Abstract
Prescribed burning is a management tool used for both management of fuel loads and for ecological purposes across fire prone areas. While in temperate areas wildfires usually occur during the hottest summer months, prescribed burns are generally conducted in autumn and spring, when conditions are more suitable for controlling fire. Orchids maintain avoidance mechanisms, such as persisting as dormant tubers during the predominant fire season, and therefore may be at risk from prescribed burns occurring during their active life cycle period. Using a glasshouse experiment, we investigated the impacts of fire season on the Australian orchid species Pterostylis curta. This approach allowed us to i) implement seasonal burns and relate impacts to quantifiable above and belowground life cycle stages of the study species, ii) isolate and assess the role of smoke, and iii) control for fire intensity and life stage of the study species at each of the treatment levels to enable robust comparison focused on fire season effects. We found that late autumn burns caused complete failure of a cohort in our glasshouse study. Heat alone was not the driver of tuber mortality, because soil heating was similar across all burn seasons, and plants burnt in the three other seasons were able to re-emerge strongly in the growing season after fire. Furthermore, a lack of post-fire emergence was due to tuber mortality, not dormancy. Our results highlight that there is likely an interaction between fire-related heat and the life cycle stage at which burning occurs, especially replacement tuber initiation, that drives post-fire demography. We show that orchids like P. curta had the lowest risk of negative impacts when burnt in the later stages of their growing season, and that an understanding of finer-scale phenological cycles can inform more robust fire management of orchid species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fire plays an important role in shaping plant communities around the world (Whelan 1995; Bond & van Wilgen 1996; McLauchlan et al. 2020). Species have adapted to persist under particular fire regimes, consisting of the frequency, seasonality and severity (among other elements) of fire at any given site (Gill 1975; Keeley et al. 2006). However, fire regimes are shifting due to climate change and other anthropogenic drivers, including urbanisation and associated increases in ignitions and prescribed fire management (Penman et al. 2011; Balch et al. 2017). While the impacts of changing fire regimes, particularly increasing fire frequency, are well studied, far fewer studies have investigated the impacts of shifts in other components of the fire regime, such as seasonality (Nolan et al. 2021; Tangney et al. 2022; Thomsen & Ooi 2022).
Fires in temperate systems generally occur during the hottest months of the year, from late spring to early autumn (Bradstock 2010). In contrast, prescribed burns, for hazard reduction or ecological purposes, for practical reasons are conducted outside of these peak hot seasons (Penman et al. 2011; Paroissien & Ooi 2021; Thomsen et al. 2023). Depending on a plant species’ given traits, they may be impacted differently by fires occurring outside the historic fire season, and may be subject to neutral, negative or even positive effects on persistence (Tangney et al. 2022).
In Australia, there is a broad perception that many terrestrial orchids are pyrophytic, where abundance or the proportion of plants flowering is promoted by wildfire, allowing plants to take advantage of minimal competition post-fire (Coates & Duncan 2009; Jasinge et al. 2018a). However, negative impacts of fire are also commonly found (Jones & Clements 2002; Duncan 2012; Jasinge 2018b), suggesting that multiple factors can drive response, including different fire regime elements. This assumption is supported by the reporting of mixed responses to fire for species within the same genus. For example, studies on Pterostylis in Australia have reported positive effects of fire on abundance of plants emerged (Jasinge et al. 2018b), neutral effects (Quarmby 1999; Duncan 2012) or negative effects, particularly on emergence and flowering (Jones & Clements 2002; Duncan 2012). The similar phenological attributes of species across this genus therefore suggests that different fire regime components may drive variation in response.
Reductions in orchid abundance post-fire may result from tuber mortality due to heat-related damage (Backhouse and Jeanes 1995; Quarmby 1999), or tuber dormancy following disturbance (Shefferson et al. 2005). Alternatively, it has been suggested that orchid mycorrhizal fungi (OMF), which form an essential symbiotic relationship driving nutrient acquisition of many orchid species (McCormick 2018), can have their abundance decreased after fire, potentially reducing growth and emergence of orchids (Jasinge 2018a; Dove & Hart 2017). Additionally, there are often assumptions made about how different components of fire (e.g. smoke or heat) impact orchids. For example, chemicals found in smoke, such as ethylene, may stimulate flowering and seed germination in many plant species including orchids (Dixon et al. 1995; Lamont and Downes 2011; Ritmejerytė et al. 2018) and is thought to be the contributing factor driving increased vegetative growth in Pterostylis revoluta (Jasinge et al. 2018b) and in several shrub species (Moreira et al. 2010). Assessing the impacts of fire on orchids is made difficult due to the cryptic below-ground stages of their life cycle, and the post-fire response and mechanisms driving these are often assumed and interpreted from above-ground life cycle components. Excavating wild orchids in situ to assess pre- and post-fire impacts is difficult and, particularly for threatened species or those with small populations, destructive sampling would increase threat (Brundrett 2016; Jasinge et al. 2018a). Without empirically assessing above- and below-ground traits, the fate of terrestrial orchids and the impacts of fire can be misinterpreted.
Past studies investigating fire season impacts on orchids have tended to interpret findings around phenological stages to make broad recommendations for management across seasonal timeframes. Many terrestrial orchid species in fire-prone areas are adapted to summer fires, maintaining avoidance mechanisms such as persisting as dormant tubers during the predominant fire season (Weston et al. 2005). Fires occurring during the active growing season will therefore negatively impact species (Jasinge et al. 2018a, b), such as those reported in Western Australia (WA) for Leporella fimbriata and Pyrorchis nigricans after late autumn fires (Brundrett 2019). However, fine-scale phenological changes may inform more precise response to fire season. For example, Pterostylis sanguinea, also in WA, sprouts prior to the growing season and maintains a shoot just below the surface (Brundrett 2014), potentially making it extremely sensitive to early autumn fires. A better understanding of finer-scale fire season impacts would provide land managers with greater scope to successfully implement burns that have positive or neutral (rather than negative) impacts on the species under their management. Seasons span three months and testing for impacts of intra-season burning will provide key insights on best conservation and fire management practices.
Using a glasshouse experiment, we investigated the impacts of fire season on the Australian orchid species Pterostylis curta. This approach allowed us to i) implement seasonal burns and relate impacts to quantifiable life cycle stages of the study species, ii) isolate and assess the role of smoke, and iii) control for fire intensity and life stage of the study species at each of the treatment levels to enable robust comparison focused on fire season effects. Implemented fires for ecological purposes are a key management tool and conservation agencies need to ensure that burns do not negatively impact threatened species. Understanding when burns can be implemented is critical for maintaining populations of threatened species, with orchids representing a large proportion of this group. In fact, the presence of orchids in some regions, dictate the frequency and timing of prescribed burns scheduled in that area (DEW 2013; DBCA 2023). By understanding developmental life stages at key prescribed burning times, we aim to inform fire managers of more precise timepoints, linked to life cycle events, that prescribed burning can take place when particular orchid species are present. Specifically, we aimed to answer the questions:
-
(a)
How does burning in autumn and spring, both early and late in each season (key prescribed burning times), impact re-emergence and growth in the following growing season?
-
(b)
What stages of the life cycle, both above and below-ground, are critical for determining response of Pterostylis curta after different prescribed burning times?
-
(c)
Are heat- or smoke-related mechanisms key drivers of post-fire recovery?
Methods
Study species and experimental design
Pterostylis curta R.Br. is a terrestrial orchid species distributed across sclerophyll forests predominantly on the east coast of Australia, including the states of Queensland, New South Wales, Tasmania and Lord Howe Island, but also occurs in South Australia. Its distribution extends outside Australia into New Caledonia, its northernmost extent (Atlas of Living Australia 2023). Plants emerge around March (early autumn), sprouting from a fleshy, globose tuber not usually bigger than one centimetre long. Plants will then produce a rosette of 2–6 leaves, followed by flowering from July to October, and senescing shortly after the flowering period over the hot summer months. Replacement tubers are formed at the end of short droppers from May to October. Pterostylis curta can also multiply clonally by producing multiple daughter tubers that are formed at the end of lateral stolonoid roots.
To test the impacts of fire season on Pterostylis curta, we conducted an experiment in the glasshouse at the University of New South Wales (UNSW) in Sydney, Australia. Glasshouse conditions reflected ambient daily temperatures. Pots were hand watered to capacity approximately every three days. We used 134 individual tubers of mixed provenance, purchased from a private collector in New South Wales (NSW) with no tuber production information available. Tubers were planted asymbiotically to a depth of 4 cm,to mimic the natural soil depth that tubers are often found in the field (Batty et al. 2006; Bell 2020), and allow for consistent levels of insulation from heat during fire treatments (Jasinge et al. 2018b). Plastic forestry tubes (5 cm × 5 cm × 12 cm) were used and filled with well-draining soil (Osmocote© Native potting mix sieved with both a 6 mm and 3 mm grade sieve). The potting mix was sterilised using steam at 100 °C for 30 min prior to sowing. All tubers were weighed and measured (length and width) prior to planting.
Emergence of rosettes occurred primarily from the end of February to March (Australian late summer – early autumn). Date of leaf emergence was recorded for each individual tuber, and the number of rosettes, maximum leaf width and length, and any signs of flowering were recorded for all plants throughout the experiment. Plants were then randomly assigned to two groups. The first group, of 32 plants, were used for destructive sampling at four time points during the study period, to better understand the seasonal life cycle of above-ground vegetative growth and below-ground root and tuber development in the absence of fire. Data were collected from eight of these 32 individuals in early autumn, late autumn, early spring and late spring, to provide detail of above- and below-ground development of plants that corresponded with time points at which the second group of plants were burnt. Leaf measurements were taken using callipers, and plants then dug up to record the presence and fresh weights of any tubers, including newly developed daughter tubers and lateral roots (combined weight). Tubers or roots were cleaned of excess soil and weighed using an analytical balance.
To test the impacts of fire season or smoke, the remaining group of 102 plants were randomly assigned to one of four seasonal fire treatments (early autumn, late autumn, early spring, or late spring), a smoke treatment, or left untreated as a control. The life stage (dormant, sprouted or flowering onset) of all plants were recorded prior to assignment, with each treatment containing a similar range of stages. For the fire treatments, pots were placed in concrete Besser blocks filled with sand (Experimental design set up shown in Fig. 1) and then covered with approximately 20 g of dried Eucalyptus spp. leaves to provide fuel biomass and create smoke. An LPG gas welding torch was then used to apply flames directed at the soil surface, burning any sprouted plants, for three minutes. A temperature logger (Thermochron iButton) was placed in the sand substrate at a depth of 4 cm to record soil heating temperature at tuber level (Table 1). Due to the limited number of tubers available, the smoke treatment was applied in early autumn only, by applying 20 ml of 2% smoke water per pot (Collette and Ooi 2017). Plants were then placed back in the glasshouse until the end of the experiment and were monitored through to September 2022, to allow individuals time to go through a complete life cycle post-fire. Re-emergence, number of rosettes, maximum leaf width and length, and estimates of flowering onset were recorded. Re-emergence, in the context of this study is defined as the ability of an individual orchid to emerge in the following season from a replacement tuber. Any pots where plants had not sprouted were checked for dormant tubers.
Experimental set up for burning of Pterostylis curta individuals. Up to 20 plastic pots (forestry tubes) containing one individual plant each were burnt during each treatment timepoint. Up to four pots were placed in each concrete block. Builders sand was then used to cover and protect plastic pots from the flames. All pots were covered with dried Eucalyptus leaves to provide fuel for burning
Statistical analysis
To assess the effects of the seasonal fire treatment on orchid response, we used Generalised Linear Models (GLM), with the main predictor, season of fire, as a single fixed factor with five levels (control, early autumn, late autumn, early spring, late spring). We used a binomial distribution model with proportional or binary responses as the dependent factor (re-emergence and presence of flowering scapes), a poisson distribution for count responses (number of leaves) and a gaussian distribution for continuous variables (leaf length and width). Due to complete separation in models for success of re-emergence, an empirical logistic transformation was used from the RVAideMemoire package (Herve 2023). To test whether smoke was the critical driver of orchid response during burning, we used the early autumn fire treatment only in a two-factor design. To assess if there was any difference in leaf size/number, or fresh weight of tubers or roots at each fire season timepoint, we used a GLM and included time of year as the fixed factor with a gaussian distribution. Model significance for all analyses was estimated using the Anova function in the R package car (Fox and Weisbery 2019) and was considered significant if P < 0.05. Pairwise comparisons were made with the emmeans package to discern any differences between factor levels when models were significant (Length et al. 2023). All data were analysed using R (v4.2.3; R Core Team 2023). Non-significant statistics can be found in Supplementary information (Table S1).
Results
Tuber dynamics over time (with no fire or smoke manipulation)
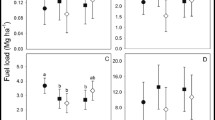
Leaf and below-ground storage structures varied in size over time (Table 2) (Fig. 2). Number of leaves (χ2 = 36.289, Df = 3, P < 0.001), and leaf width (χ2 = 8.35, Df = 3, P = 0.039) differed significantly between the fire season time points, increasing in number/size over the growing season to peak in late spring. However, leaf length showed no significant difference between fire season time points. Mean initial tuber fresh weight was highest in late autumn to early spring and decreased by late spring, although differences in weight were not significant, likely due to the small sample size (n = 5–10). Lateral root development had initiated by early autumn and senesced by late spring. Combined fresh weights of lateral roots showed no difference between seasons. Production of replacement tubers initiated in late autumn and were fully developed by late spring, with fresh weight of replacement tubers increasing over the growing season (χ2 = 167.27, Df = 3, P = < 0.005). In late autumn, only 20% of plants had developed a replacement tuber, whereas by late spring 100% of plants had developed a replacement tuber (Table 2). For those plants producing multiple daughter tubers, onset of the second (replacement) tuber did not begin until late spring (Table 2).
Impacts of fire season
Rates of re-emergence were significantly impacted by season of burn (χ2 = 12.773, Df = 4, P = 0.012), with late autumn burns resulting in 100% mortality of tubers (Fig. 2). Pairwise comparisons showed re-emergence rates after late autumn burns were significantly lower than all other treatments (P < 0.05) except the early autumn burn. All other treatments resulted in similar re-emergence rates to the control (P > 0.8). For total number of rosettes produced post-fire, plants in the control treatment produced more individuals per pot (1.8 ± 0.84) than those that received the early autumn or early spring burn treatments (1 ± 0.0). Plants re-emerging after late spring burns produced similar numbers of rosettes to the control (1.5 ± 0.84). No dormant tubers were found in pots with no re-emergence. We found no difference in leaf length or leaf width between fire season (Fig. 3). Only two plants initiated any scape development, one in the control treatment and the other in the late spring treatment.
Smoke versus fire as drivers of P. curta response
There were no significant differences in the number of plants re-emerging in the year after treatment application between plants that received the fire treatment, the smoke water treatment, or the control, although the burn treatment produced a slightly lower response overall (Fig. 4). Of those plants that did re-emerge, pots that received a smoke water or fire treatment only grew one rosette, whereas control pots had up to 3 rosettes (mean 1.8 ± 0.84). No significant differences were found for leaf length or leaf width (Fig. 5). See (Fig. 6)
Life cycle stages of Pterostylis curta at critical fire season burn times. Photos show an example of individuals measured at each time point. A early autumn showing the initial tuber and early leaf and lateral root development, with initiation of a dropper, B late autumn showing increased leaf and lateral root growth, C early spring showing development of a replacement daughter tuber while initial tuber is still present and increased lateral root growth (note that initial tuber and replacement tuber have been cut off main plant), and D late spring showing peak leaf growth, increased size of replacement tuber, increased growth of lateral root with production of a second daughter tuber. Photo credit: Tricia Ong
Discussion
This study shows that the timing of fire in Australian temperate climate regions can impact Pterostylis curta in quite striking ways. Most significantly, we found that late autumn burns caused complete failure of a cohort in our glasshouse study, suggesting that burns in these seasons may have severe impacts on local abundance of species like P. curta in the field. Response after early autumn burning was also reduced, although not significantly so. For individuals that did not re-emerge, subsequent excavation found that no dormant tubers remained, meaning that mortality rather than dormancy reduced emergence after the late autumn burns. One of our key findings is that heat alone is not the driver of tuber mortality, because soil heating was similar across all burn seasons, and plants burnt in the three other seasons had high levels of re-emergence in the first growing season after fire. More likely, there is an interaction between fire-related heat and the life cycle stage at which burning occurs, especially replacement tuber initiation (especially replacement tuber initiation), that drives post-fire demography.
The cryptic below-ground dynamics of terrestrial orchids makes it difficult to predict both the population responses to and the impacts of fire, and above-ground stages are therefore often used as surrogates to inform suitable potential periods for burning (Jasinge et al. 2018b). For example, many in situ studies have concluded that burning in autumn/winter, during the active growing season of species like P. curta, may kill orchid individuals by destroying aboveground biomass when carbohydrate reserves are too depleted to allow for successful emergence the following season (Jasinge et al. 2018a). In our glasshouse experiment we found no difference in re-emergence rates in the first post-fire growing season between the control and early autumn and early spring burns, where the majority of plants were actively growing during the fire treatment. Vulnerability to particular fire seasons is likely due to timing of initial stem production, with this stage being most vulnerable to mortality from fire. The physical stages within the growing season are therefore important for predicting subsequent re-emergence in the following season.
The mechanisms by which P. curta could re-emerge in the first post-fire growing season appeared to differ, depending on the time of year plants were burned. Fire in early autumn occurred at a critical time point when P. curta is just initiating growth and no replacement tubers have developed. We conclude that emergence after early autumn burns is therefore from initial tubers that have retained enough resources to allow production of a replacement tuber which can support re-emergence the following year, however, further investigation is needed to clarify this. We found that in late autumn, energy is being put into the development of both significant leaf growth (much larger leaves in late versus early autumn) and growth of a replacement tuber, however not enough resources remain in the initial tuber, or have been stored into the replacement tuber, to enable successful emergence the following season. In contrast, replacement/daughter tuber development at the time of spring burns is sufficient to enable successful re-emergence. This highlights the importance of understanding fundamental phenological stages, both above- and below-ground, to guide best burning practices for orchid species.
Further evidence for resources driving response to timing of burns can be seen in the number of tubers produced. All pots were originally sown with a single tuber, and only a single rosette was produced at the initiation of the experiment. Other studies using Pterostylis have also shown that multiple tuber production results in multiple sprouts (Brundrett 2014); we therefore assumed that the numbers of rosettes emerging per pot in the post-fire growing season was indicative of tuber numbers. Early autumn and early spring burnt plants produced only one rosette per pot in the growing season following fire treatment, compared to the control pots that had 1–3 rosettes per pot. Although the early autumn and early spring burned pots still had a high proportion of plants re-emerging in the following post-fire growing season, the lower rosette abundance was presumably linked to the limited time available for multiple tuber production. In contrast, pots burnt in late spring had produced similar rosette abundance to the controls, after having had almost the full growing season to produce multiple tubers. This provides strong evidence that fire season can limit subsequent tuber development, with early autumn and early spring burns allowing persistence of individuals, but only with enough resources to replace themselves via a single tuber, whereas late spring burned plants (as well as unburned plants) could multiply clonally via the development of daughter tubers.
One unexpected result was found for rosette abundance after smoke water application, which was applied during the early autumn treatment and compared to plants burned in the same season. Surprisingly, rosette number in the following post-fire growing season was reduced for both the burned and smoke only treatments, to a single rosette per pot, compared to the control pots which usually contained multiple rosettes (mean 1.8 ± 0.84). Unlike individuals burnt during treatments, smoke-treated aboveground biomass was not destroyed and able to continue growing post-treatment, presumably with enough time to produce multiple daughter tubers. Smoke water application may have therefore inhibited tuber development. A potential mechanism behind this could be high smoke concentration, which inhibits germination even for some smoke responsive species (Gupta et al. 2020). Alternatively, it could be related to the inhibition of OMFs by smoke, reported by Jasinge et al. (2018a), which could reduce subsequent vegetative growth. This highlights that much more work is needed to understand the role of different fire cues, and potentially their interactions with OMFs, in driving orchid population dynamics. Leaf growth was not impacted by burning or smoke application, suggesting that the inhibiting mechanisms are specific to tuber production. Decreased growth observed in the field is therefore likely to be driven by other factors, such as drought (Jasinge et al. 2018b).
Glasshouse studies can provide results that are critical to understanding plant ecology, and as shown here can uncover dynamics difficult to observe in natural populations. A key finding in our study was that a lack of re-emergence after burn treatments was due to tuber mortality and not dormancy. This means that, at least for P. curta, any post-fire reduction in abundance observed after fire during in situ studies is a result of tuber mortality and likely to reflect population decline. Our study therefore highlights the importance of developing methods, such as the empirical assessment we have made ex situ, to interpret observations made during in situ studies of fires or other disturbances impacting the recovery of orchid populations.
Currently most recommendations for prescribed burning of orchids are to wait until late spring, when they have finished flowering and are already dormant. While for highly threatened populations this scenario would create the lowest risk of reducing local population persistence, our findings highlight that it is possible to provide more nuanced advice to managers, such as levels of risk around different life cycle stages particularly where prescribed burning windows are limited. Prescribed burning will continue to occur from early autumn to late spring when conditions are cooler and allow safer implementation, and so creating management advice that allows a better-informed understanding of level of risk to species within this schedule is key. Generally, burning orchids like P. curta at the latter stages of their growing season (early and late spring for P. curta) seems to have the lowest risk, however there appears to be a window of opportunity for occasionally burning in early autumn, which contains less risk than late autumn burns; but some negative impacts remain. We recommend that P. curta and other orchid species with a similar phenology should not be burnt in late autumn.
Data availability
The datasets related to this study are available at https://doi.org/10.6084/m9.figshare.24750129.
References
Adams PB (2018) Destructive effect of fire on terrestrial orchid populations at Warrandyte. Victoria the Victorian Naturalist 135(6):171–177
Atlas of Living Australia (2023) Species pages: Pterostylis curta. https://bie.ala.org.au/species/https://id.biodiversity.org.au/taxon/apni/51412092. Accessed: 1st September 2023
Backhouse GN, Jeanes JA (1995) The orchids of victoria. Melbourne University Press, Melbourne
Balch JK, Bradley BA, Abatzoglou JT, Nagy RC, Fusco EJ, Mahood AL (2017) Human-started wildfires expand the fire niche across the United States. Proc Natl Acad Sci 114(11):2946–2951. https://doi.org/10.1073/pnas.1617394114
Batty AL, Brundrett MC, Dixon KW, Sivasithamparam K (2006) In situ symbiotic seed germination and propagation of terrestrial orchid seedlings for establishment at field sites. Aust J Bot 54(4):375–381
Bell SA (2020) Translocation of threatened terrestrial orchids into non-mined and post-mined lands in the Hunter Valley of New South Wales. Australia Restoration Ecology 28(6):1396–1407
Bond WJ, van Wilgen BW (1996) Fire and plants. Chapman & Hall, London
Bradstock RA (2010) A biogeographic model of fire regimes in Australia: current and future implications. Glob Ecol Biogeogr 19(2):145–158. https://doi.org/10.1111/j.1466-8238.2009.00512.x
Brown NAC (1993) Promotion of germination of fynbos seeds by plant-derived smoke. New Phytol 123(3):575–583. https://doi.org/10.1111/j.1469-8137.1993.tb03770.x
Brundrett MC (2014) Identification and ecology of southwest Australian orchids. Western Australian Naturalists’ Club Inc
Brundrett MC (2016) Using vital statistics and core-habitat maps to manage critically endangered orchids in the Western Australian wheatbelt. Aust J Bot 64(1):51–64. https://doi.org/10.1071/BT15087
Brundrett MC (2019) A comprehensive study of orchid seed production relative to pollination traits, plant density and climate in an urban reserve in Western Australia. Diversity 11(8):123
Çatav ŞS, Küçükakyüz K, Tavşanoğlu Ç, Pausas JG (2018) Effect of fire-derived chemicals on germination and seedling growth in Mediterranean plant species. Basic Appl Ecol 30:65–75. https://doi.org/10.1016/j.baae.2018.05.005
Coates F, Duncan M (2009) Demographic variation between populations of Caladenia orientalis–a fire-managed threatened orchid. Aust J Bot 57(4):326–339. https://doi.org/10.1071/BT08144
Collette JC, Ooi MKJ (2017) Germination ecology of the endangered species Asterolasia buxifolia (Rutaceae): smoke response depends on season and light. Aust J Bot 65(3):283–291. https://doi.org/10.1071/BT17025
Department of Biodiversity, Conservation and Attraction (DBCA) (2023) Fire Information Note – Geophytes, Including Orchids. https://www.dbca.wa.gov.au/sites/default/files/2022-10/Fire%20Information%20Note%20-%20Geophytes%2C%20Including%20Orchids.pdf. Accessed: 19 February 2024.
Department for Environment and Water (DEW) (2013) Ecological Management Guidelines For Native Vegetation in South Australia. Government of South Australia, pp 1–40
Department of Climate Change, Energy, the Environment and Water (DCCEEW) (2023) EPBC Act List of Threatened Flora. Australian Government, Canberra
Dixon KW, Roche S, Pate JS (1995) The promotive effect of smoke derived from burnt native vegetation on seed germination of Western Australian plants. Oecologia 101:185–192. https://doi.org/10.1007/BF00317282
Dove NC, Hart SC (2017) Fire reduces fungal species richness and in situ mycorrhizal colonization: a meta-analysis. Fire Ecology 13:37–65
Duncan M (2012) Response of orchids to bushfire: black saturday victoria 2009: natural values fire recovery program. Department of Sustainability and Environment, Victoria
Fox J, Weisberg S (2019) An R Companion to Applied Regression, 3rd edn. Sage, Thousand Oaks CA
Gaskett AC, Gallagher RV (2018) Orchid diversity: spatial and climatic patterns from herbarium records. Ecol Evol 8(22):11235–11245. https://doi.org/10.1002/ece3.4598
Gill AM (1975) Fire and the Australian flora: a review. Aust for 38:4–25. https://doi.org/10.1080/00049158.1975.10675618
Gupta S, Hrdlička J, Ngoroyemoto N, Nemahunguni NK, Gucký T, Novák O, Kulkarni MG, Doležal K, Van Staden J (2020) Preparation and standardisation of smoke-water for seed germination and plant growth stimulation. J Plant Growth Regul 39:338–345
Herve M (2023) RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R package version 0.9–83–2, https://CRAN.R-project.org/package=RVAideMemoire.
Jasinge NU, Huynh T, Lawrie AC (2018a) Consequences of season of prescribed burning on two spring-flowering terrestrial orchids and their endophytic fungi. Aust J Bot 66(4):298–312. https://doi.org/10.1071/BT17179
Jasinge NU, Huynh T, Lawrie AC (2018b) Changes in orchid populations and endophytic fungi with rainfall and prescribed burning in Pterostylis revoluta in Victoria. Australia Annals of Botany 121(2):321–334. https://doi.org/10.1093/aob/mcx164
Jones DL, Clements MA (2002) Australian orchid research a review of volume 4: pterostylis. Australian Orchid Foundation, Melbourne, p 5
Keeley JE, Fotheringham CJ, Baer-Keeley M (2006) Demographic patterns of postfire regeneration in Mediterranean-climate shrublands of California. Ecol Monogr 76:235–255. https://doi.org/10.1890/0012-9615(2006)076[0235:DPOPRI]2.0.CO;2
Khatoon A, Rehman SU, Aslam MM, Jamil M, Komatsu S (2020) Plant-derived smoke affects biochemical mechanism on plant growth and seed germination. Int J Mol Sci 21(20):7760. https://doi.org/10.3390/ijms21207760
Lamont BB, Downes KS (2011) Fire-stimulated flowering among resprouters and geophytes in Australia and South Africa. Plant Ecol 212:2111–2125. https://doi.org/10.1007/s11258-011-9987-y
Lenth R (2023) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.8.5. https://CRAN.R-project.org/package=emmeans
McCormick MK, Whigham DF, Canchani-Viruet A (2018) Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytol 219(4):1207–1215
McLauchlan KK, Higuera PE, Miesel J et al (2020) Fire as a fundamental ecological process: research advances and frontiers. J Ecol 108(5):2047–2069. https://doi.org/10.1111/1365-2745.13403
Nolan RH, Collins L, Leigh A, Ooi MK, Curran TJ, Fairman TA, Resco de Dios V, Bradstock R (2021) Limits to post-fire vegetation recovery under climate change. Plant, Cell Environ 44(11):3471–3489. https://doi.org/10.1111/pce.14176
Paroissien R, Ooi MK (2021) Effects of fire season on the reproductive success of the post-fire flowerer Doryanthes excelsa. Environ Exp Bot 192:104634. https://doi.org/10.1016/j.envexpbot.2021.104634
Penman TD, Christie FJ, Andersen AN, Bradstock RA, Cary GJ, Henderson MK, Price O, Tran C, Wardle GM, Williams RJ, York A (2011) Prescribed burning: how can it work to conserve the things we value? Int J Wildland Fire 20(6):721–733. https://doi.org/10.1071/WF09131
Quarmby JP (1999) Recovery plan for twelve threatened orchids in the Lofty Block region of South Australia 2010. Department of Environment and Natural Resources, South Australia
R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ritmejerytė E, Obvintseva A, Huynh T (2018) The effect of smoke derivatives and carbon utilisation on symbiotic germination of the endangered Pterostylis despectans (orchidaceae). Lankesteriana 18(3):169–177. https://doi.org/10.15517/lank.v18i3.34534
Shefferson RP, Kull T, Tali K (2005) Adult whole-plant dormancy induced by stress in long-lived orchids. Ecology 86(11):3099–3104. https://doi.org/10.1890/05-0586
Tangney R, Paroissien R, Le Breton TD, Thomsen A, Doyle CAT, Ondik M, Miller RG, Ooi MKJ (2022) Success of post-fire plant recovery strategies varies with shifting fire seasonality. Communications Earth & Environment 3(1):126. https://doi.org/10.1038/s43247-022-00453-2
Tatarenko IV, Kondo K (2003) Seasonal development of annual shoots in some terrestrial orchids from Russia and Japan. Plant Species Biol 18(1):43–55. https://doi.org/10.1046/j.1442-1984.2003.00087.x
Thomas PB, Morris EC, Auld TD (2007) Response surfaces for the combined effects of heat shock and smoke on germination of 16 species forming soil seed banks in south-east Australia. Austral Ecol 32(6):605–616. https://doi.org/10.1111/j.1442-9993.2007.01730.x
Thomsen AM, Ooi MKJ (2022) Shifting season of fire and its interaction with fire severity: impacts on reproductive effort in resprouting plants. Ecol Evol 12(3):e8717. https://doi.org/10.1002/ece3.8717
Thomsen AM, Davies RJ, Ooi MKJ (2023) Using multiple plant functional types to assess response to prescribed burn season in Mediterranean-climate vegetation. Appl Veg Sci 26(4):e12750. https://doi.org/10.1111/avsc.12750
Weston PH, Perkins AJ, Entwisle TJ (2005) More than symbioses: orchid ecology, with examples from the Sydney region. Cunninghamia 9(1):1–15
Whelan RJ (1995) The Ecology of Fire. Cambridge University Press, Cambridge
Wraith J, Pickering C (2019) A continental scale analysis of threats to orchids. Biol Cons 234:7–17. https://doi.org/10.1016/j.biocon.2019.03.015
Zhang Z, Yan Y, Tian Y, Li J, He JS, Tang Z (2015) Distribution and conservation of orchid species richness in China. Biol Cons 181:64–72. https://doi.org/10.1016/j.biocon.2014.10.026
Acknowledgements
This project was funded by an Australian Research Council Linkage Project grant (LP180100741) awarded to MKJO, a Holsworth Wildlife Research Endowment awarded to AMT, and an Australian Orchid Foundation grant awarded to AMT. Thank you to Joel Cohen for donating Pterostylis curta tubers, Tricia Ong and Mercedes Ondik for assistance in initial planting of tubers and data collection, Richard Thomson and Les Nesbitt for advice on growing orchids, and Guy Taseski for assistance in the glasshouse.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This project was funded by an Australian Research Council Linkage Project grant (LP180100741), awarded to MKJO, a Holsworth Wildlife Research Endowment awarded to AMT, and an Australian Orchid Foundation grant awarded to AMT.
Author information
Authors and Affiliations
Contributions
All authors conceived the ideas and designed the research. Alexandria M Thomsen carried out the experimental design. Alexandria M Thomsen analysed the data, and led the writing of the manuscript, with critical input contributed by R JP Davies and Mark KJ Ooi. All authors reviewed and gave approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Simon Pierce.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thomsen, A.M., Davies, R.J.P. & Ooi, M.K.J. The impacts of inter- and intra-seasonal burns on the terrestrial orchid Pterostylis curta. Plant Ecol (2024). https://doi.org/10.1007/s11258-024-01437-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11258-024-01437-2