Abstract
Flower strips are a fundamental part of agri-environment schemes in the Common Agricultural Policy (CAP). Although vegetation is central for many arthropod groups, a few studies have evaluated the effects of flower strip structural and functional attributes on arthropod communities. In this study, we explored the relationship between flower strip attributes and the abundance of different arthropod functional groups in annual flower strips located in an organic apple orchard. We surveyed plant and arthropod communities in 30 1 m × 6 m plots. In each plot, we collected data on species composition and vegetation structure (e.g., total cover, density, number of floral displays). For each plant species, we also retrieved data on leaf palatability and nutritional value. Arthropods were collected using sweep netting technique. Structural and functional attributes of the flower strip revealed a crucial role in regulating arthropod abundance, which however depended on the specific arthropod functional group. We identified three main attributes (plant species richness, composition, and vegetation density) of flower strips that should be considered when implementing multifunctional flower strips. Specifically, plant species richness to ensure complementarity of resources and niches, plant species composition to ensure complementary floral resources, and vegetation density to ensure sheltering microhabitats and suitable microclimatic conditions and to increase the density of floral resources. Our results suggest that by considering structural and functional attributes of flower strips, it is possible to design multifunctional flower strips with greater effectiveness as measures for ecological intensification.
Similar content being viewed by others
Introduction
Over the past few years, ecological intensification has emerged as a nature-based approach to support agricultural production while reducing harmful environmental impacts (Garibaldi et al. 2019). The main assumption of ecological intensification is that by managing specific components of biodiversity, it is possible to enhance agricultural productivity by either complementing or replacing high-input management practices, with the ultimate goal of reducing environmental impacts and associated costs without limiting crop production (Kleijn et al. 2019).
The presence of semi-natural habitats such as hedgerows and perennial semi-natural grasslands in agroecosystems has been shown to be effective in sustaining communities of beneficial arthropods and preserving associated ecosystem services (Tscharntke et al. 2021). Semi-natural habitats are created and maintained for their ability to provide feeding resources as well as shelter, overwintering, and nesting sites to beneficial arthropods, which are generally rarefied in intensive agricultural systems (Landis 2017; Geiger et al. 2009). In addition, the extent of natural and semi-natural habitats in the landscape influences the effectiveness of agri-environmental measures serving as reservoirs and source populations for beneficial arthropods (Grab et al. 2018; Eeraerts 2023).
Among the most widely implemented semi-natural habitats in agri-environmental measures are flower strips, a fundamental part of agri-environment schemes in the Common Agricultural Policy (CAP). Flower strips are usually established to improve pollination service and have been shown to actually increase the richness and abundance of pollinators, especially bees and hoverflies (e.g., Campbell et al. 2017b; Ganser et al. 2021; Lowe et al. 2021). Flower strips can also support biological control, positively influencing parasitoids and predators by supplying nectar and pollen for adults, serving as overwintering refugia or providing an additional prey source for larvae (Wäckers and van Rijn 2012; Holland et al. 2016; Ganser et al. 2019). However, the effect of flower strips on beneficial arthropods depends on several factors related to both flower strip attributes (e.g., plant species composition, plant community structural, and functional attributes, such as leaf area, leaf toughness and palatability, and leaf C and N content) and the target arthropods. For example, von Königslöw et al. (2022) reported a different effect of the same flower strip on hoverflies and bees in an apple orchard. Hence, despite the large body of research on the effect of flower strips on beneficial arthropods, there are still many doubts on how to design and manage flower strips to promote multifunctionality, i.e., the ability of flower strips to simultaneously support a diverse arthropod community thereby assuring the supply of different ecosystem services (e.g., Bommarco et al. 2013; Albrecht et al. 2020).
Although vegetation is central for many animal species groups, including arthropods (Holland et al. 2016; Kotze et al. 2022), it is often disregarded in studies on flower strips focusing on fauna (Schmidt et al. 2022). For example, small-scale variation in plant community cover and height has been shown to provide greater diversity of resources, shelter, and nesting sites (Woodcock et al. 2007) and support a higher arthropod diversity compared to more structurally simplified plant communities (Threlfall et al. 2017; Mata et al. 2021). Plant species composition also has a major impact on the arthropod community, as different floral morphologies have different levels of accessibility and different arthropod taxa may have different mouthparts and abilities to deal with different floral morphologies (e.g., Branquart and Hemptinne 2000; Araj and Wratten 2015). In addition, while perennial grasses support predators by providing relatively stable refugia during the cold season, the richness, and abundance of entomophilous forbs may be crucial for both parasitoids and pollinators, as they provide floral resources such as nectar and pollen that enhance their survival and fecundity (Bianchi and Wäckers 2008; Fantinato et al. 2021).
Most studies on functional traits have focused on their relationships with arthropod communities (Greenop et al. 2023), but studies on plant functional traits in agroecosystems are still limited (Perović et al. 2018), although they are expected to play a crucial role in influencing arthropod community composition. Plant functional traits associated with plant resource use, growth, and life history strategies (e.g., leaf area, leaf toughness and palatability, and leaf C and N content) are often studied to explore plant–environment and plant–plant interactions (e.g., Blumenthal et al. 2020; Fantinato et al. 2023); however, being also related to leaf nutritional value (e.g., C:N ratio; Pérez-Harguindeguy et al. 2013) and resistance to herbivory (e.g., leaf toughness; Pérez-Harguindeguy et al. 2013), these traits can help explain the richness and abundance of herbivorous (Storkey et al. 2013) and saprophagous arthropods (Ganault et al. 2022). Similarly, the classification of arthropods into functional groups has the potential to disentangle the complex relationships between arthropods and their functions within ecosystems (Balzan et al. 2014).
Considering the potential contribution of flower strips to the supply of multiple ecosystem services, the aim of this study was to uncover structural and functional attributes of flower strips that can guide flower strip design to maximize their role in the maintenance of different functional groups of beneficial arthropods in the agricultural systems. We hypothesize that structural and functional attributes of the flower strip differently affect arthropod community composition and the abundance of different functional groups of arthropods. We expect that structural and functional attributes of the flower strip play a different role in regulating arthropod abundance depending on the specific functional group considered. For example, the quantity of floral resources conceivably is a crucial factor in targeting pollinator and parasitoid functional groups, while this same attribute is likely to have no influence on, e.g., herbivores that are supposed to be affected by functional attributes related to leaf nutrient content or leaf toughness.
Materials and methods
Study site
The study was conducted at a 2.7 ha organic apple orchard (45º24′28.872"N 11º9′34.416" E) in Caldiero (Verona, north-eastern Italy). The orchard is organic certified and conforms to all EU and Italian regulations ruling the production of organic crops, including EU Regulation 2018/848/EU and LN 9.03.2022, n. 23.
The soil composition of the orchard is clay loam. The mean annual temperature is 14.3 °C with a mean high temperature of 31.3 °C in July and a low of -1.1 °C in January. The average annual rainfall is 756.3 mm, peaking in May and November and lowering in January and March.
The apple trees in the orchard are organized in regular rows, each of them 2 m apart. Each apple tree has branches arranged along the row and are spaced 3 m apart. The main apple cultivar grown in the orchard is “Gala” and M9 is use as rootstock. To control Cydia pomonella (L.) presence and damage on apple fruits, pheromone disruption was applied. Typical fungicide treatments were applied as needed during the period between April and June to control Powdery mildew and Apple scab damages. All treatments were performed with chemical products allowed by organic certification.
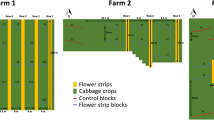
In the apple orchard, flower strips were sown every second intermediate interrow, while spontaneous herbaceous vegetation was left in the other interrows (Fig. 1). The seed mix used for the flower strips was a commercial seed mix consisting of a total of 19 plant species (2 grasses and 17 forbs), including both annual and biennial species whose main purpose was to attract pollinator insects (Online Resource 2). The flower strips were sown in March and tillage was done at the endo of May of the same year for green manure purposes. Before and during sowing, the soil was worked using a ripper and a rotary harrow with a seeder.
Data collection
In the first week of May 2022, we established 30 1 m × 6 m sampling plots within the interrows of the apple orchard sown with the seed mix. We sampled plant and arthropod communities within each 1 m × 6 m sampling plot on two consecutive days in the second week of May 2022, which corresponded to the peak of biomass production of the flower strip. To avoid edge effects, each plot was placed 6 m from the nearest end of the interrow. Each plot was further divided into six smaller sub-plots of 1 m × 1 m (Fig. 1).
For each 1 m × 1 m sub-plot, we recorded structural attributes of the plant community: percentage cover of vascular plants (%), plant height measured with a rigid ruler at the center of the sub-plot (cm) and canopy closure (%). Canopy closure was estimated by measuring the Leaf Area Index (LAI) using a convex model of a spherical crown densiometer. Canopy closure provides an estimate of the complexity of canopy stratification and the light quantity that reaches the ground; that is, the highest values of canopy closure correspond to monostratified canopies that intercept most of the light (Jennings et al. 1999; Liira et al. 2002).
In addition, at the level of the whole sampling plot (1 m × 6 m) we recorded and identified to the species level each vascular plant species and their cover (%) and the total number of floral displays of entomophilous plant species as an indicator of the quantity of available floral resources. Depending on the plant species, a floral display consisted of a single flower (e.g., Linum usitatissimum L.), a flowering head (e.g., Trifolium incarnatum L. inflorescence) or a group of flowers appearing together in a recognizable visual unit (e.g., Vicia villosa Roth).
We collected the arthropod community using a sweeping net with 10 sweeping shots per sampling plot (1 m × 6 m), on sunny days with no or little wind. Sampling was conducted the day after the sampling of the vegetation, twice per day, from 10 a.m. to 1 p.m. (morning) and from 1 p.m. to 4 p.m. (afternoon), to ensure the collection of arthropods that exhibit different daily periods of activity (Fantinato et al. 2018). To avoid damage to the plant community, we used 40-cm diameter entomological net instead of a classic sweeping net (Burgio et al. 2016). After collection, the arthropods were placed in plastic bags and taken to the laboratory for identification (Table S1). The collected arthropods were classified at the lowest possible taxonomic level. Each taxonomic group was then assigned to a functional group based on expert knowledge and literature sources. Identified arthropod functional groups included the following trophic groups: chewing herbivores, sap-sucking herbivores, pollen and nectar feeders, parasitoids, predators, aquatic saprophages, and terrestrial saprophages (Goulet and Huber 1993; Marshall 2012, 2018). In addition, we also included the category of pollinator due to their important ecological service.
In case arthropods belonged to different functional groups at different stages of their life cycle, we considered only the trophic condition of larvae since usually it is the longest stage in their lifecycle. Thus, regarding, e.g., Argidae that are chewing herbivores as larvae and pollen and nectar feeders as adults, we considered them as chewing herbivore. When a taxon included species of different trophic levels, we considered the dominant category. For example, since most of Chalcidoidea families are parasitoids, all collected Chalcidoidea were included in this group, although Chalcidoidea also includes Agaonidae with herbivore larvae. We considered pollinators to be all taxa that correspond to bees and non-bee pollinators according to current literature (e.g., Marshall 2012; Ollerton 2017; Requier et al. 2023); where data were not available, we included in this group those taxa in which adults regularly visit flowers as flower visitors and that are larger than 3 mm (e.g., Rader et al. 2011). If arthropods belonged to the functional group of pollinators in addition to their trophic group, we counted them in both functional groups (i.e., the sum of individuals included in the functional groups is higher than the total number of individuals recorded). Individuals of Aphididae and Formicidae were not counted because the collection technique we used (i.e., sweeping net) is not suitable for quantifying their abundance (Dewar et al. 1982; Zina et al. 2022).
Plant functional traits
To investigate the relationship between functional traits of plants and functional groups of arthropods, we selected and extracted three plant traits from the global database TRY 3.0 (Kattge et al. 2020) that are related to plant size, leaf toughness or palatability, and nutrient content. We selected Leaf Area (LA) (mm2), Leaf dry matter content (LDMC) (g/g), and Leaf nitrogen content per dry mass (LNC) (mg/g) (Mooney and Gulmon 1982; Pérez-Harguindeguy et al. 2013; Storkey et al. 2013). All trait data were checked, and we excluded data related to studies in which plant individuals were grown under controlled environmental conditions. For each 1 m × 6 m sampled plot, the community-weighted mean (CWM; Eq. 1) of each selected plant trait was calculated as the average trait value in the community weighted by species abundance (Garnier et al. 2004):
where CWM (trait x) is the CWM for a x trait, pi is the relative cover of species i in the community, and xi is the trait value for the species i.
Data analysis
To investigate the relationship between the structural and functional attributes of flower strips and the abundance of individual arthropods of each functional group (i.e., chewing herbivores, parasitoids, pollinators, predators, and terrestrial saprophages) we performed GLMMs (package lme4). For each arthropod functional group, we included the abundance of individuals as dependent variable and the total cover of vascular plants (%), plant height (cm), canopy closure, plant species richness, total number of floral displays of entomophilous plants, and CMWs of functional traits (CMWLA, CMWLDMC, CMWLNC) as independent variables. The total cover of vascular plants (%), plant height (cm), and canopy closure were calculated at plot level by averaging the values recorded in the six 1 m × 1 m sub-plots. Since the arthropod samples collected in the morning and afternoon were treated as replicates, plot identity was included in the model as random factor. Negative binomial distribution (function glmer.nb, log as link function) was chosen for all arthropod functional groups as data were overdispersed (dispersion test function; package AER; O’Hara and Kotze 2010).
For each GLMM, we followed the independent variable selection procedure for the mixed-effects models (Zuur et al. 2010). At the beginning of the selection procedure, we included all the independent variables. We checked for possible collinearity between the independent variables using Spearman’s rank correlations and retained those variables that were not highly correlated with each other (i.e., Spearman’s r < 0.7). Since plant height (cm) and canopy closure were collinear (r > 0.7), we decided to include in the model only the canopy closure, because canopy closure provides a comprehensive picture of the complexity of canopy stratification, which also contains the information conveyed by the mean vegetation height. Using a stepwise backward selection, we eliminated the non-significant variables from the model at each step until only terms with P < 0.05 remained in the model. The analysis was performed for each functional group of arthropods, with the exception of sap-sucking herbivores and aquatic saprophages. As the Aphidoidea were not counted, the results for the group of sap-sucking arthropods would have been incomplete and unreliable, as Aphidoidea are also sap-suckers. As for the group of aquatic saprophages, these organisms, which spend most of their trophic life under water, are not necessarily associated with the presence of terrestrial flower strips. All analyses were performed using R version 4.2.2 (R Core Team 2019).
Results
Overall, 32 plant species were recorded in the sampling plots (Online Resource 2). Forbs dominated over grasses both in number (27 vs. 5, respectively) and in mean coverage per plot (75.5 ± 10.19% vs. 21.8 ± 10.76%, respectively). Although commercial plant species accounted for only 44% of the total plant species pool recorded, they contributed an average of 92.46 ± 5.85% (mean ± SD) to the total cover of vascular plants, while spontaneous plant species contributed only 6.84 ± 0.72%. The most abundant plant species were Vicia villosa (25.50 ± 18.06%), Secale cereale L. (16.47 ± 12.46%), and Taraxacum officinale (Weber) ex Wiggers (13.59 ± 23.00%).
Although sown with the same seed mix, the structural and functional attributes of the flower strips differed (Table 1; Online Resource 3), with canopy closure and the number of floral displays the most variable attributes.
Regarding the arthropod community, we collected a total of 3,952 individuals belonging to 9 orders (i.e., Araneae, Coleoptera, Dermaptera, Diptera, Hemiptera, Hymenoptera, Lepidoptera, Neuroptera, Orthoptera) (Online Resource 1). Herbivores were the most abundant functional group (46.32%, of which 40.66% were chewing herbivores and 5.66% were sap-sucking herbivores), followed by saprophages (31.36%; of which 27.64% were terrestrial and 3.72% aquatic) and parasitoids (14.07%). The less abundant functional groups were pollinators (4.69%) and predators (3.55%). At the plot level, the abundance of individuals belonging to different functional groups was found to vary greatly between the flower strips (Table 2).
Different arthropod functional groups responded differently to different structural and functional attributes of flower strips (Table 3). The abundance of chewing herbivores showed a significant positive relationship with plant species richness and CWMLNC, while their abundance decreased at increasing CWMLDMC (Table 3; Fig. 2a-b-c). The abundance of parasitoids showed a significant positive relationship with total vegetation cover (%) and canopy closure (Fig. 2d-e) and a significant negative relationship with the number of floral displays (Table 3; Fig. 2f). The abundance of pollinators showed a significant positive relationship with both total cover of vascular plants (%) and number of visual displays (Table 3; Fig. 2g-h). The abundance of terrestrial saprophages significantly decreased in abundance at increasing CWMLDMC and number of floral displays, while their abundance increased at increasing CWMLNC (Table 3; Fig. 2i, j, k). The abundance of predators did not show any significant relationship with structural and functional attributes of flower strips. Finally, CWMLA showed no significant relationship with the abundance of any arthropod group.
Relationship between vegetation structure variables (Total cover of vascular plants (%), Number of floral displays, Canopy closure, Plant species richness) and community-weighted mean of plant functional traits (CWMLDMC, CWMLNC) of arthropods functional groups (chewing herbivores, parasitoid, pollinator, saprophage). Lines represent the estimates of the Generalized Mixed Linear Models (GLMMs). Black points are original data points, while the gray band represents 95% confidence interval around the regression line. Only significant results are shown in the figure
Discussion
In the present study, we have shown that flower strips can support a diversified set of functional groups of arthropods thereby proving the potential to be a multifunctional landscape element in agroecosystems. The results showed that the abundance of arthropods belonging to different functional groups is influenced by structural and functional attributes of the strips. Interestingly, however, the role played by the attributes of the flower strips on the abundance of arthropods changed depending on the functional group considered. Namely, while the abundance of chewing herbivores and terrestrial saprophages was more related to the functional attributes of the flower strip, i.e., the toughness/palatability of the leaves and nutritional value of the plant species, the abundance of parasitoids and pollinators was more related to the structural attributes of the plant community, such as the total cover of vascular plants, the canopy closure, and the number of floral displays.
As expected, the abundance of pollinators increased with the number of floral displays. This result confirms previous studies that found that flower abundance and associated availability of pollen and nectar favors a richer and more abundant pollinator community (e.g., Bartholomée et al. 2020; Feltham et al. 2015), highlighting the crucial role of flower strips in increasing pollination capacity (Feltham et al. 2015; Korpela et al. 2013) and crop pollination of adjacent fields (Albrecht et al. 2020). However, pollinator abundance was also positively correlated to the total cover of vascular plants, i.e., to vegetation density; thus, not only the number of floral displays but also greater plant species cover contributed to greater pollinator abundance. As flower strips are usually dominated by entomophilous forbs, higher vascular plant cover leads to dense flower patches that are more attractive to pollinators because they allow them to reduce travel time among sparse patches and save energy (Elliott and Irwin 2009; Schütz et al. 2022).
A similar trend was observed for parasitoids, whose abundance increased with increasing cover of vascular plants and canopy closure of the flower strips, namely, with higher plant density. Parasitoids may prefer dense flower strips since they provide sheltering microhabitats as well as stable and suitable microclimatic conditions to complete their life cycle (Langellotto and Denno 2004). Conversely, and contrary to what has been observed in previous studies (e.g., Campbell et al. 2017a; Pollier et al. 2019), we found that parasitoid abundance was negatively related to the number of floral displays. Indeed, several studies have found that parasitoid richness and abundance are higher when associated with abundant floral resources (Jervis et al. 1993; Dib et al. 2012). However, as reported by Wäckers (2005), some plant species, such as Vicia sepium L., may be unattractive or even repulsive to foraging parasitoids. In addition, mouthpart features of most parasitoids (Wäckers 2004) limit their feeding to exposed nectaries and generally to flowers with simple floral morphologies (Willmer 2011). The negative relationship between parasitoid abundance and the number of floral displays observed in this study could therefore be due to the complexity of floral morphologies of the dominant plant species (i.e., Vicia villosa, Vicia sativa, and Lathyrus oleraceus Lam.). This suggests that when establishing flower strips to attract and maintain parasitoids, it is crucial not only to consider flower abundance and associated pollen and nectar availability but also carefully select plant species based on their floral morphologies.
As stated above, the abundance of chewing herbivores and terrestrial saprophages was more related to the functional attributes of the flower strip. Chewing herbivores represented the most abundant functional group with abundance increasing in flower strips with higher plant species richness, lower CWMLDMC, and higher CWMLNC. While the relationship with leaf palatability and nutritional value was somehow expected, the relationship with plant species richness highlights an interesting issue. The increase in herbivore abundance at increasing plant species richness suggests that the presence of different plant species can provide resources for herbivores with different feeding preferences (species-specific relationships); that is, a greater variety of resources supports a greater diversity of consumers. This result is certainly fascinating from a biodiversity perspective but must be treated with caution from an agronomic perspective, as undesirable pest species can also benefit from the food sources. On the other hand, species-rich flower strips can also serve as a sink for herbivorous arthropods, which tend to feed within the strip rather than on crops. In addition, the abundance of generalist herbivores can also provide alternative prey sources for pests, leading to increased control of agricultural pests. Therefore, only ‘appropriate diversity helps’ (Gurr et al. 2005) and the creation of flower strips requires careful assessment of structural and functional attributes to meet the needs of beneficial arthropods without promoting pest species (Winkler et al. 2003).
Terrestrial saprophages showed a trend similar to that of chewing herbivores with a negative relationship with CWMLDMC and a positive relationship with CWMLNC. Terrestrial saprophages play an important role in the functioning of terrestrial ecosystems being key regulators of plant litter decomposition. The relationship between terrestrial saprophage abundance and nitrogen content and palatability of plant biomass has been mostly described considering the litter layer (e.g., David and Gillon 2009; Purse et al. 2012), and litter nitrogen content has been shown to strongly influence the abundance of terrestrial saprophages (Haddad et al. 2000; Moço et al. 2010). However, it has to be considered that defensive or supporting structures that increase leaf toughness and reduce palatability remain in plant tissue after plant death and thus influences the properties of litter biomass (Kazakou et al. 2009). We can thus assume that the relationships between terrestrial saprophages and litter are mediated by the functional composition of the strip, which thus becomes a crucial feature to be considered for the maintenance of this functional group of arthropods. The fact that the number of saprophages decreased with the number of floral displays was somewhat surprising and indicates a relationship that still needs to be explored and deserves further investigation. A possible explanation arises when we consider that most of the terrestrial saprophages collected in the present study belong to taxa that feed on pollen and nectar as adults and usually visit flowers with open nectaries. The Drosophilidae, for example, which were the most abundant among the terrestrial saprophages, are generally unable to manipulate flowers with complex morphology and, in temperate regions, are more likely to visit species from the Apiaceae or Asteraceae family (Larson et al. 2001), which were underrepresented in the species pool of the flower strips. Thus, also in this case, species composition in terms of floral morphology and nectar accessibility, becomes a crucial attribute to consider when designing a flower strip.
Finally, we could not find any relationship between the structural and functional attributes of the flower strips and the abundance of predators. Predators depend upon stable environmental conditions and their establishment requires more time compared to other functional groups of arthropods (e.g., pollinators; Hussain et al. 2021). Tscharntke et al. (2016) pointed out that agri-environmental measures may not effectively support biocontrol agents for various reasons. The flower strips in the study area are frequently disturbed by farming practices, e.g., grass mowing and the passage of agricultural vehicles for work on apple trees. These practices may lead to continuous disturbance and limit the suitability of the habitat for predators (Thorbek and Bilde 2004).
Experimental data on the provision of ecosystem services and especially on multifunctionality are still rare (Schütz et al. 2022). Although our study only provides a temporal snapshot of conditions during the peak of the growing season of annual flower strips, we can outline some attributes of flower strips that should be taken into account to increase the effectiveness in ecological intensification and to implement multifunctional flower strips:
-
(1)
Plant species richness: as species richness and functional richness are inherently linked, creating flower strips with higher plant species richness can help maximize the complementarity of resources and niches and thus improve their ability to support larger and more diverse arthropod communities. It is important to note that flower strips with a high species richness may attract larger numbers of herbivorous arthropods that could potentially become pests of associated crops;
-
(2)
Plant species composition: the selection of plant species is crucial for the promotion of floral resources that can support different beneficial arthropod groups with different resource requirements and feeding habitats. The selection of plant species in the seed mix should be carefully considered in relation to the beneficial arthropod groups. Particular attention should be paid to the floral morphology of flowering plant species, as not all morphologies are suitable for beneficial arthropod groups that do not have specialized mouthparts, such as parasitoids;
-
(3)
Vegetation density: the management of flower strips is crucial for the creation and maintenance of an optimal vegetation structure, on which the presence of multiple ecological niches for different beneficial arthropod groups depends. Agricultural practices have a significant impact on arthropod groups, which require a stable, stratified, and closed habitat. It is therefore important to manage flower strips in a way that avoids excessive disturbance in the early stages of vegetative development. In this way, a multi-layered and dense vegetation of the flower strips is created, providing protective microhabitats and suitable microclimatic conditions that allow the different arthropods to complete their life cycle. The age of the flower strip deserves a final remark. Annual flower strips are certainly appealing to farmers who can adapt their location to crop rotation; however, due to frequent farming practices they are subjected to, annual flower strips fail in assuring suitable overwintering and nesting opportunities for many arthropod groups, including predators. Conversely, if properly managed, perennial structures are likely to offer more stable habitats thereby effectively contributing to promote local arthropod population growth over time.
Data Availability
The data that support the findings of this study are available from the authors upon reasonable request.
References
Albrecht M, Kleijn D, Williams NM, Tschumi M et al (2020) The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: a quantitative synthesis. Ecol Lett 23:1488–1498. https://doi.org/10.1111/ele.13576
Araj S-E, Wratten SD (2015) Comparing existing weeds and commonly used insectary plants as floral resources for a parasitoid. Biol Control 81:15–20. https://doi.org/10.1016/j.biocontrol.2014.11.003
Balzan MV, Bocci G, Moonen AC (2014) Augmenting flower trait diversity in wildflower strips to optimise the conservation of arthropod functional groups for multiple agroecosystem services. J Insect Conserv 18:713–728. https://doi.org/10.1007/s10841-014-9680-2
Bartholomée O, Aullo A, Becquet J, Vannier C, Lavorel S (2020) Pollinator presence in orchards depends on landscape-scale habitats more than in-field flower resources. Agric Ecosyst Environ 293:106806. https://doi.org/10.1016/j.agee.2019.106806
Bianchi FJJA, Wäckers FL (2008) Effects of flower attractiveness and nectar availability in field margins on biological control by parasitoids. Biol Control 46:400–408. https://doi.org/10.1016/j.biocontrol.2008.04.010
Blumenthal DM, Mueller KE, Kray JA, Ocheltree TW, Augustine DJ, Wilcox KR (2020) Traits link drought resistance with herbivore defence and plant economics in semi-arid grasslands: The central roles of phenology and leaf dry matter content. J Ecol 108:2336–2351. https://doi.org/10.1111/1365-2745.13454
Bommarco R, Kleijn D, Potts SG (2013) Ecological intensification: harnessing ecosystem services for food security. Trends Ecol Evol 28:230–238. https://doi.org/10.1016/j.tree.2012.10.012
Branquart E, Hemptinne J-L (2000) Selectivity in the exploitation of floral resources by hoverflies (Diptera: Syrphinae). Ecography (cop) 23:732–742. https://doi.org/10.1111/j.1600-0587.2000.tb00316.x
Burgio G, Marchesini E, Reggiani N, Montepaone G, Schiatti P, Sommaggio D (2016) Habitat management of organic vineyard in Northern Italy: the role of cover plants management on arthropod functional biodiversity. Bull Entomol Res 106:759–768. https://doi.org/10.1017/S0007485316000493
Campbell AJ, Wilby A, Sutton P, Wäckers F (2017a) Getting more power from your flowers: multi-functional flower strips enhance pollinators and pest control agents in apple orchards. InSects 8:1–18. https://doi.org/10.3390/insects8030101
Campbell AJ, Wilby A, Sutton P, Wäckers FL (2017b) Do sown flower strips boost wild pollinator abundance and pollination services in a spring-flowering crop? A case study from UK cider apple orchards. Agric Ecosyst Environ 239:20–29. https://doi.org/10.1016/j.agee.2017.01.005
David JF, Gillon D (2009) Combined effects of elevated temperatures and reduced leaf litter quality on the life-history parameters of a saprophagous macroarthropod. Glob Chang Biol 15:156–165. https://doi.org/10.1111/j.1365-2486.2008.01711.x
Dewar AM, Dean GJ, Cannon R (1982) Assessment of methods for estimating the numbers of aphids (Hemiptera: Aphididae) in Cereals. Bull Entomol Res 72:675–685. https://doi.org/10.1017/S0007485300008701
Dib H, Libourel G, Warlop F (2012) Entomological and functional role of floral strips in an organic apple orchard: hymenopteran parasitoids as a case study. J Insect Conserv 16:315–318. https://doi.org/10.1007/s10841-012-9471-6
Eeraerts M (2023) A minimum of 15% semi-natural habitat facilitates adequate wild pollinator visitation to a pollinator-dependent crop. Biol Conserv 278:109887. https://doi.org/10.1016/j.biocon.2022.109887
Elliott SE, Irwin RE (2009) Effects of flowering plant density on pollinator visitation, pollen receipt, and seed production in Delphinium barbeyi (Ranunculaceae). Am J Bot 96:912–919. https://doi.org/10.3732/ajb.0800260
Fantinato E, Del Vecchio S, Giovanetti M, Acosta ATR, Buffa G (2018) New insights into plants co-existence in species-rich communities: the pollination interaction perspective. J Veg Sci 29:6–14. https://doi.org/10.1111/jvs.12592
Fantinato E, Sonkoly J, Török P, Buffa G (2021) Patterns of pollination interactions at the community level are related to the type and quantity of floral resources. Funct Ecol 35:2461–2471. https://doi.org/10.1111/1365-2435.13915
Fantinato E, Fiorentin R, Della Bella A, Buffa G (2023) Growth-survival trade-offs and the restoration of non-forested open ecosystems. Glob Ecol Conserv 41:e02383. https://doi.org/10.1016/j.gecco.2023.e02383
Feltham H, Park K, Minderman J, Goulson D (2015) Experimental evidence that wildflower strips increase pollinator visits to crops. Ecol Evol 5:3523–3530. https://doi.org/10.1002/ece3.1444
Ganault P, Barantal S, Coq S, Hättenschwiler S, Lucas S, Decaëns T, Nahmani J (2022) Leaf litter morphological traits, invertebrate body mass and phylogenetic affiliation explain the feeding and feces properties of saprophagous macroarthropods. Eur J Soil Biol. https://doi.org/10.1016/j.ejsobi.2021.103383
Ganser D, Knop E, Albrecht M (2019) Sown wildflower strips as overwintering habitat for arthropods: Effective measure or ecological trap? Agric Ecosyst Environ 275:123–131. https://doi.org/10.1016/j.agee.2019.02.010
Ganser D, Albrecht M, Knop E (2021) Wildflower strips enhance wild bee reproductive success. J Appl Ecol 58:486–495. https://doi.org/10.1111/1365-2664.13778
Garibaldi LA, Pérez-Méndez N, Garratt MPD, Gemmill-Herren B, Miguez FE, Dicks LV (2019) Policies for ecological intensification of crop production. Trends Ecol Evol 34:282–286. https://doi.org/10.1016/j.tree.2019.01.003
Garnier E, Cortez J, Billès G, Navas ML, Roumet C, Debussche M, Laurent G, Blanchard A, Aubry D, Bellmann A, Neill C, Toussaint JP (2004) Plant functional markers capture ecosystem properties during secondary succession. Ecology 85:2630–2637. https://doi.org/10.1890/03-0799
Geiger F, Wäckers FL, Bianchi FJJA (2009) Hibernation of predatory arthropods in semi-natural habitats. Biocontrol 54:529–535. https://doi.org/10.1007/s10526-008-9206-5
Goulet H, Huber JT (1993) Hymenoptera of the World: An Identification Guide to Families. Centre for Land and Biological Resources Research Ottawa, Ontario
Grab H, Poveda K, Danforth B, Loeb G (2018) Landscape context shifts the balance of costs and benefits from wildflower borders on multiple ecosystem services. Proc R Soc B Biol Sci. https://doi.org/10.1098/rspb.2018.1102
Greenop A, Woodcock BA, Pywell RF (2023) Using functional traits to predict pollination services. J Pollinat Ecol 35:194–206
Gurr GM, Wratten SD, Tylianakis J, Kean J, Keller M (2005) Providing plant foods for natural enemies in farming systems: balancing practicalities and theory. In: Wäckers FL, Van Rijn PCJ, Bruin J (eds) Plant-Provided Food for Carnivorous Insects: a Protective Mutualism and its Applications. Cambridge University Press, Cambridge, pp 326–347
Haddad NM, Haarstad J, Tilman D (2000) The effects of long-term nitrogen loading grassland insect communities. Oecologia 124:73–84. https://doi.org/10.1007/s004420050026
Holland JM, Bianchi FJ, Entling MH, Moonen AC, Smith BM, Jeanneret P (2016) Structure, function and management of semi-natural habitats for conservation biological control: a review of European studies. Pest Manag Sci 72:1638–1651. https://doi.org/10.1002/ps.4318
Hussain RI, Brandl M, Maas B, Rabl D, Walcher R, Krautzer B, Entling MH, Moser D, Frank T (2021) Re-established grasslands on farmland promote pollinators more than predators. Agric Ecosyst Environ 319:107543. https://doi.org/10.1016/j.agee.2021.107543
Jennings SB, Brown ND, Sheil D (1999) Assessing forest canopies and understorey illumination: Canopy closure, canopy cover and other measures. Forestry 72:59–73. https://doi.org/10.1093/forestry/72.1.59
Jervis MA, Kidd NAC, Fitton MG, Huddleston T, Dawah HA (1993) Flower-visiting by hymenopteran parasitoids. J Nat Hist 27:67–105. https://doi.org/10.1080/00222939300770051
Kattge J, Bönisch G, Díaz S et al (2020) TRY plant trait database – enhanced coverage and open access. Glob Chang Biol 26:119–188. https://doi.org/10.1111/gcb.14904
Kazakou E, Violle C, Roumet C, Pintor C, Gimenez O, Garnier E (2009) Litter quality and decomposability of species from a Mediterranean succession depend on leaf traits but not on nitrogen supply. Ann Bot 104:1151–1161. https://doi.org/10.1093/aob/mcp202
Kleijn D, Bommarco R, Fijen TPM, Garibaldi LA, Potts SG, van der Putten WH (2019) Ecological intensification: bridging the gap between science and practice. Trends Ecol Evol 34:154–166. https://doi.org/10.1016/j.tree.2018.11.002
Korpela EL, Hyvönen T, Lindgren S, Kuussaari M (2013) Can pollination services, species diversity and conservation be simultaneously promoted by sown wildflower strips on farmland? Agric Ecosyst Environ 179:18–24. https://doi.org/10.1016/j.agee.2013.07.001
Kotze DJ, Lowe EC, MacIvor JS, Ossola A et al (2022) Urban forest invertebrates: how they shape and respond to the urban environment. Urban Ecosyst 25:1589–1609. https://doi.org/10.1007/s11252-022-01240-9
Landis DA (2017) Designing agricultural landscapes for biodiversity-based ecosystem services. Basic Appl Ecol 18:1–12. https://doi.org/10.1016/j.baae.2016.07.005
Langellotto GA, Denno RF (2004) Responses of invertebrate natural enemies to complex-structured habitats: a meta-analytical synthesis. Oecologia 139:1–10. https://doi.org/10.1007/s00442-004-1497-3
Larson BMH, Kevan PG, Inouye DW (2001) Flies and flowers: taxonomic diversity of anthophiles and pollinators. Can Entomol 133:439–465. https://doi.org/10.4039/Ent133439-4
Liira J, Zobel K, Mägi R, Molenberghs G (2002) Vertical structure of herbaceous canopies: the importance of plant growth-form and species-specific traits. Plant Ecol 163:123–134. https://doi.org/10.1023/A:1020365402855
Lowe EB, Groves R, Gratton C (2021) Impacts of field-edge flower plantings on pollinator conservation and ecosystem service delivery – A meta-analysis. Agric Ecosyst Environ 310:107290. https://doi.org/10.1016/j.agee.2020.107290
Marshall SA (2012) Flies: the natural history & diversity of Diptera. Firefly Books, Richmond Hill, Ontario SE
Marshall SA (2018) Beetles: the natural history and diversity of Coleoptera. Firefly Books, Buffalo, New York SE
Mata L, Andersen AN, Morán-Ordóñez A, Hahs AK et al (2021) Indigenous plants promote insect biodiversity in urban greenspaces. Ecol Appl 31:1–17
Moço MKS, Gama-Rodrigues EF, Gama-Rodrigues AC, Machado RCR, Baligar VC (2010) Relationships between invertebrate communities, litter quality and soil attributes under different cacao agroforestry systems in the south of Bahia, Brazil. Appl Soil Ecol 46:347–354. https://doi.org/10.1016/j.apsoil.2010.10.006
Mooney HA, Gulmon SL (1982) Constraints on leaf structure and function in reference to herbivory. Bioscience 32:198–206. https://doi.org/10.2307/1308943
O’Hara RB, Kotze DJ (2010) Do not log-transform count data. Methods Ecol Evol 1:118–122. https://doi.org/10.1111/j.2041-210x.2010.00021.x
Ollerton J (2017) Pollinator diversity: distribution, ecological function, and conservation. Annu Rev Ecol Evol Syst 48:353–376. https://doi.org/10.1146/annurev-ecolsys-110316-022919
Pérez-Harguindeguy N, Diaz S, Garnier E, Lavorel S et al (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234. https://doi.org/10.1071/BT12225
Perović DJ, Gámez-Virués S, Landis DA, Wäckers F, Gurr GM, Wratten SD, You MS, Desneux N (2018) Managing biological control services through multi-trophic trait interactions: review and guidelines for implementation at local and landscape scales. Biol Rev 93:306–321. https://doi.org/10.1111/brv.12346
Pollier A, Tricault Y, Plantegenest M, Bischoff A (2019) Sowing of margin strips rich in floral resources improves herbivore control in adjacent crop fields. Agric for Entomol 21:119–129. https://doi.org/10.1111/afe.12318
Purse BV, Gregory SJ, Harding P, Roy HE (2012) Habitat use governs distribution patterns of saprophagous (litter-transforming) macroarthropods - A case study of British woodlice (Isopoda: Oniscidea). Eur J Entomol 109:543–552
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at https://www.R-project.org/
Rader R, Edwards W, Westcott DA, Cunningham SA, Howlett BG (2011) Pollen transport differs among bees and flies in a human-modified landscape. Diversity Distrib 17:519–529. https://doi.org/10.1111/j.1472-4642.2011.00757.x
Requier F, Pérez-Méndez N, Andersson GKS, Blareau E, Merle I, Garibaldi LA (2023) Bee and non-bee pollinator importance for local food security. Trends Ecol Evol 38:196–205. https://doi.org/10.1016/j.tree.2022.10.006
Schmidt A, Fartmann T, Kiehl K, Kirmer A, Tischew S (2022) Effects of perennial wildflower strips and landscape structure on birds in intensively farmed agricultural landscapes. Basic Appl Ecol 58:15–25. https://doi.org/10.1016/j.baae.2021.10.005
Schütz L, Wenzel B, Rottstock T, Dachbrodt-Saaydeh S, Golla B, Kehlenbeck H (2022) How to promote multifunctionality of vegetated strips in arable farming: a qualitative approach for Germany. Ecosphere 13:1–21. https://doi.org/10.1002/ecs2.4229
Storkey J, Brooks D, Haughton A, Hawes C, Smith BM, Holland JM (2013) Using functional traits to quantify the value of plant communities to invertebrate ecosystem service providers in arable landscapes. J Ecol 101:38–46. https://doi.org/10.1111/1365-2745.12020
Thorbek P, Bilde T (2004) Reduced numbers of generalist arthropod predators after crop management. J Appl Ecol 41:526–538. https://doi.org/10.1111/j.0021-8901.2004.00913.x
Threlfall CG, Mata L, Mackie JA, Hahs AK, Stork NE, Williams NSG, Livesley SJ (2017) Increasing biodiversity in urban green spaces through simple vegetation interventions. J Appl Ecol 54:1874–1883. https://doi.org/10.1111/1365-2664.12876
Tscharntke T, Karp DS, Chaplin-Kramer R, Batáry P, DeClerck F, Gratton C, Hunt L, Ives A, Jonsson M, Larsen A, Martin EA, Martínez-Salinas A, Meehan TD, O’Rourke M, Poveda K, Rosenheim JA, Rusch A, Schellhorn N, Wanger TC, Wratten S, Zhang W (2016) When natural habitat fails to enhance biological pest control – Five hypotheses. Biol Conserv 204:449–458. https://doi.org/10.1016/j.biocon.2016.10.001
Tscharntke T, Grass I, Wanger TC, Westphal C, Batary P (2021) Beyond organic farming - harnessing biodiversity-friendly landscapes. Trends Ecol Evol 36(10):919–930. https://doi.org/10.1016/j.tree.2021.06.010
von Königslöw V, Fornoff F, Klein A-M (2022) Pollinator enhancement in agriculture: comparing sown flower strips, hedges and sown hedge herb layers in apple orchards. Biodivers Conserv 31:433–451. https://doi.org/10.1007/s10531-021-02338-w
Wäckers FL (2004) Assessing the suitability of flowering herbs as parasitoid food sources: flower attractiveness and nectar accessibility. Biol Control 29:307–314. https://doi.org/10.1016/j.biocontrol.2003.08.005
Wäckers FL (2005) Suitability of (extra-)floral nectar, pollen, and honeydew as insect food sources. Plant-Provided Food for Carnivorous Insects. Cambridge University Press, Cambridge, pp 17–74
Wäckers FL, van Rijn PCJ (2012) Pick and mix: selecting flowering plants to meet the requirements of target biological control insects. Biodivers Insect Pests Key Issues Sustain Manag. https://doi.org/10.1002/9781118231838.ch9
Willmer P (2011) Pollination and floral ecology. University Press, Princeton
Winkler K, Wäckers F, Valdivia L, Larraz V, Van Lenteren J (2003) Strategic use of nectar sources to boost biological control. IOBC WPRS Bull 29:209–214
Woodcock BA, Potts SG, Westbury DB, Ramsay AJ, Lambert M, Harris SJ, Brown VK (2007) The importance of sward architectural complexity in structuring predatory and phytophagous invertebrate assemblages. Ecol Entomol 32:302–311. https://doi.org/10.1111/j.1365-2311.2007.00869.x
Zina V, Duarte G, Fonseca A, Conde S, Teresa Ferreira M, Carlos Franco J, Rosário Fernandes M (2022) Land use system, invasive species and shrub diversity of the riparian ecological infrastructure determine the specific and functional richness of ant communities in Mediterranean river valleys. Ecol Indic. https://doi.org/10.1016/j.ecolind.2022.109613
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210x.2009.00001.x
Funding
Gabriella Buffa and Edy Fantinato were supported by the Ca’ Foscari University of Venice, MUR (Ministero dell'Università e della Ricerca) and EU in the framework of the European LIFE project LIFE19 NAT/IT/000848 PollinAction. Sebastiano Favarin was supported by PON “Ricerca e Innovazione” 2014 – 2020, MUR. Daniele Sommaggio was supported by LIFE19 NAT/IT/000848.
Author information
Authors and Affiliations
Contributions
Gabriella Buffa, Edy Fantinato and Sebastiano Favarin conceived the idea and formulated the study design; Edy Fantinato, Sebastiano Favarin and Maddalena Masiero carried out the fieldwork. Daniele Sommaggio, Edy Fantinato, Gabriella Buffa and Sebastiano Favarin analysed and interpreted the data. Gabriella Buffa, Edy Fantinato, Sebastiano Favarin and Daniele Sommaggio wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by James Millington.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Favarin, S., Sommaggio, D., Fantinato, E. et al. Ecological intensification: multifunctional flower strips support beneficial arthropods in an organic apple orchard. Plant Ecol (2024). https://doi.org/10.1007/s11258-024-01402-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11258-024-01402-z