milder winters.
Nevertheless, it is necessary to mention at this point that the germination and establishment of the seedlings could have been affected by the conditions of the experimental dishes. Although the dishes were adjusted to match the moisture conditions as closely as possible to the surrounding soil and light conditions, the highest number of seedlings at the experimental site was observed around the field depressions, where water was retained the longest after rain. These areas were moister than the rest of the site. Therefore, we believe that low seedling survival in experimental dishes is primarily influenced by soil moisture, consistent across the site except for the wettest areas.
Some publications also mention predation as an important factor in reducing the chance of seedling establishment. In the field, Engler et al. (2011) observed that half of the collected R. japonica seeds had cracked seed coats and missing endosperm, which they attributed to birds feeding on the seeds. Additionally, Bailey et al. (1995) reported bird predation; most of the seeds of R. japonica were eaten by house sparrows (Passer domesticus). These authors also noted that a cold, humid winter negatively affects seedling survival. Thus, although R. japonica may produce abundant viable seeds (Bailey 1994; Engler et al. 2011; Forman and Kesseli 2003), a substantial proportion may fall prey to predation, while germination of the remaining seeds or seedlings establishment may be limited by drought, light, frost and other environmental stressors (Bailey et al. 1995; Engler et al. 2011; Saad et al. 2011). According to our study, fluctuating soil moisture appears to be the most limiting factor of the factors mentioned.
Genome size and ploidy levels
The study seedlings originated from open-pollinated R. japonica seeds, making it impossible to determine the specific pollen donor accurately. However, pollination probably occurred either through male R. ×bohemica plants found in the study area (Online Resource Fig. S9, S10), resulting in a backcross, or through F. aubertii (abundantly represented in a 1 km radius of the study area) leading to the formation of Reyllopia ×conollyana hybrids. R. japonica and the potential pollen donors R. ×bohemica were subjected to genome size analysis, which revealed expected DNA ploidy levels (octaploid for R. japonica and hexaploid for R. ×bohemica) consistent with previous findings (Mandák et al. 2003; Suda et al. 2010). Genome analysis of 30 seedlings (20 seedlings from EXP1, one surviving seedling from EXP2, and nine freely growing seedlings from the study locality) yielded more interesting information. Genome size exhibited a wide range, from 5.17 to 11.95 (see Table 2). Thirteen seedlings had genome sizes corresponding to octoploid plants. These seedlings may result from different backcrosses with unreduced or irregular pollen grains from the hexaploid R. ×bohemica or diploid F. aubertii, leading to 54—154 chromosomes. The potential hybridisation output for pentaploid seedling is the crossing of gametes of R. japonica (1n = 44) with those of F. aubertii (1n = 10), likely resulting in hybrid exhibiting 2n = 54 (i.e., hybrid as R. ×conollyana). Genome sizes similar to hexaploids could result from crosses between octoploid R. japonica (1n = 44) and unreduced gametes of F. aubertii (1n = 20), leading to hybrids with 2n = 64, which might be present in the locality but were not detected. The genome sizes approaching octaploidy should be result of crosses between octoploid R. japonica (1n = 44) and hexaploid R. ×bohemica (1n = 33), leading to hybrids with 2n = 77. The variability in seed genome size and composition appears to have minimal impact on the viability of the study plants, as evidenced by a 68% survival rate of seedlings under controlled conditions (EXP1) after six months (Fig. 4).
Conclusions
Based on previous studies and our results, the establishment of seedlings of R. japonica is site-specific. The low number of germinated seeds and established seedlings appears to result from unfavourable environmental conditions during seedling germination and growth rather than an inappropriate genetic or cytological make-up. Our observations suggest that water stress might be an important factor affecting seedling mortality.
In the context of climate change, considering the high ability of R. japonica to produce large numbers of viable hybrid seeds, it is possible that rare established seedlings may become more common. Adult fertile aneuploid individuals (male-fertile R. ×bohemica) have already been reported (Mereďa et al. 2023). Thus, there is a risk that evolutionary processes could create new plants that quickly spread to new locations and better adapt to specific environments than their parental taxa. Therefore, it is essential to identify areas where these processes may occur, study them, and develop new management strategies that may be required to prevent the emergence and spread of new clones from seedlings, especially along damp riverbanks.
Data availability
Not applicable.
References
Abbott RJ (1992) Plant invasions, interspecific hybridisation and the evolution of new plant taxa. Trends Ecol Evol 7:401–405. https://doi.org/10.1016/0169-5347(92)90020-C
Akaike H (1978) A Bayesian analysis of the minimum AIC procedure. Ann Inst Stat Math 30:9–14. https://doi.org/10.1007/BF02480194
Bailey JP (1994) The reproductive biology and fertility of Fallopia japonica (Japanese Knotweed) and its hybrids in the British Isles. In: De Waal LC, Child LE, Wade PM, Brock JH (eds) Ecology and management of invasive riparian plants. John Wiley and Sons, Chichester, pp 141–158
Bailey JP, Stace CA (1992) Chromosome number, morphology, pairing, and DNA values of species and hybrids in the genus Fallopia (Polygonaceae). Plant Syst Evol 180:29–52. https://doi.org/10.1007/BF00940396
Bailey JP, Child LE, Wade M (1995) Assessment of the genetic variation and spread of British populations of Fallopia japonica and its hybrid Fallopia ×bohemica. In: Pysek P, Prach K, Rejmánek M, Wade PM (eds) Plant invasions: general aspects and special problems. SPB Academic Publishing, Amsterdam, pp 141–150
Bailey JP, Bímová K, Mandák B (2007) The potential role of polyploidy and hybridisation in the further evolution of the highly invasive Fallopia taxa in Europe. Ecol Res 22:920–928
Bailey JP, Bímová K, Mandák B (2009) Asexual spread versus sexual reproduction and evolution in Japanese Knotweed s.l. sets the stage for the “battle of the Clones.” Biol Invasions 11:1189–1203. https://doi.org/10.1007/s10530-008-9381-4
Baskin CC, Baskin JM (2014) Seeds: Ecology, biogeography, and evolution of dormancy and germination, 2nd edn. Academic Press
Basset C, Abou Najm M, Ghezzehei T, Hao X, Daccache A (2023) How does soil structure affect water infiltration? A meta-data systematic review. Soil Tillage Res 226:1–15
Beerling DJ, Bailey JP, Conolly AP (1994) Fallopia japonica (Houtt.) Ronse Decraene. J Ecol 82:959
Bímová K, Mandák B, Pyšek P (2003) Experimental study of vegetative regeneration in four invasive Reynoutria taxa (Polygonaceae). Plant Ecol 166:1–11. https://doi.org/10.1023/A:1023299101998
Bronick CJ, Lal R (2005) Soil structure and management: A review. Geoderma 124:3–22
Carter MR, Gregorich EG (2007) Soil Sampling and Methods of Analysis, 2nd edn. CRC Press. https://doi.org/10.1201/9781420005271
Cerabolini B, De Andreis R, Ceriani RM, Pierce S, Raimondi B (2004) Seed germination and conservation of endangered species from the Italian Alps: Physoplexis comosa and Primula glaucescens. Biol Conserv 117:351–356
Chen F, Zhou W, Yin H, Luo X, Chen W, Liu X, Wang X, Meng Y, Feng L, Qin Y, Zhang C, Yang F, Yong T, Wang X, Liu J, Du J, Liu W, Yang W, Shu K (2020) Shading of the mother plant during seed development promotes subsequent seed germination in soybean. J Exp Bot 71:2072–2084. https://doi.org/10.1093/jxb/erz553
Clements DR, Larsen T, Grenz J (2016) Knotweed management strategies in north america with the advent of widespread hybrid bohemian knotweed, regional differences, and the potential for biocontrol via the psyllid Aphalara itadori Shinji. Invasive Plant Sci Manag 9:60–70. https://doi.org/10.1614/IPSM-D-15-00047.1
Engler J, Abt K, Buhk C (2011) Seed characteristics and germination limitations in the highly invasive Fallopia japonica s.l. (Polygonaceae). Ecol Res 26:555–562
Fay PA, Schultz MJ (2009) Germination, survival, and growth of grass and forb seedlings: Effects of soil moisture variability. Acta Oecologica 35:679–684
Forman J, Kesseli RV (2003) Sexual reproduction in the invasive species Fallopia japonica (Polygonaceae). Am J Bot 90:586–592
Grime JP, Hodgson JG, Hunt R (1988) Comparative Plant Ecology: A Functional Approach to Common British Species, 1st edn. Springer, Dordrecht, London
Guisan A, Thuiller W (2005) Predicting species distribution: Offering more than simple habitat models. Ecol Lett 8:993–1009
Hodálová I, Mártonfiová L, Skokanová K, Španiel S, Mereďa P (2022) Fallopia ×moravica (Polygonaceae), a new hybrid between Fallopia compacta and F. sachalinensis. Phytotaxa 572:123–143
Kołodziejek J (2019) (2019) Growth performance and emergence of invasive alien Rumex confertus in different soil types. Sci Reports 91(9):1–13. https://doi.org/10.1038/s41598-019-56068-9
Mandák B, Pyšek P, Lysák M, Suda J, Krahulcová A, Bímová K (2003) Variation in DNA-ploidy levels of Reynoutria taxa in the Czech Republic. Ann Bot 92:265–272
Mandák B, Bímová K, Plačková I (2006) Genetic structure of experimental populations and reproductive fitness in a heterocarpic plant Atriplex tatarica (Chenopodiaceae). Am J Bot 93:1640–1649. https://doi.org/10.3732/AJB.93.11.1640
Mereďa P, Mártonfiová L, Skokanová K, Španiel S, Hodálová I (2023) Cytogeography of invasive knotweeds (Fallopia sect. Reynoutria) in central Europe: rare aneuploids and evidence for a climatically determined distribution. Preslia 95:241–266
Mikulic-Petkovsek M, Veberic R, Hudina M, Misic E (2022) HPLC-DAD-MS Identification and Quantification of Phenolic Components in Japanese Knotweed and American Pokeweed Extracts and Their Phytotoxic Effect on Seed Germination. Plants 11:3053
Murrell C, Gerber E, Krebs C, Parepa M, Schaffner U, Bossdorf O (2011) Invasive knotweed affects native plants through allelopathy. Am J Bot 98:38–43
Nentwig W, Bacher S, Kumschick S, Pyšek P, Vilà M (2018) More than “100 worst” alien species in Europe. Biol Invasions 20:1611–1621. https://doi.org/10.1007/s10530-017-1651-6
Otto F (1990) Chapter 11 DAPI Staining of Fixed Cells for High-Resolution Flow Cytometry of Nuclear DNA. Methods Cell Biol 33:105–110. https://doi.org/10.1016/S0091-679X(08)60516-6
Parepa M, Fischer M, Krebs C, Bossdorf O (2014) Hybridization increases invasive knotweed success. Evol Appl 7:413–420. https://doi.org/10.1111/eva.12139
Pepe M, Gratani L, Fabrini G, Varone L (2020) Seed germination traits of Ailanthus altissima, Phytolacca americana and Robinia pseudoacacia in response to different thermal and light requirements. Plant Species Biol 35:300–314. https://doi.org/10.1111/1442-1984.12286
Pessarakli M (2015) Plant Responses under Environmental Stress Conditions. Adv Plants Agric Res 2:276–286
R Development Core Team (2019) R: A language and environment for statistical computing
Saad L, Tiébré MS, Hardy OJ, Mahy G, Vanderhoeven S (2011) Patterns of hybridization and hybrid survival in the invasive alien Fallopia complex (Polygonaceae). Plant Ecol Evol 144:12–18. https://doi.org/10.5091/plecevo.2011.444
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332. https://doi.org/10.1146/annurev.ecolsys.32.081501.114037
Schönswetter P, Suda J, Popp M, Weiss-Schneeweiss H, Brochmann C (2007) Circumpolar phylogeography of Juncus biglumis (Juncaceae) inferred from AFLP fingerprints, cpDNA sequences, nuclear DNA content and chromosome numbers. Mol Phylogenet Evol 42:92–103. https://doi.org/10.1016/J.YMPEV.2006.06.016
Siebold PF (1848) Extrait du catalogue et du prix-courant des plantes du Japon et des Indes-Orientales et Occidentales Neerlandaises. Jaarb van K Ned Maatsch tot Aanmoediging van den Tuinbouw 38–49
Suda J, Krahulcová A, Trávnícek P, Krahulec F (2006) Ploidy level versus DNA ploidy level: An appeal for consistent terminology. Taxon 55:447–450. https://doi.org/10.2307/25065591
Suda J, Trávníček P, Mandák B, Berchová-Bímová K (2010) Genome size as a marker for identifying the invasive alien taxa in Fallopia section Reynoutria. Preslia 82:97–106
TIBCO (2017) TIBCO Statistica® In Computer program (13.3.0). TIBCO Software Inc
Tiébré MS, Bizoux JP, Hardy OJ, Bailey JP, Mahy G (2007) Hybridization and morphogenetic variation in the invasive alien Fallopia (Polygonaceae) complex in Belgium. Am J Bot 94:1900–1910. https://doi.org/10.3732/ajb.94.11.1900
Vašátko T (2023) Elektronický digitální povodňový portál [Electronic digital flood portal]. https://www.edpp.cz/
Vojík M (2023) Alien and invasive plant species in parks and gardens of the Czech Republic (PhD Thesis). Czech University of Life Sciences Prague, pp 138
Vrchotová N, Šerá B (2008) Allelopathic properties of knotweed rhizome extracts. Plant, Soil Environ 54:301–303
Walters C, Wheeler LM, Grotenhuis JM (2005) Longevity of seeds stored in a genebank: species characteristics. Seed Sci Res 15:1–20. https://doi.org/10.1079/SSR2004195
Wander M (2004) Soil Organic Matter Fractions and Their Relevance to Soil Function. Soil Organic Matter in Sustainable Agriculture. CRC Press, Boca Raton, pp 67–102
Acknowledgements
We thank the Ecological Department of the Faculty of Environmental Sciences for renting ClimaBoxes, and Environmental English for language correction.
Funding
Open access publishing supported by the National Technical Library in Prague. This study was financially supported by the project “DivLand” (No. SS02030018) from the Technology Agency of the Czech Republic, by a grant donated by the Ministry of Education, Youth and Sports of the Czech Republic (CZ.02.1.01/0.0/0.0/16_026/0008403) and supported by Norway through the Norway Grants “Be plant wise—do not support invasive species” (No. 3211100006).
Author information
Authors and Affiliations
Contributions
All persons entitled to authorship have been so named, and all authors have seen and agreed to the submitted version of the manuscript. Martina Kadlecová: Conceptualisation, data collection and processing, writing—original draft; Martin Vojík: Data collection and text revision; Jaroslav Vacula: Graphic outputs; Kateřina Berchová Bímová: Expert consultation and revision of the text.
Corresponding author
Ethics declarations
Conflict of interest
There are no potential conflicts with Ethical Standards and conflicts of interest (financial or non-financial). All financial supporting resources are listed.
Animal research
Not applicable.
Consent to Participate
Not applicable.
Consent to publish
Not applicable.
Plant reproducibility
Not applicable.
Clinical trials registration
Not applicable.
Additional information
Communicated by Giulia Albani Rocchetti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kadlecová, M., Vojík, M., Vacula, J. et al. Grab to fill the gap: key factors influencing Reynoutria japonica germination and seedling establishment in the secondary distribution range. Plant Ecol (2024). https://doi.org/10.1007/s11258-024-01438-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11258-024-01438-1