Abstract
Nitrogen (N) and phosphorus (P) availability affect plant sexual reproduction performance. Seed as the main product of sexual reproduction is expected to be affected by N and P availability in parent plant. We experimentally test how parental N:P stoichiometry affected seed characteristics and performance of two grassland species. Seeds of a common species (Holcus lanatus) and an endangered species (Parnassia palustris) were collected from parent plants under two different N:P ratio growth conditions in a grassland reserve in the Netherlands. We measured the following traits of the two species from the two parental N:P ratio growth conditions: seed N concentration and content, seed P concentration and content, seed length, seed area, seed weight, seed germination, and offspring survival. No significant inter-location difference was found in seed weight of the collected seeds for either of the two species. However, the stronger P-limited conditions at the locations of the parent plants affected seed length and seed area negatively in H. lanatus and positively in P. palustris. Stronger P-limited conditions also decreased seed P concentration and content and increased seed N:P ratio of H. lanatus, but no inter-location nutrient difference was found in seeds of P. palustris. Surprisingly, contrary to what could be expected from seed P concentration and content there was no inter-location difference in seed germination for H. lanatus. For P. palustris, stronger P-limited conditions in the parental environment significantly enhanced seed germination, whereas stronger P-limited conditions in the offspring environment decreased offspring survival, suggesting that P limitation may negatively affect P. palustris rejuvenation. Our results suggest the possibility of different influences of parental N:P ratio and especially of P limitation on seed characteristics and performance of a common and an endangered species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seeds are the main product of plant’s sexual reproduction and are rich in mineral nutrients which the adult plant translocates to its seeds to provide nutrition for seedlings in the early stages of establishment before the development of a root system (Lamont and Groom 2013). Among these nutrients, nitrogen (N) and phosphorus (P) are the most essential and have been researched extensively (Henery and Westoby 2001; Lamont and Groom 2013). Studies have shown that nutrient shortages in parent plants lead to increased allocation of plant minerals to seeds [except in the case of iron (Fe)] (Lamont and Groom 2013), although the relative allocation of biomass to the seeds has been shown to be constant (Fenner 1986a). Moreover, the fraction of a plant’s total content of a particular element that the plant allocates to its seeds varies widely, depending on the elements. In Fenner’s greenhouse experiment (1986), it ranged from 4% of total potassium (K) to 38% of total P in plants on 100% Hoagland solution, while in the most nutrient-deprived plants it ranged from 2.5% of total Fe to 52% of total P. However, both aforementioned cases indicate that a high percentage of the plant’s P content is invested in sexual reproduction.
Various studies have also pointed to the influence of nutrient supply of parent plants on seed traits and seed performance, such as seed size, dormancy, germination, and dispersal, which are vital components in the plant life cycle (Fenner 1983; Harper 1977; Thompson 1987). Early work on Senecio vulgaris, a short-lived monocarpic plant, showed that seed nutrient concentrations were largely buffered from the differences in parental nutrient status (Fenner 1986b). However, various other studies indicated that mineral nutrient availability of parent plants was positively correlated with seed mass (Willson and Price 1980; Gray and Thomas 1982; Parrish and Bazzaz 1985; Marshall 1986; Wulff 1986a, b, c; Galloway 2001), germination time (Aarssen 1989; Aarssen and Burton 1990) and germination success (Galloway 2001). However, a contrasting result was obtained by Beadle (1962) and Lamont and Groom (2013) who found that plants on nutrient-poorer soils had larger seeds. Threatened species seem to be especially sensitive to mineral nutrient availability. Fujita et al. (2014) showed that threatened species persisting under severe P limitation invested less in sexual reproduction. Similarly, seed development in several threatened orchid species was reported to respond positively to increased nutrient supply under controlled conditions (Paul et al. 2012; Figura et al. 2021).
Nutrient-specific effects on seed traits and performance have been widely documented. Greenhouse experiments with pea and watercress indicated that seeds of plants grown under high P supply were some 15% larger than those of plants grown under low P supply (Austin 1966a). Another study reported that when N was deficient, seed and fruit production of agricultural crops decreased dramatically (Tonitto et al. 2006). In terms of seed germination, Austin (1966a) showed that seeds from plants deficient in major elements can be impaired in germination compared to seeds from plants grown in complete nutrient cultures. However, Stewart et al. (1997) reported that adding P had no effect on seed germination of two cattail species. With regard to protected species, Figura et al. (2021) suggested negative effects of nitrate on symbiotic threatened orchid germination, while Bochenková et al. (2015) suggested no direct effect of N, but a possible influence of low P of low seed germination of endangered species Pulsatilla pratensis. The importance of a balanced N and P supply to seed germination was also pointed out by several studies showing that seed germination is restricted by P limitation (Hrdličková et al. 2011; Hejcman et al. 2012). Furthermore, the positive influence of a high nutrient content of seeds on seedling size is also well established (Austin 1966b; Lamont and Groom 2013). Therefore, it seems that the nutrient supply is critical for seed traits and performance, and thereby plant dispersal, and nutrients stored in the seed before the development of the root system for absorbing nutrients from soil may impact seedling establishment and may also cause changes in seedling growth. However, it has been difficult to predict species-specific responses to varying conditions of major nutrients, particularly for endangered species.
Soil N and P are considered as the most critical elements for plant growth and sexual reproduction performance (Verhoeven et al. 1996) and are being influenced by human activity worldwide (Steffen et al. 2015). These changes in N and P availability in the environment can probably shift the main type of nutrient limitation (Von Oheimb et al. 2010), leading to changes in plant species composition in natural vegetation (Stevens et al. 2004) and in plant trait composition, including plants’ investment in sexual reproduction (Fujita et al. 2014). Several studies have found that N limitation occurs frequently with higher productivity and that N-limited ecosystems harbor competitive fast-growing species whereas P-limited herbaceous ecosystems are mostly characterized by low productivity and slow-growing species (Wassen et al. 2005; Fujita et al. 2014; Roeling et al. 2018). Moreover, under P limitation, higher numbers and percentages of endangered plant species have been found (Wassen et al. 2005), whereas plant species growing under P limitation have been found to invest significantly less in sexual reproduction than species grown under N limitation, e.g., later flowering starting time, shorter flowering period, and lower seed production (Fujita et al. 2014; Wang et al. 2019). These findings are in line with previous studies that have reported that endangered species have shorter flowering periods (Lahti et al. 1991), smaller seed mass (Murray et al. 2002), and poorer dispersal ability (Farnsworth and Ogurcak 2008). Another study showed that deficiency of P led to a strong reduction in the amount of flowers produced (Brouwer et al. 2001).
Because of their low investment in sexual reproduction as a strategy to save on P, it seems likely that compared to species persisting under N limitation, species persisting under P limitation may have lost their capacity to effectively disperse. This would make them more vulnerable to environmental change and extinction (Fujita et al. 2014). However, most studies in this area have been done under controlled conditions in greenhouse experiments or are based on average traits from databases, but studies that focus on traits and performance of seeds produced in natural vegetation under different nutrient conditions and that include intraspecific responses are scarce.
We therefore carried out a greenhouse experiment with seeds of both a common species (Holcus lanatus) and an endangered species (Parnassia palustris) collected from plants growing at two field locations differing in N:P ratio in the above-ground vegetation, indicating differences in the relative availability of N and P (Wassen et al. 1995). We aimed to test if differences in relative N and P availability of parent plants (indicated by differences in above-ground N:P ratio) affect certain seed traits of the two species (seed size, seed weight, seed N and P concentrations and contents, seed germination) and offspring plant survival at the end of the life cycle, under nutrient treatments varying in absolute and relative N and P supply. Secondly, we aimed to explore if there were interspecific effects between the common and the endangered species. Our hypothesis was that seed traits of both the common and the endangered species responded to their parental N:P ratio. However, we expected that those responses would differ between the common and the endangered species: i.e., that high N:P would restrict the seed characteristics and performance of the common species (i.e., smaller seeds, lighter seeds, lower N and P concentrations and contents in the seeds, and lower germination and offspring survival), but would promote them in the endangered species, due to the fact that endangered species persist under P-limited conditions (Wassen et al. 2005). Moreover, we assumed that with increasing N:P ratio, the plant survival of H. lanatus would decrease and the plant survival of P. palustris would increase.
Materials and methods
Study area and location selection
Seeds were collected from two sampling locations at the Middenduin nature reserve (MD1, 52°16′N 5°9′E and MD2, 52°23′N 4°35′E) located in the western Netherlands and owned by the State Forestry Service. Prescreening of these locations indicated that they differed in N:P ratio of the above-ground biomass, while soil conditions and species composition were comparable. The two sampling locations were located in close proximity, with contiguous natural vegetation between them. We therefore assumed that plant species at both locations could be considered part of the same genetic population. The area harbors herb-rich low-productive grassland, with a high species diversity and occurrence of rare species such as P. palustris L., Epipactis palustris L., and Rhinanthus minor L. The climate is temperate: mean annual temperature is 11 °C, mean annual minimum temperature is − 9 °C, and mean annual maximum temperature is 33 °C, and the mean annual precipitation is 765 mm (http://projects.knmi.nl/klimatologie/daggegevens/selectie.cgi).
Study species
H. lanatus L. is a common perennial velvety grass species, often found in nutrient-rich environments throughout Europe. The flowers are wind-pollinated and usually out-crossing. The hermaphroditic inflorescences produce numerous seeds that are shed from June to early autumn (Watt 1978) and remain viable for over 5 years (www.cabi.org/isc/datasheet/114824).
P. palustris L. is an endangered perennial herb, with a basal cluster of leaves and several straight stems up to 30 cm high, each carrying one flower at the top. The hermaphroditic flowers are insect-pollinated. Seeds ripen between September and October and are shed the most in September (Schat 1983). This species declined during the last century, which has resulted in genetic isolation and a threatened status in many European countries (Bossuyt 2007).
Plot selection, vegetation harvesting, and seed collection
One 2 × 2 m plot containing both H. lanatus and P. palustris was selected from each of the two locations, i.e., there were in total two plots at the two locations. We took the conventional vegetation recording of estimating cover of all species presenting in the two 2 × 2 m plots. The coverages of H. lanatus and P. palustris were 1% and 0.05%, respectively, in the plot at location MD1 and 0.25% and 1.5%, respectively, in the plot at location MD2 (for species composition of the two plots see Online Resource Table S1). On June 20, 2014, which was the peak of the growing season of that year, living above-ground vegetation was harvested in three 20 × 20 cm squares at the three random corners of the two plots at the two locations (Wassen et al. 1995, 2005). The harvested vegetative materials were taken to the lab immediately for drying. Between June and September 2014, all mature seeds of H. lanatus and P. palustris inside of each plot were collected. Seeds of the two species from the two plots were stored separately in four glass bottles with a silica gel pack inside of each bottle and stored in the fridge at 4 °C before the latter measurement.
Seed length, area, and weight
As indicators for seed size, we measured seed length, cross-sectional area, and weight. Random seeds of H. lanatus from locations MD1 and MD2, respectively, were placed on a flat sheet of white paper next to a ruler. Representative digital images of seeds (457 for seed length of seed from location MD1; 435 for seed area of seed from location MD1; 674 for seed length of seed from location MD2; 666 for seed area of seed from location MD2) were taken using a Nikon D5200 camera (Nikon Corp., Japan) 1 m vertically above the seeds.
Random seeds of P. palustris from locations MD1 and MD2, respectively, were placed on the plain stand of a Nikon SMZ800N magnification unit (Nikon Cor., Japan). Representative digital images of seeds (569 for seed length of seed from location MD1; 508 for seed area of seed from location MD1; 438 for seed length of seed from location MD2; 375 for seed area of seed from location MD2) were taken using a Nikon Digital Sight DS-Fi1 camera with photonic F3000 light.
To quantify seed size, the following two attributes were calculated from seed images by ImageJ software:
-
Seed length defined as the longest distance between two points on the edge of a seed.
-
Seed area defined by the vertical projection area of a seed.
Seed length and area of each species from each location were then calculated by averaging all the measured seeds.
Ten batches of 100 seeds of H. lanatus from each of the two locations (MD1 and MD2) and 10 batches of 200 seeds of P. palustris also from these locations, were weighed. The weight of 100 seeds of H. lanatus from each location and the weight of 200 seeds of P. palustris, also from each location, were then calculated by averaging the resulting 10 batch weights.
Determination of N concentration, P concentration, and N:P ratio in above-ground biomass and in seeds
Above-ground vegetative material was dried at 70 °C for 48 h, and ground into powder with a Retsch MM400 mixer mill, and passed through a 0.5 mm sieve. Three subsamples of dried seeds from each bottle containing seeds of each species from each location were randomly collected (the weight of each subsample was around 1 g). Seed samples were also ground into powder with a Retsch MM400 mixer mill.
Total N concentration of dried plant material and seed material was measured with a C/N elemental analyzer (NA1500, Carlo Erba-Thermo Fisher Scientific); total P concentration of dried plant material and seed material was measured by a TXRF Spectrometer (S2, PICOFOX, Bruker).
We use these plant nutrient concentrations—referred to in this paper as plant N and plant P—as indicators of plant-available nutrient concentrations (Wassen et al. 1995). Based on above-ground N:P ratios we determined whether the vegetation at the locations of seed collection was N-limited (N:P < 13.5), P-limited (N:P > 16), or N and P co-limited (13.5 ≤ N:P ≤ 16) (Güsewell and Koerselman 2002; Olde Venterink et al. 2003).
Determination of seed N content and P content
Seed N and P contents of H. lanatus were calculated by multiplying seed N and P concentrations of H. lanatus, respectively, by the average weight of 100 seeds of H. lanatus; seed N and P contents of P. palustris were calculated by multiplying seed N and P concentrations of P. palustris, respectively, by the average weight of 200 seeds of P. palustris.
Germination experiment
Seeds for germination were stored in the fridge (4 °C) for 10.7 weeks. Healthy and mature seeds of H. lanatus and P. palustris were selected individually using a Nikon SMZ800N magnification unit (Nikon Cor., Japan) (for H. lanatus, we excluded those which were molded and insect-damaged. Moreover, unripe seeds, for instance those with very green seed coat were also excluded; for P. palustris, we excluded those with obviously smaller seed size than the majority, as well as those whose shapes were obviously malformed). For H. lanatus, we selected 5044 seeds from location MD1 and 5515 from location MD2; for P. palustris, we selected 5112 seeds from location MD1 and 6721 from location MD2. These seeds were sown on moist quartz sand in separate germination chambers, marked with labels of species names and locations information, and covered by a piece of glass to retain moisture. Germination chambers were kept in the greenhouse with a relatively high and fluctuating temperature and natural light conditions. After 22 days, when no more new seedlings emerged (every new occurring seedling was labeled by gently fixing one little pin beside it), the final numbers of seedlings of H. lanatus and P. palustris from locations MD1 and MD2 were counted. The germination of each species from each location was calculated by dividing the number of seedlings by the number of seeds sown.
Plant survival at the end of the life cycle, under different nutrient treatments
A random selection of seedlings was transferred to pots (volume 8 L for H. lanatus and 3 L for P. palustris) containing a mixture of quartz sand and natural dune sand collected close to the study areas (for detailed information, see Wang et al. 2019). Four seedlings were planted in each pot and incubated in the greenhouse. Nutrient solutions were supplied following Güsewell (2005).
A full factorial combination with six nutrient treatments was applied, with three nutrient supply ratios [N:P = 5 (low relative N supply), N:P = 15 (co-limitation), and N:P = 45 (low relative P supply)] and two absolute nutrient supply levels: low and high. The treatments were defined by the total amounts of N and P applied per plant throughout the cultivation process (in mg) and were calculated as:
in which L is the overall supply level (geometric mean of N and P supply). L was 13.4 mg for low supply level and 40.3 mg for high supply level, similar to the first-year nutrient treatments of Güsewell (2005).
For each nutrient treatment with seeds of one species from one location, there were four pots. The total number of pots was 2 species × 2 locations × 3 nutrient supply ratios × 2 nutrient supply levels × 4 pot replicates = 96. Nutrient solutions were applied weekly (for detailed information, see Wang et al. 2019). N was supplied as KNO3 and Ca(NO3)2, and P was provided as KH2PO4. Both KNO3 and KH2PO4 supplied part of the K, and the remaining K was added by supplying KCl (Güsewell 2005). The other essential macronutrients, such as calcium (Ca) and micronutrients, such as Fe and copper (Cu), were applied in non-limiting concentrations and were supplied in the same amounts to all treatments weekly. Details of the nutrient treatments are given in Online Resource Table S1. Chlorine (Cl) was the only element that was not supplied in fixed amounts to all treatments. We have indicated the range of Cl addition in Online Resource Table S1.
In addition to receiving nutrient solutions, plants were watered frequently with demineralized water to prevent drying out. The pots were switched around regularly to randomize possible differences in light, temperature, and moisture conditions in the greenhouse.
For both species, the number of dead individuals was recorded at the end of the experiment, after monitoring a full life cycle from seed to seed. Survival of each species from each location in each pot was then calculated by dividing the number of surviving plants by the total number of original seedlings (which was 4).
Data analysis
One-tailed t tests were used to compare the difference of the following parameters between location MD1 and location MD2: N and P concentrations in the above-ground biomass, N and P concentrations, and N and P contents of seed material of H. lanatus, respectively, as well as of seed material of P. palustris, respectively, seed traits (length, area, weight) of H. lanatus, respectively, as well as of P. palustris, respectively, and the survival of P. palustris under various nutrient treatments.
The between-location differences in seed germination of H. lanatus and P. palustris were analyzed using a Chi-square test. The difference in plant survival of P. palustris per location and for the two locations together, under different nutrient treatments, was tested in one-way ANOVA with standard post hoc test of Gabriel’s procedure to analyze differences between treatments with the combination of different nutrient supply ratios and levels. Differences were considered to be statistically different at P ≤ 0.05. All tests were performed with SPSS 24.0 software (SPSS, Chicago, USA); the figures were also created in SPSS.
Results
N and P concentrations of above-ground biomass
P concentration in the above-ground biomass was significantly higher at location MD1 than at location MD2, but no significant difference was found for N (Table 1). The two locations differed significantly in the relative availability of N and P, as indicated by N:P ratio of the above-ground biomass. The N:P ratio in above-ground plant material was 22.3 at location MD1 and 40.5 in MD2, indicating that at both locations plant growth was limited by P but that location MD2 was more strongly P-limited than location MD1 (Güsewell and Koerselman 2002).
Seed N and P concentrations and N and P contents
As with N concentration in the above-ground biomass, there was no difference in seed N concentration for either H. lanatus or P. palustris between locations (Table 2). The seed P concentration of H. lanatus from the more severely P-limited location (MD2) was significantly higher than that from location MD1, but there was no significant difference in the seed P concentration of P. palustris between locations (Table 2). Unlike the N:P ratio of above-ground biomass and thus contrary to expectations, the seed N:P ratio of H. lanatus from the strongly P-limited location MD2 was significantly lower than that from location MD1 (Table 2). No significant inter-location difference was found for the seed N:P ratio of P. palustris.
The seed N and P contents of H. lanatus and P. palustris showed the same tendency as seed N and P concentrations: no significant inter-location difference was found for seed N or P content of P. palustris, but the seed P content of H. lanatus from location MD2 was significantly higher than that from location MD1 (Table 2).
Seed length, area, and weight
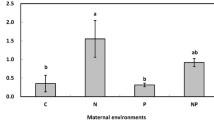
Parental N:P ratio influenced seed length and area; the influences differed between H. lanatus and P. palustris. Parental N:P ratio did not influence seed weight in either species (Fig. 1). Seeds from the more severely P-limited location (location MD2) were shorter and smaller in the case of H. lanatus, but longer and bigger in the case of P. palustris (Fig. 1).
Comparisons of seed length, seed area, and seed weight of H. lanatus and P. palustris from locations MD1 and MD2. Data are means ± SD. Significant differences between locations are indicated by different lowercase letter(s). Values with the same lower letter(s) are not significantly different (P = 0.05)
Germination
Seed germination differed remarkably between the two species: average germination was 6.3% for H. lanatus and 59.6% for P. palustris. When comparing seeds with a different parental N:P ratio (location MD1 versus location MD2), no significant difference was found for the germination of H. lanatus, whereas seed germination of P. palustris from location MD2 was significantly higher than that from location MD1 (Table 3).
Offspring plant survival at the end of the life cycle
H. lanatus grew successfully under all the nutrient treatments, irrespective of the parental N:P ratio. At the end of the experiment, only four plants, all in a single pot in treatment 45H (i.e., N:P ratio of 45, high supply level), had died, resulting in an overall survival of 97.9% for H. lanatus.
For P. palustris, again, no effect of parental N:P ratio was detected, i.e., offspring plant survival of P. palustris did not differ significantly between location MD1 and location MD2 (Fig. 2A). We therefore pooled the results of locations MD1 and MD2 before further analyzing the survival of P. palustris at different N:P ratios and supply levels (Fig. 2B).
Offspring plant survival (%) of P. palustris from locations MD1 and MD2 under different nutrient treatments (A); offspring plant survival (%) of P. palustris under different nutrient treatments, with locations MD1 and MD2 pooled (B). Values without character indicate no significant difference between locations MD1 and MD2 under the same nutrient treatment (P = 0.05) (A); values indicated by the same character are not significantly different (P = 0.05) (B)
After pooling, the plant survival of P. palustris was found to decrease with increasing N:P ratio, irrespective of nutrient supply level (Fig. 2B): i.e., the survival of P. palustris was the highest (c. 81.3%) under the treatments with an N:P ratio of 5 (N limitation) and the lowest (c. 6.3%) under the treatments with an N:P ratio of 45 (P limitation).
Discussion
In this experiment, we tested the effects of nutrient conditions of parent plants on certain seed traits (seed length, seed area, seed weight, seed N and P concentrations and contents), seed germination, and survival of plants grown from these seeds under various nutrient treatments, for a common species (H. lanatus) and an endangered species (P. palustris) from two field locations with two different N:P ratio growth conditions. The N:P ratio in the above-ground biomass was 22.3 for location MD1 and 40.5 for location MD2. The latter ratio indicates very strong P limitation whereas the former is closer to an N and P availability that is considered balanced (14.75: cf. Güsewell and Koerselman 2002). The results for H. lanatus showed that stronger P-limited conditions for the parent plants decreased P concentration and content in seeds and increased N:P ratio. No differences in nutrients in seeds from different parental N:P ratios were found for P. palustris. Stronger P-limited conditions promoted the seed germination ability of P. palustris significantly but not for H. lanatus. Additionally, no significant inter-location difference in seed weight was found for either species, while for seeds from parent plants under stronger P-limited conditions, seed length, and area were restricted in H. lanatus but promoted in P. palustris. Furthermore, no influence of parental N:P ratio on offspring plant survival was found.
Intraspecific differences between parental N:P ratios of H. lanatus and P. palustris
Seed traits such as seed length, size, mass, and mineral nutrient concentration have been shown to correlate with seed quality, seed germination, and seedling establishment (Vera 1997; Ellison 2001; Cordazzo 2002; Bu et al. 2007; Wu and Du 2007; Kaya et al. 2008; White and Veneklaas 2012). Our comparisons of seed traits of H. lanatus and P. palustris collected from the two locations with different parental N:P ratios confirmed our hypothesis that parental N:P ratio may affect seed trait expression and seed performance.
In detail, seeds of H. lanatus from the more P-limited location (location MD2) were shorter and smaller than those from the location with a more balanced N:P ratio (location MD1) (Fig. 1). This is in line with a previous greenhouse experiment on H. lanatus that showed that seeds produced under P limitation (N:P = 45) were significantly smaller and shorter than seeds produced under N limitation and N and P co-limitation (N:P = 5 and N:P = 15) (Wang et al. 2019). The shorter length and smaller seeds of H. lanatus at location MD2 imply that conditions with a low relative P availability in parent plants may reduce the seed quality of common species, possibly because P limitation restricts the vegetative growth of the parent plant (Liao and Yan 1999; Wang et al. 2021). Moreover, this greenhouse experiment on the investment in sexual reproduction of H. lanatus under a gradient of N:P ratios indicated that P limitation dramatically restricted seed number and weight per plant (Wang et al. 2019). In contrast, in P. palustris, P limitation did not reduce seed length and seed area but promoted them: seeds from the more P-limited location (location MD2) were longer and larger in area than those from the more balanced N:P ratio (location MD1) (Fig. 1). This is in line with the finding by Lamont and Groom (2013) that seeds were larger in poorer soils. However, contrary to their other conclusion that seeds produced on poorer soils are more nutrient rich, in our study we found no inter-location difference in N concentration, P concentration, N content, or P content in the seeds of P. palustris (Table 2).
The larger seed size might therefore be one of the reasons that seeds of P. palustris from location MD2 had more successful germination than those from location MD1, especially given the positive effect of seed size on seed germination suggested by Schat (1983). However, contrary to expectations, seed germination of H. lanatus did not differ significantly between our two locations, even though, as noted above, there were inter-location differences in seed size and P concentration in the seeds. The lack of difference in germination of H. lanatus from different parental N:P ratios contrasts with findings from other studies. For instance, Hejcman et al. (2012) showed that when parent plants had been grown under conditions of insufficient P and K, the seed germination of Rumex crispus was lower than when parent plants received a balanced supply of N, P, and K. Since P is an element essential for plant vegetative growth (Wang et al. 2021) as well as for sexual reproduction (Fujita et al. 2014; Wang et al. 2019), the lack of inter-location difference in germination of seeds of H. lanatus might be explained by the observation that although the seeds from the stronger P-limited location were smaller, they also had a higher P concentration, which may have counterbalanced the negative effects of a smaller seed size.
Where the seedling establishment of H. lanatus under the various nutrient treatments was relatively stable, with a high offspring plant survival of 97.9%, the offspring plant survival of P. palustris varied greatly under different nutrient treatments: the highest survival was at N:P 5 (indicating severe N limitation) and the lowest was at N:P 45 (indicating severe P limitation) (Fig. 2). In addition, throughout their life cycle, the surviving individuals under N:P 45 also showed poor growth and a stunted appearance compared to the individuals under N:P 5 and 15. According to Tilman’s (1982) resource ratio hypothesis, a species whose requirement ratio of multiple resources is closer to the supply ratio of the resources will be likely to persist better than a species with a more divergent ratio. We may therefore deduce that an N:P ratio of 5 is more optimal for P. palustris than a ratio of 15 or 45—which is opposite to our original assumption that the endangered species P. palustris would prefer P limitation. This unexpected conclusion might be explained by the fact that although P limitation has proved to be a condition under which endangered species persist, this does not mean that all endangered species predominantly confined to P-limited ecosystems. In fact, 51% of the endangered species in the dataset of Fujita et al. (2014) occurred under N:P ratios in above-ground biomass that were below 16, indicating N limitation or N and P co-limitation. The apparent preference of the endangered species P. palustris for N limitation during its growth contrasts with our finding that the seed characteristics of this species was promoted under P limitation. However, under natural conditions the negative influence of P limitation on the growth and survival of this endangered species observed in our experiments may still might be offset, compared to the situation under N limitation where large number of competitors around exist. Moreover, despite the better characteristics and performance of seeds of P. palustris from the more P-limited site, i.e., longer and bigger seeds, as well as higher germination success (Fig. 1, Table 3), we noticed that plants of P. palustris growing from seeds from the more P-limited site (location MD2) were more easily infected by fungi, i.e., offspring of P. palustris growing from seeds from parent plants grown under P-limited conditions is more susceptible to fungal infection than those from parent plants grown under more balanced N:P ratio growth conditions. Although at the end of the growing season there was no significant inter-site difference in the offspring plant survival of P. palustris (Fig. 2A), the higher infection rate may affect its competitive ability when grown in competition with other species (Alexander and Holt 1998).
Interspecific differences between H. lanatus and P. palustris
Seeds of the common species H. lanatus were generally bigger than those of the endangered species P. palustris (Fig. 1). This is consistent with the theory suggested by Mitchley and Grubb (1986) that competitive superiority of large-seeded species allows them to become common, while small-seeded species are fugitive occupants of relatively rare microsites. As mentioned in the discussion above, under stronger P limitation, the seed quality of P. palustris tended to improve (longer and bigger seeds, with higher germination), whereas the seed quality of H. lanatus suffered (shorter and smaller seeds). A possible explanation for this opposite intraspecific responses between the common and the endangered species is that, endangered species are more sensitive to P enrichment, as endangered species occur more frequently under conditions of P limitation (Wassen et al. 2005), where plants invest less in sexual reproduction than they do under conditions of N limitation or co-limitation (Fujita et al. 2014). As P is proportionally the most important element in plant seeds (Van Andel and Vera 1977; Fenner 1986a), the bigger seed size of P. palustris in more P-limited conditions may be a strategy to acquire and store sufficient P for the next generation.
The somewhat counterintuitive finding that germination of the larger seeds of H. lanatus was much lower than that of the smaller seeds of P. palustris (Table 3) which is in line with the finding of Hanley et al. (2003) that the germination for fire-following species was greater for the smaller-seeded species than for the larger-seeded species at high temperature. However, to our knowledge, the relationship between seed size and germination of non-fire-following species has rarely been studied, which probably deserve further research. Moreover, the low germination of seeds of H. lanatus might also derive from the restriction of parental P-limited growth conditions. Interestingly, although germination was lower for H. lanatus and higher for P. palustris, offspring plant survival of these two species was very different: 98% for H. lanatus but only 42% for P. palustris. The low germination but high offspring plant survival of H. lanatus in our research is consistent with the result Isselstein et al. (2002) reported for this species. In general, the pattern of plant mortality risk depends largely on specific tolerances, which may strongly differ between species: for instance, P. palustris requires particular environmental conditions (Schat 1983), whereas H. lanatus has a much broader ecological amplitude (Veeranjaneyulu and Ramadas 1982). The relatively low plant survival of P. palustris (especially under P limitation) (Fig. 2, Online Resource Fig. S1) and the fact that its plants are small (Bonnin et al. 2002) indicate that rejuvenation of P. palustris is hampered by P limitation and probably puts this species at a strong competitive disadvantage vis-à-vis common species like H. lanatus and might be a factor accounting for P. palustris being endangered in the wild.
Conclusion
In conclusion, this experiment shows potential influences of parental N:P ratio, especially P limitation in the parental environment, on seed characteristics, and performance for both of the common species and the endangered species. However, seed quality of the endangered species was improved under P limitation in the parental environment, while the common species suffered under P limitation. Remarkably, for P. palustris, seed germination was significantly enhanced under stronger P-limited conditions in the parental environment, whereas the offspring survival decreased under stronger P-limited conditions in the offspring environment, suggesting that P limitation negatively affects P. palustris rejuvenation. Our results point to the potential importance of the management to reduce P availability to conserving endangered species because of its potential effects on reproduction and dispersal. However, since only two plant species were studied and so little research has been done on the relations between parental N and P concentration and seed characteristics and performance, it is difficult to draw generalizable conclusions. We therefore suggest that future research examines more common and endangered species to better understand the potential impact of eutrophication on the conservation status of plant species by affecting their sexual reproduction success and dispersal capacity.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Aarssen LW (1989) Competitive ability and species coexistence: a “plant”s-eye’ view. Oikos 56:386–401. https://doi.org/10.2307/3565625
Aarssen LW, Burton SM (1990) Maternal effects at four levels in Senecio vulgaris (Asteraceae) grown on a soil nutrient gradient. Am J Bot 77:1231–1240. https://doi.org/10.2307/2444634
Alexander HM, Holt RD (1998) The interaction between plant competition and disease. Perspect Plant Ecol Evol Syst 1:206–220. https://doi.org/10.1078/1433-8319-00059
Austin RB (1966a) The influene of the phosphorus and nitrogen nutrition of pea plants on the growth of their progeny. Plant Soil 3:359–368. https://doi.org/10.1007/bf01374044
Austin RB (1966b) The growth of watercress (Rorippa nasturtium aquaticum (L) Hayek) from seed as affected by the phosphorus nutrition of the parent plant. Plant Soil 1:113–120. https://doi.org/10.1007/bf01373077
Beadle NCW (1962) Soil phosphate and the delimitation on plant communities in eastern Australia II. Ecology 43:281–288. https://doi.org/10.2307/1931983
Bochenková M, Hejcman M, Karlík P (2015) Effect of plant community on recruitment of Pulsatilla pratensis in dry grassland. Sci Agric Bohem 43:127–133. https://doi.org/10.7160/sab.2012.430402
Bonnin I, Colas B, Bacles C, Holl AC, Hendoux F, Destiné B, Viard F (2002) Population structure of an endangered species living in contrasted habitats: Parnassia palustris (Saxifragaceae). Mol Ecol 11:979–990. https://doi.org/10.1046/j.1365-294x.2002.01499.x
Bossuyt B (2007) Genetic rescue in an isolated metapopulation of a naturally fragmented plant species, Parnassia palustris. Conserv Biol 21:832–841. https://doi.org/10.1111/j.1523-1739.2006.00645.x
Brouwer E, Hans B, Roelofs JGM (2001) Nutrient requirements of ephemeral plant species from wet, mesotrophic soils. J Veg Sci 12:319–326. https://doi.org/10.2307/3236845
Bu H, Chen X, Xu X, Liu K, Jia P, Du G (2007) Seed mass and germination in an alpine meadow on the Eastern Tsinghai-Tibet plateau. Plant Ecol 191:127–149. https://doi.org/10.1007/s11258-006-9221-5
Cordazzo CV (2002) Effect of seed mass on germination and growth in three dominant species in Southern Brazilian coastal dunes. Braz J Biol 62:427–435. https://doi.org/10.1590/s1519-69842002000300005
Ellison AM (2001) Interspecific and intraspecific variation in seed size and germination requirements of Sarracenia (sarraceniaceae). Am J Bot 88:429–437. https://doi.org/10.2307/2657107
Farnsworth EJ, Ogurcak DE (2008) Functional groups of rare plants differ in levels of imperilment. Am J Bot 95:943–953. https://doi.org/10.3732/ajb.0800013
Fenner M (1983) Seed ecology. Chapman and Hall, London
Fenner M (1986a) The allocation of minerals to seeds in Senecio vulgaris plants subjected to nutrient shortage. J Ecol 74:385–392. https://doi.org/10.2307/2260262
Fenner M (1986b) A bioassay to determine the limiting minerals for seeds from nutrient-deprived Senecio vulgaris plant. J Ecol 74:497–505. https://doi.org/10.2307/2260270
Figura T, Tylova E, Jersakova J, Vohik M, Ponert J (2021) Fungal symbionts may modulate nitrate inhibitory effect on orchid seed germination. Mycorrhiza 31:231–241. https://doi.org/10.1007/s00572-021-01021-w
Fujita Y, Olde Venterink H, van Bodegom PM, Douma JC, Heil GW, Hölzel N, Jabłońska E, Kotowski W, Okruszko T, Pawlikowski P, de Ruiter PC, Wassen MJ (2014) Low investment in sexual reproduction threatens plants adapted to phosphorus limitation. Nature 505:82–86. https://doi.org/10.1038/nature12733
Galloway LF (2001) The effect of maternal and paternal environments on seed characters in the herbaceous plant Campanula americana (Campanulaceae). Am J Bot 88:832–840. https://doi.org/10.2307/2657035
Gray D, Thomas TH (1982) Seed germination and seedling emergence as influenced by the position of development of the seed on, and chemical applications to, the parent plant. The physiology and biochemistry of seed development. Dormancy and Germination. Elsevier, Amsterdam, pp 111–136
Güsewell S (2005) High nitrogen: phosphorus ratios reduce nutrient retention and second-year growth of wetland sedges. New Phytol 166:537–550. https://doi.org/10.1111/j.1469-8137.2005.01320.x
Güsewell S, Koerselman W (2002) Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect Plant Ecol Evol Syst 5:37–61. https://doi.org/10.1078/1433-8319-0000022
Hanley ME, Unna JE, Darvill B (2003) Seed size and germination response: a relationship for fire-following plant species exposed to thermal shock. Oecologia 134:18–22. https://doi.org/10.1007/s00442-002-1094-2
Harper JL (1977) Population biology of plants. Academic Press, London
Hejcman M, Kristalova V, Cervena K, Hrdlickova J, Pavlu V (2012) Effect of nitrogen, phosphorus and potassium availability on mother plant size, seed production and germination ability of Rumex crispus. Weed Res 52:260–268. https://doi.org/10.1111/j.1365-3180.2012.00914.x
Henery ML, Westoby M (2001) Seed mass and seed nutrient content as predictors of seed output variation between species. Oikos 92:479–490. https://doi.org/10.1034/j.1600-0706.2001.920309.x
Hrdličková J, Hejcman M, Křišťálová V, Pavlů V (2011) Production, size, and germination of broad-leaved dock seeds collected from mother plants grown under different nitrogen, phosphorus, and potassium supplies. Weed Biol Manag 11:190–201. https://doi.org/10.1111/j.1445-6664.2011.00420.x
Isselstein J, Tallowin JRB, Smith REN (2002) Factors affecting seed germination and seedling establishment of fen-meadow species. Restor Ecol 10:173–184. https://doi.org/10.1046/j.1526-100x.2002.00045.x
Kaya M, Kaya G, Kaya MD, Atak M, Saglam S, Khawar KM, Ciftci CY (2008) Interaction between seed size and NaCl on germination and early seedling growth of some Turkish cultivars of chickpea (Cicer arietinum L.). J Zhejiang Univ B 9:371–377. https://doi.org/10.1631/jzus.b0720268
Lahti T, Kemppainen E, Kurtto A, Uotila P (1991) Distribution and biological characteristics of threatened vascular plants in Finland. Biol Conserv 55:299–314. https://doi.org/10.1016/0006-3207(91)90034-7
Lamont BB, Groom PK (2013) Seeds as a source of carbon, nitrogen, and phosphorus for seedling establishment in temperate regions: a synthesis. Am J Plant Sci 4:30–40. https://doi.org/10.4236/ajps.2013.45a005
Liao H, Yan X (1999) Seed size is closely related to phosphorus use efficiency and photosynthetic phosphorus use efficiency in common bean. J Plant Nutr 22:877–888. https://doi.org/10.1080/01904169909365679
Marshall DL (1986) Effect of seed size on seedling success in three species of Sesbania (Fabaceae). Am J Bot 73:457–464. https://doi.org/10.1002/j.1537-2197.1986.tb12063.x
Mitchley J, Grubb PJ (1986) Control of relative abundance of perennials in chalk grassland in Southern England: I. Constancy of rank order and results of pot- and field-experiments on the role of interference. J Ecol 74:1139–1166. https://doi.org/10.2307/2260240
Murray BR, Thrall PH, Gill AM, Nicotra AB (2002) How plant life-history and ecological traits relate to species rarity and commonness at varying spatial scales. Austral Ecol 27:291–310. https://doi.org/10.1046/j.1442-9993.2002.01181.x
Olde Venterink H, Wassen MJ, Verkroost AWM, De Ruiter PC (2003) Species richness-productivity patterns differ between N-, P-, and K-limited wetlands. Ecology 84:2191–2199. https://doi.org/10.1890/01-0639
Parrish JAD, Bazzaz FA (1985) Nutrient content of Abutilon theophrasti seeds and the competitive ability of the resulting plants. Oecologia 65:247–251. https://doi.org/10.1007/bf00379224
Paul S, Kumaria S, Tandon P (2012) An effective nutrient medium for asymbiotic seed germination and large-scale in vitro regeneration of dendrobium hookerianum, a threatened orchid of northeast India. AoB Plants 12:1–7. https://doi.org/10.1093/aobpla/plr032
Roeling IS, Ozinga WA, van Dijk J, Eppinga MB, Wassen MJ (2018) Plant species occurrence patterns in Eurasian grasslands reflect adaptation to nutrient ratios. Oecologia 186:1055–1067. https://doi.org/10.1007/s00442-018-4086-6
Schat H (1983) Germination of some dune ecology slack pioneers. Acta Bot Neerl 32:203–212. https://doi.org/10.1111/j.1438-8677.1983.tb01701.x
Steffen W, Richardson K, Rockstrom J, Cornell SE, Fetzer I, Bennett EM, Biggs R, Carpenter SR, de Vries W, de Wit CA, Folke C, Gerten D, Heinke J, Mace GM, Persson LM, Ramanathan V, Reyers B, Sorlin S (2015) Planetary boundaries: guiding human development on a changing planet. Science 347:1259855–1259855. https://doi.org/10.1126/science.1259855
Stevens CJ, Dise NB, Mountford JO, Gowing DJ (2004) Impact of nitrogen deposition on the species richness of grasslands. Science 303:1876–1879. https://doi.org/10.1126/science.1094678
Stewart H, Miao SL, Colbert M, Carraher CE Jr (1997) Seed germination of two cattail (Typha) species as a function of everglades nutrient levels. Wetlands 17:116–122. https://doi.org/10.1007/bf03160723
Thompson K (1987) Seeds and seed banks. New Phytol 106:23–34. https://doi.org/10.1111/j.1469-8137.1987.tb04680.x
Tilman D (1982) Resource competition and community structure. Princeton University Press, Princeton
Tonitto C, David MB, Drinkwater LE (2006) Replacing bare fallows with cover crops in fertilizer-intensive cropping systems: a meta-analysis of crop yield and N dynamics. Agric Ecosyst Environ 112:58–72. https://doi.org/10.1016/j.agee.2005.07.003
Van Andel J, Vera F (1977) Reproductive allocation in Senecio sylvaticus and Chamaenerion angustifolium in relation to mineral nutrition. J Ecol 65:747–758. https://doi.org/10.2307/2259377
Veeranjaneyulu K, Ramadas V (1982) Heavy metal tolerance in normal populations of some weed species. Indian J Plant Physiol 25:400–406
Vera ML (1997) Effects of altitude and seed size on germination and seedling survival of heathland plants in North Spain. Plant Ecol 133:101–106. https://doi.org/10.1023/A:1009729201384
Verhoeven JTA, Koerselman W, Meuleman AFM (1996) Nitrogen- or phosphorus-limited growth in herbaceous, wet vegetation: relations with atmospheric inputs and management regimes. Trends Ecol Evol 11:494–497. https://doi.org/10.1016/s0169-5347(96)10055-0
Von Oheimb G, Power SA, Falk K, Friedrich U, Mohamed A, Krug A, Boschatzke N, Härdtle W (2010) N:P ratio and the nature of nutrient limitation in Calluna-dominated heathlands. Ecosystems 13:317–327. https://doi.org/10.1007/s10021-010-9320-y
Watt TA (1978) The biology of Holcus lanatus L. (Yorkshire fog) and its significance in grassland. Herb Abstr 48:195– 204
Wang S, van Dijk J, Wassen MJ (2019) Sexual reproduction traits of Holcus lanatus L. and Parnassia palustris L. in response to absolute and relative supply of nitrogen and phosphorus. Environ Exp Bot 168:103813. https://doi.org/10.1016/j.envexpbot.2019.103813
Wang S, Van Dijk J, De Boer HJ, Wassen MJ (2021) Source and sink activity of Holcus lanatus in response to absolute and relative supply of nitrogen and phosphorus. Funct Plant Biol 48:493–502. https://doi.org/10.1071/FP20118
Wassen MJ, Olde Venterink HGM, de Swart EOAM (1995) Nutrient concentrations in mires vegetation as a measure of nutrient limitation in mire ecosystems. J Veg Sci 6:5–16. https://doi.org/10.2307/3236250
Wassen MJ, Olde Venterink H, Lapshina ED, Tanneberger F (2005) Endangered plants persist under phosphorus limitation. Nature 437:547–550. https://doi.org/10.1038/nature03950
White PJ, Veneklaas EJ (2012) Nature and nurture: the importance of seed phosphorus content. Plant Soil 357:1–8. https://doi.org/10.1007/s11104-012-1128-4
Willson MF, Price PW (1980) Resource limitation of fruit and seed production in some Asclepias species. Can J Bot 58:2229–2233. https://doi.org/10.1139/b80-257
Wu G, Du G (2007) Germination is related to seed mass in grasses (Poaceae) of the eastern Qinghai-Tibetan Plateau, China. Nord J Bot 25:361–365. https://doi.org/10.1111/j.0107-055x.2007.00179.x
Wulff RD (1986a) Seed size variation in Desmodium paniculatum. II. Effects on seedling growth and physiological performance. J Ecol 74:99–114. https://doi.org/10.2307/2260351
Wulff RD (1986b) Seed size variation in Desmodium paniculatum. I. Factors affecting seed size. J Ecol 74:87–97. https://doi.org/10.2307/2260350
Wulff RD (1986c) Seed mass variation in Desmodium paniculatum: effects on reproductive yield and competitive ability. J Ecol 74:115–121. https://doi.org/10.2307/2260352
Acknowledgements
We acknowledge the China Scholarship Council (CSC) for a doctoral scholarship to Shuqiong Wang (CSC NO. 201406140142). We thank Ineke Roeling for the original seed collection and storing, Ton Markus for improving all the figures, and Joy Burrough for the professional English checking of a near-final draft of the manuscript.
Funding
The research was supported with funding from the China Scholarship Council (CSC), Grant No. 201406140142.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SW. The first draft of the manuscript was written by SW and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Verbal informed consent for publication was obtained from all participants.
Additional information
Communicated by Sandor Bartha.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, S., van Dijk, J. & Wassen, M.J. Influence of parental nitrogen : phosphorus stoichiometry on seed characteristics and performance of Holcus lanatus L. and Parnassia palustris L.. Plant Ecol 222, 1129–1142 (2021). https://doi.org/10.1007/s11258-021-01166-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-021-01166-w