Abstract
Resource allocation studies of clonal plants whose individuals form networks of interconnected ramets are very challenging. In addition, the presence of mycorrhizal fungi may further modify resource allocation. In this study, we sought to determine whether the impact of mycorrhizas on resource allocation occurs throughout the entire ramet network. The optimal division of resources is decisive for the potential ecological and evolutionary success of an individual. The study was carried out under uniform conditions on Hieracium pilosella (L.) networks with the same age. Data related to growth and reproduction were collected from the total of 47 mother plants and 210 daughter ramets that they generated. The differences between AMF-inoculated and non-inoculated plants with respect to continuous variables were tested using a one-way ANOVA. Mother plants that were inoculated with a mycorrhizal fungus displayed lower belowground dry biomass than the non-inoculated plants. The presence of the fungus did not affect the allocation of resources to other examined traits of the mother plants. The daughter ramets formed by the fungus-inoculated plants had larger rosette diameters and produced more inflorescence heads than did the non-mycorrhizal daughter ramets. In our study, the positive effect of mycorrhizas on resource allocation was observed only in the next vegetatively produced generation. The distribution of resources is a dynamic process and, from an evolutionary standpoint, it cannot be approached as a mere balance of benefits and losses observed at a given time point.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycorrhizas, the symbiotic interactions between plants and fungi, are universally observed in nature. In fact, over 80% of land plants partake in mycorrhizal relationships (Smith and Read 2008; Wang and Qiu 2006). Plants involved in the interaction with mycorrhizal fungi benefit from a more substantial supply of phosphorus and nitrogen in comparison to plants where no such interaction takes place (Van Der Heijden et al. 2003; Vogelsang et al. 2006). In turn, mycorrhizal fungi access the carbohydrates produced by the plant (Miller et al. 2002). The exchange of resources may lead to varied responses of the plant (from positive to negative ones), which will depend on the environmental circumstances as well as the species of the plant and the fungus (Johnson et al. 1997; Streitwolf-Engel et al. 2001; Sudová and Vosátka 2008; Sudová 2009; Liang et al. 2018).
The optimal distribution of the limited amount of available resources to supply growth, reproduction, and defense is the key strategy in the life of any organism (Cody 1966). A plant that has access to an increased pool of resources due to the presence of mycorrhizal fungi can evenly allocate the surplus to the growth, reproduction, and defense, channel it to one process or, as is the case of perennials, store it for use in the subsequent years of its life (Uchinomiya and Iwasa 2014; Orians et al. 2018). The pattern of distribution may be easily reconstructed in spatially isolated specimens that proliferate using one reproductive mode. However, the pattern is much more difficult in clonal plants in which a specimen comprises units known as ramets (a mother plant and daughter ramets) connected by means of rhizomes or runners. Once interconnected, they form a network that may comprise up to several thousand ramets (e.g., DeWoody et al. 2008). However, the length of the connections between the ramets will vary, depending on the growth form, i.e., phalanx or guerrilla growth. The former growth pattern is characterized by short connections, which yield complexes of closely clustered ramets. The latter growth pattern produces longer connections; therefore, the ramets are situated at a conspicuous distance from one another, with the network loosely covering the area (Lovett-Doust 1981; Schmid and Harper 1985).
Due to the networked structure, clonal plants benefit from multidirectional resource transport and signaling (Stuefer et al. 1996; de Kroon et al. 2005). The analysis of resource distribution in plants with such a networked architecture in itself is problematic, but the issue is further compounded by the presence of mycorrhizal fungi. To date, it has been found that the inoculation of clonal plants with mycorrhizal fungi enhances their vegetative reproduction (Varma and Schüepp 1994; Cassells et al. 1996). The findings have since been employed in the cultivation of species from the genus Fragaria (Smith 1995; Sinclair et al. 2014). Additionally, several studies have demonstrated that the effects of the interaction between clonal plants and arbuscular mycorrhizal fungi (AMF) differ depending on the fungal species (Streitwolf-Engel et al. 1997, 2001; Sudová and Vosátka 2008; Sudová 2009; Vannier et al. 2016). In addition, fungal inoculation, regardless of the species, not only modifies the vegetative reproduction of the inoculated plant but also impacts its growth rate, length of connections, ramets, or biomass (e.g., Varma and Schüepp 1994; Streitwolf-Engel et al. 2000).
However, clonal plants rely on vegetative reproduction, producing daughter ramets, and sexual reproduction, generating seeds. Consequently, the analysis of resource distribution should consider both processes. Thus, our study attempted to assess the impact of mycorrhizal fungi on resource allocation in the mother plants and all stolon-connected daughter ramets, which comprise the circular network that develops in mouse-ear hawkweed (Hieracium pilosella L.). Thus, the experiment spanned the entire plant (as opposed to two-ramet complexes only), analyzing resources allocated to vegetative and sexual reproduction.
Given the findings of studies that examined the impact of mycorrhizal fungi on growth and reproduction in clonal plants (Du et al. 2009), it was expected that (1) the presence of mycorrhizal fungi in the inoculated ramets would result in increased vegetative reproduction (number of runners and rosettes) and generative reproduction (number of inflorescences) compared with the non-inoculated ramets and (2) the effect would be observed simultaneously in all ramets.
Material and methods
The study species
Mouse-ear hawkweed (Hieracium pilosella L.) is a perennial plant in the daisy family (Asteraceae), which is a species indigenous to Europe and parts of Asia and is invasive in North America, Australia, and New Zealand (Beaumont et al. 2009). The leaves are gathered into a rosette at the base, from which a leafless stem sprouts to a height of 5–30 cm and is usually crowned with a single inflorescence. After ripening, the flower head stem contains purple-black achenes. Hieracium pilosella L. proliferates sexually via seeds and vegetatively using aboveground runners that grow out of axillary meristems. A rosette of leaves develops on the top of the runner and subsequently takes root. The plant may further reproduce vegetatively or produce seed-bearing flowers (Bishop and Davy 1985). Hieracium pilosella L. tends to prefer the guerrilla growth form, in which one finds the mother plant in the center of the expanding network with daughter plants encircling it, forming a round spatial pattern.
Description of the experiment
Two groups of plants were examined: I—H. pilosella with mycorrhizas and II—H. pilosella without mycorrhizas. The material, achenes of H. pilosella, was obtained in Kletno in southern Poland (GPS 16.863611, 50.261111). The achenes were surface-sterilized in sodium hypochlorite and ethanol and subsequently placed in dishes with agar media (1.4%). The dishes were placed in a phytotron chamber, where they were illuminated for 16 h/day with the temperature maintained at 21 °C during the day and 15 °C at night at 60% humidity. After 30 days half of the seedlings were transferred individually to separate trays containing steamed (three times in the temperature of 90 °C) and autoclaved (in the temperature of 121 °C) soil inoculated with the mycorrhizal fungus Glomus intraradices (Schenck and Smith). Every plant received 4 g of fungal inoculate that contained 35 fungal propagules. The remaining seedlings were also transferred individually to separate trays with steamed, non-inoculated soil. These plants that were planted in non-inoculated soil were placed on a separate table in the phytotron. The tables with control trays were 2 m away from the tables with experimental trays containing AM fungi. The trays, measuring 50 cm × 36 cm × 8 cm, were filled with a total of 12.6 L of soil. The material remained in the uniform conditions of the phytotron chamber throughout the duration of the culture. The soil culture lasted 100 days, i.e., until new inflorescences had fully developed. The data collected encompassed 47 plants from the mother-plant pool (including 23 plants inoculated with Glomus intraradices and 24 non-inoculated plants), as well as the 210 daughter ramets they produced. Glomus intraradices colonizes plants over 6 weeks (Smith and Read 2008). This is why all our measurements of plants, concerning their size and reproduction, were conducted after 12 weeks since inoculation, after reproduction was completed.
Measurement and AMF detection
The size of seedlings cultured in vitro was measured prior to planting in the AMF-inoculated soil. The date of planting was adopted as day 1 of the culture. Following the period of blooming and vegetative growth, i.e., on day 100, the plants were removed from the soil and cleaned. Subsequent measurements included the diameter of the mother-plant rosette, number of leaves in the rosette, dry mass of the aboveground and belowground sections of the mother-plant rosette, number of daughter ramets, runner length, diameter of the daughter-ramet rosette, and number of leaves in the daughter-ramet rosette. In addition, the dry mass of the aboveground and belowground parts of the mother plants, runners, daughter-ramet rosettes, and roots of the daughter ramets were also measured. The dry mass was obtained by placing the material in a laboratory drier at 80 °C for 6 h. After the first drying cycle, the samples were weighed, dried again for 30 min, and reweighed. The procedure was repeated until the last measurement was equal to the previous one. In addition, throughout the culture, shoots with inflorescences were cut to evaluate how many were produced by each of the mother plants and daughter ramets.
At the end of the culture, the roots of the mother plants and their corresponding daughter ramets were randomly selected from inoculated plants to detect Glomus intraradices. After cleaning, the roots were preserved in 50% ethanol and stored at 4 °C. The roots were then thoroughly washed in distilled water and placed in 10% KOH (24 h). Following repeated washing in distilled water, the samples were placed in 5% HCl (2 h) and subsequently stained with aniline blue (0.05%). Excess staining agent was drained, and the roots were cut into 1-cm-long sections. The slides were prepared using lactic acid. Observations were performed using an Olympus BX53 microscope (Olympus, Warsaw, Poland).
Statistical analyses
The mother plants were treated as independent experimental units. The measurements were assigned to a given mother plant, including those of the daughter ramets, whose characteristics were averaged prior to the assignment. Differences between the AMF-inoculated and non-inoculated plants with respect to continuous variables (e.g., the root dry biomass and length of the runners) were tested using a one-way ANOVA. When the dependent variable represented counts (number of inflorescence heads and number of daughter ramets), a general linear model with Poisson distribution and a logarithm link function was used. All statistical analyses were performed using SPSS ver. 24.0 (IBM Corp. 2016).
Results
The impact of AMF inoculation
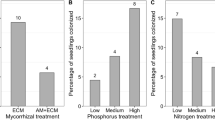
Microscopic examination confirmed the presence of Glomus intraradices in all studied inoculated samples. There were no initial differences in seedling size between the fungus-inoculated plants and the non-inoculated ones (F1,45 = 0.053, p = 0.819). The initial size of the seedlings did not affect the size and reproduction of the ramets from inoculated and non-inoculated groups. After 100 days of growth, the mother plants inoculated with the fungus exhibited significantly lower belowground dry biomass than the non-inoculated plants (F1,45 = 15.095, p < 0.001) (Fig. 1). However, the presence of the mycorrhizal fungus had no effect on resource allocation to other functions of the mother plants, including rosette diameter (F1,45 = 0.508, p = 0.479), aboveground dry biomass (F1,45 = 0.712, p = 0.403), the number of inflorescence heads (χ2 = 2.143, df = 1, p = 0.143), and the number of daughter ramets (χ2 = 0.059, df = 1, p = 0.807). The total length of the runners produced did not differ between the two groups of mother plants (F1,45 = 0.250, p = 0.620), but the average dry biomass of the runner was lower in the plants with induced mycorrhizas (F1,45 = 4.136, p = 0.048). The positive effect of the mycorrhizas on resource allocation was observed in the next vegetatively produced generation. The daughter ramets formed by the fungus-inoculated (M+) plants had a larger rosette diameter (F1,35 = 5.212, p = 0.029) (Fig. 1) and produced more inflorescence heads than the non-mycorrhizal (M−) daughter ramets (χ2 = 8.140, df = 1, p = 0.004; \(\stackrel{-}{x}\)M+ = 1.13, \(\stackrel{-}{x}\)M− = 0.37). The presence of the mycorrhizal fungus had no effect on both aboveground dry biomass (F1,35 = 0.274, p = 0.604) and on root dry biomass of the daughter ramets (F1,44 = 0.343, p = 0.561).
Discussion
Most studies that show the impact of AMF on the growth and reproduction of clonal plants have been conducted on arrays of two or several interconnected ramets. Consequently, the effects of the interdependence between AMF and a mother plant and daughter ramets were evaluated on the basis of artificially generated fragments of a plant’s network. In our study, we created an experimental arrangement that enabled the comprehensive growth of a H. pilosella network in a manner analogous to that of natural conditions. We chose H. pilosella, because this species obtains strong benefits from AMF for phosphorus supply and growth (Derelle et al. 2012). As a result, we were able to observe the effects of the AMF inoculation of mother plants in the next vegetatively produced generation. No major effects of mycorrhizas were determined with respect to the reproduction of the mother plants. However, the daughter ramets produced by the infected mother plants exhibited larger rosettes and more extensive generative reproduction than the daughter ramets from the uninfected mother plants.
The spreading mycorrhizal network formed around the roots of mother plants also reaches the daughter ramets, where its effects are detectable. Runners are a particular kind of dispersal vector, depending on which daughter-ramet networks remain in contact with the fungus as they expand (Vannier et al. 2016). Clearly, the mouse-ear hawkweed actively benefits from the mycorrhizal network. Research by Onipchenko and Zobel (2000) demonstrated that clonal plants utilize the products of the mycorrhizal network primarily when a plant does not need to produce long connections between the ramets. Such a situation is encountered in habitats where there is available space, and all the ramets have relatively equal access to nutrients. Additionally, a circular network structure tends to be preferred in such circumstances, which is exactly what the mouse-ear hawkweed does. The newly grown ramets are concentrated around the mother plants with an average length of runners of 12.33 cm. According to Onipchenko and Zobel (2000), the mycorrhizal network and the architecture of the clonal network represent two alternative strategies that ensure the plant can access nutrients in the soil.
Another possible explanation of the stronger AMF effect on daughter ramets than on mother plants could be a prolonged establishment phase of the fungus in host tissues. However, this mechanism seems of lower importance in accounting for our results. One of the relationships we revealed is an effect of AMF inoculation on belowground parts of mother plants. This indicates that, even taking into account the lag in AMF establishment, the amount of AMF was adequate to alter allocation pattern of mother plants.
Studies conducted by Du et al. (2009) show that the mycorrhizas boost the growth and vegetative reproduction of Trifolium repens in a nutrient-rich and well-illuminated environment. Alternatively, poor nutrient availability and deficient lighting translate into considerably reduced enhancement of plant growth and reproduction. When access to light is limited, the AMF import carbon, thereby decreasing the plant growth potential (Wright et al. 1998). Mycorrhizal colonization under a high-nutrient condition may be adverse to plant growth (Koide and Schreiner 1992; DeLucia et al. 1997). In addition to the environmental factors, the intensity of the positive and negative impact of the mycorrhizas is correlated with the development of the plant. The strength and scale of interdependencies between a fungal and plant species fluctuates over the course of the lifespan of the plant; therefore, the outcomes of their interaction can manifest at later stages of the plant’s development (Ronsheim 2012).
It is likely that the distribution of nutrients changes as the H. pilosella network grows. If the mother plant supplies resources to daughter ramets while receiving none in return, the process depletes the resource pool that could be allocated to sustain its interaction with the AMF. Consequently, mycorrhizal fungi do not stimulate growth and reproduction in the mother plant. Furthermore, the daughter ramets have access to a greater pool of resources that enable them to feed the mycorrhizal fungi, which then support the growth and reproduction in the daughter ramets. This result would explain the differences observed between the AMF-inoculated daughter ramets and the non-inoculated ones. Alternatively, it needs to be noted that when comparing studies conducted on distinct plant species, the manner in which the plant proliferates is considered. The variable length of the connections between the ramets is reflected in the diverse growth mode on the continuum from the phalanx to guerrilla forms (Lovett-Doust 1981; Harper 1986). The most expedient form in a given case is dictated by the habitat conditions of the plant in question (e.g., Humphrey and Pyke 2003; Chen et al. 2011). Analyses of species that tend to follow the guerrilla growth pattern must consider whether the daughter ramets grow in more or less the same time, forming a circular network around the mother plant, such as in H. pilosella, or whether they develop in a linear fashion, such as in Trisetum distichophyllum or Carex bigelowii. The formation of an encircling network in the plant may incur a higher energetic expenditure due to the production of several runners at the same time than a plant that consecutively produces daughter ramets. Additionally, in a circular network, mycorrhizal fungi can simultaneously colonize several new ramets instead of just one. This outcome leads to the question of whether the efficiency of the plant–fungus relationship changes depending on the growth type. This finding could explain why the effects of mycorrhizas are so divergent when examined in different species, development stages, and environmental conditions.
The distribution of resources is a dynamic process and from an evolutionary standpoint cannot be approached as a mere balance of benefits and losses observed at a given time point because the impact of mycorrhizas on the allocation of resources may not be observed in the mother plant or even the first daughter ramets but may become visible in the successive new ramets.
References
Beaumont LJ, Gallagher RV, Thuiller W, Downey PO, Leishman MR, Hughes L (2009) Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers Distrib 15:409–420
Bishop GF, Davy AJ (1985) Density and the commitment of apical meristems to clonal growth and reproduction in Hieracium pilosella L. Oecol 66:417–422
Cassells AC, Mark GL, Periappuram C (1996) Establishment of arbuscular mycorrhizal fungi in autotrophic strawberry cultures in vitro. Comparison with inoculation of microplants in vivo. EDP Sci 16(10):625–632
Chen X-S, Xie Y-H, Deng Z-M, Li F, Hou Z-Y (2011) A change from phalanx to guerrilla growth form is an effective strategy to acclimate to sedimentation in a wetland sedge species Carex brevicuspis (Cyperaceae). Flora 206:347–350
Cody ML (1966) A general theory of clutch size. Evolution 20:174–184
de Kroon H, Huber H, Stuefer JF, van Groenendael JM (2005) A modular concept of phenotypic plasticity in plants. New Phytol 166(1):73–82
DeLucia EH, Callaway RM, Thomas EM, Schlesinger WH (1997) Mechanisms of phosphorus acquisition for ponderosa pine under different climatic regimes. Ann Bot 79:111–120
Derelle D, Declerck S, Genet P, Dajoz I, van Aarle IM (2012) Association of highly and weakly mycorrhizal seedlings can promote the extra- and intraradical development of a common mycorrhizal network. FEMS Microbiol Ecol 79:251–259
DeWoody J, Rowe CA, Hipkins VD, Mock KE (2008) "Pando" lives: molecular genetic evidence of a giant aspen clone in Central Utah. West N Am Naturalist 68(4):493–497
Du J, Yu F-H, Alpert P, Dong M (2009) Arbuscular mycorrhizal fungi reduce effects of physiological integration in Trifolium repens. Ann Bot 104(2):335–344
Harper JL (1986) Preface to “Modular Organisms: Case Studies of Growth and Form. Papers relating to a discussion meeting on growth and form in modular organisms”. Proc R Soc B 228:111.
Humphrey LD, Pyke DA (2003) Demographic and growth responses of a guerrilla and a phalanx perennial grass in competitive mixtures. J Ecol 86(5):854–865
IBM Corp (2016) IBM SPSS Statistics for Windows, Version 24. IBM Corp, Armonk
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol 135(4):575–585
Koide RT, Schreiner RP (1992) Regulation of the vesicular–arbuscular mycorrhizal symbiosis. Annu Rev Plant Biol 43:557–581
Liang J-F, An J, Gao J-Q, Zang X-Y, Yu F-H (2018) Effects of arbuscular mycorrhizal fungi and soil nutrient addition on the growth of Phragmites australis under different dryingrewetting cycles. PLoS ONE 13(1):e0191999
Lovett-Doust L (1981) Population dynamics and local specialization in a clonal plant Ranunculus repens I The dynamics of ramets in contrasiting habitats. J Ecol 69:743–755
Miller RM, Miller SP, Jastrow JD, Rivetta CB (2002) Mycorrhizal mediated feedbacks influence net carbon gain and nutrient uptake in Andropogon gerardii. New Phytol 155:149–162
Onipchenko VG, Zobel M (2000) Mycorrhiza, vegetative mobility and responses to disturbance of alpine plants in the northwestern caucasus. Folia Geobot 35(1):1–11
Orians CM, Gomez S, Korpita T (2018) Does mycorrhizal status alter herbivore-induced changes in whole-plant resource partitioning? AoB Plants 10(1):plx071.
Ronsheim ML (2012) The effect of mycorrhizae on plant growth and reproduction varies with soil phosphorus and developmental stage. Am Midl Nat 167(1):28–39
Schmid B, Harper JL (1985) Clonal growth in grassland perennials. Density and pattern-dependent competition between plants with different growth forms. J Ecol 73:793–808
Sinclair G, Charest C, Dalpé Y, Khanizadeh S (2014) Influence of colonization by arbuscular mycorrhizal fungi on three strawberry cultivars under salty conditions. Agric Food Sci 23(2):146–158
Smith DL (1995) Effect of three vesicular-arbuscular mycorrhizae species and phosphorus on reproductive and vegetative growth of three strawberry cultivars. J Plant Nutr 18:1073–1079
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, San Diego
Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1997) Clonal growth traits of two Prunella species are determined by co-occurring arbuscular mycorrhizal fungi from a calcareous grassland. J Ecol 85:181–191
Streitwolf-Engel R, Van der Heijden MG, Wiemken A, Sanders IR (2000) The ecological significance of arbuscular mycorrhizal fungal effects on clonal reproduction in plants. Ecology 82:2846–2859
Streitwolf-Engel R, Van Der Heijden MG, Wiemken A, Sanders IR (2001) The ecological significance of arbuscular mycorrhizal fungal effects on clonal reproduction in plants. Ecology 82:2846–2859
Stuefer JF, de Kroon K, Durin HJ (1996) Exploitation of environmental heterogeneity by spatial division of labour in a clonal plant. Funct Ecol 10:328–334
Sudová R (2009) Different growth response of five coexisting stoloniferous plant species to inoculation with native arbuscular mycorrhizal fungi. Plant Ecol 204:135–143
Sudová R, Vosátka M (2008) Effects of inoculation with native arbuscular mycorrhizal fungi on clonal growth of Potentilla reptans and Fragaria moschata (Rosaceae). Plant soil 308:55–67
Uchinomiya K, Iwasa Y (2014) Optimum resource allocation in the plant–fungus symbiosis for an exponentially growing system. Evol Ecol Res 16:363–372
Van Der Heijden MG, Wiemken A, Sanders IR (2003) Different arbuscular mycorrhizal fungi alter coexistence and resource distribution between co-occurring plant. New Phytol 157:569–578
Vannier N, Bittebiere AK, Vandenkoornhuyse P, Mony C (2016) AM fungi patchiness and the clonal growth of Glechoma hederacea in heterogeneous environments. Sci Rep 6:37852
Varma A, Schüepp H (1994) Infectivity and effectiveness of Glomus intraradices on micropropagated plants. Mycorrhiza 5(1):29–37
Vogelsang KM, Reynolds HL, Bever JD (2006) Mycorrhizal fungal identity and richness determine the diversity and productivity of a tallgrass prairie system. New Phytol 172:554–562
Wang B, Qiu Y-L (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16(5):299–363
Wright DP, Scholes JD, Read DJ (1998) Effects of VA mycorrhizal colonization on photosynthesis and biomass production of Trifolium repens L. Plant Cell Environ 21:209–216
Acknowledgements
This manuscript was supported by the KNOW RNA Research Centre in Poznań (No. 01/KNOW2/2014) and the Faculty of Biology Adam Mickiewicz University in Poznań, Poland. We would like to thank Prof. Katarzyna Turnau for the seeds of Hieracium pilosella (L.) and discussion about arbuscular mycorrhizal fungi. We are also thankful to the anonymous reviewers for their critical remarks due to which our manuscript could be improved.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Communicated by Mark Brundrett.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dominiak, M., Olejniczak, P. & Lembicz, M. Diversified impact of mycorrhizal inoculation on mother plants and daughter ramets in the clonally spreading plant Hieracium pilosella L. (Asteraceae). Plant Ecol 220, 757–763 (2019). https://doi.org/10.1007/s11258-019-00950-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-019-00950-z