Abstract
Generally, only the net outcome of plant–plant interactions is measured in population and community ecology research, with few attempts to determine the relative importance of negative (competition) and positive (facilitation) interactions between subordinate species. Changes in the intensity of interactions along gradients, between life-stages, or with changing densities, and the use of selective removals enhance our capacity to infer positive and negative interactions. However, the most powerful examples at least in detecting facilitation typically involve measuring changes with or without a nurse-plant or benefactor species and often involve only a very limited numbers of species. In plant competition studies, however, greater number of species are commonly tested and density-dependent series are not an uncommon tool to test for net negative interactions. Here, we develop a cost–benefit model that can be used to comprehensively calculate the average expected net gain per individual at every point in a density series provided several response variables are recorded at each density. The utility of this model is demonstrated using both hypothetical data and several empirical data sets, and it is used to infer either both positive and negative net effects. Expected net gain can also serve as an accurate estimate of mean fitness per individual at a given density provided appropriate performance measures were recorded within the primary study. Within a single density series, both facilitation and competition can occur and were detectable using this method. This approach emphasizes the current view that both negative and positive interactions play a role in shaping plant communities. Furthermore, it is evident that facilitation can be detected using the manipulative density series typically associated with competition studies and not just using the typical target nurse-plant methodology. Finally, this method is a significant advance over the current practice of tallying up single responses within a study to estimate outcomes by providing a single, synthetic measure of the net gain or cost of interactions.






Similar content being viewed by others
References
Aksenova AA, Onipchenko VG (1998) Plant interactions in alpine tundra: 13 years of experimental removal of dominant species. Ecoscience 5:258–270
Albert CH, Yoccoz NG, Edwards TC, Graham C, Zimmerman N, Thuiller W (2010) Sampling in ecology and evolution—bridging the gap between theory and practice. Ecography 33:1028–1037
Armas C, Pugnaire F (2005) Plant interactions govern population dynamics in a semiarid plant community. J Ecol 93:978–989
Armas C, Ordiales R, Pugnaire F (2004) Measuring plant interactions: a new comparative index. Ecology 85:2682–2686
Badano EI, Bustamante RO, Villarroel E, Marquet PA, Cavieres LA (2015) Facilitation by nurse plants regulates community invasibility in harsh environments. J Veg Sci 26:756–767
Bascompte J, Jordano P, Olensen JM (2006) Asymmetric coevolutionary networks faciliate biodiversity maintenance. Science 312:431–433
Baskin CC, Baskin JM (1998) Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic Press, Cambridge
Berkowitz AR, Canham CD, Kelly VR (1995) Competition vs. facilitation of tree seedling growth and survival in early successional communities. Ecology 76:1156–1168
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193
Bertness MD, Shumway SW (1993) Competition and facilitation in marsh plants. Am Nat 142:718–724
Bertness MD, Yeh SM (1994) Cooperative and competitive interactions in the recruitment of marsh elders. Ecology 75:2416–2429
Brooker RW, Kikvidze Z, Pugnaire F, Callaway RM, Choler P, Lortie CJ, Michalet R (2005) The importance of importance. Oikos 109:63–70
Brooker RW, Maestre FT, Callaway RM, Lortie CJ, Cavieres LA, Kunstler G, Liancourt P, Tielbörger K, Travis J, Anthelme F, Armas C, Coll L, Corcket E, Delzon S, Forey E, Kikvidze Z, Olofsson J, Pugnaire F, Quiroz Cl, Saccone P, Schiffers K, Seifan M, Touzard B, Michalet R (2008) Facilitation in plant communities: the past, present, and the future. J Ecol 96:18–34
Callaway RM (1995) Positive interactions among plants. Bot Rev 61:306–349
Callaway RM (2007) Positive interactions and interdependence in plant communities. Springer, Dordrecht
Callaway RM, Walker LR (1997) Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology 78:1958–1965
Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortie CJ, Michalet R, Paolini L, Pugnaire FI, Newingham B, Aschehoug ET, Armas C, Kikidze D, Cook BJ (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Chamberlain SA, Bronstein JL, Rudgers JA (2014) How context dependent are species interactions? Ecol Lett 17:881–890
Cote IM, Jennions MD (2013) The procedure of meta-analysis in a nutshell. In: Koricheva J, Gurevitch J, Mengersen K (eds) Handbook of meta-analysis in ecology and evolution. Princeton University Press, Princeton, pp 14–26
Crawley MJ (1990) The population dynamics of plants. Philos Trans R Soc Lond 330:125–140
Filazzola A, Lortie CJ (2014) A systematic review and conceptual framework for the mechanistic pathways of nurse plants. Glob Ecol Biogeogr 23:1335–1345
Goldberg DE (1996) Competitive ability: definitions, contingency, and correlated traits. Philos Trans R Soc Lond B 351:1377–1385
Goldberg DE, Turkington R, Olsvig-Whittaker L (1995) Quantifying the community-level consequences of competition. Folia Geobot Phytotax 30:231–242
Goldberg DE, Rajaniemi T, Gurevitch J, Stewart-Oaten A (1999) Empirical approaches to quantifying interaction intensity: competition and facilitation along productivity gradients. Ecology 80:1118–1131
Goldberg DE, Turkington R, Olsvig-Whittaker L, Dyer AR (2001) Density dependence in an annual plant community: variation among life history stages. Ecol Monogr 71:423–446
Goldenheim WM, Irving AD, Bertness MD (2008) Switching from negative to positive density-dependence among populations of cobble beach plant. Oecologia 158:478–482
Goodman LA (1960) On the exact variance of products. Journal of the American Statistical Association 55:708–713
Goodman LA (1962) The variance of the product of K random variables. J Am Stat Assoc 57:54–60
Grace J, Tilman D (1990) Perspectives in Plant Competition. Academic Press, Cambridge
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194
Hacker SD, Gaines SD (1997) Some implications of direct positive interactions for community species diversity. Ecology 78:1990–2003
Hamilton JG, Holzapfel C, Mahall BE (1999) Coexistence and interference between a native perennial grass and non-native annual grasses in California. Oecologia 121:518–526
He Q, Bertness MD, Altieri AH (2013) Global shifts towards positive species interactions with increasing environmental stress. Ecol Lett 16:695–706
HilleRisLambers J, Adler PB, Harpole WS, Levine JM, Mayfield MM (2012) Rethinking Community Assembly through the lens of coexistence theory. Annu Rev Ecol Evol Syst 43:227–248
Holmgren M, Scheffer M, Huston MA (1997) The interplay of facilitation and competition in plant communities. Ecology 78:1966–1975
Isbell FI, Polley HW, Wilsey BJ (2009) Species interaction mechanisms maintain grassland plant species diversity. Ecology 90:1821–1830
Jones RB (2005) TechDig. W. Digitizer
Keddy PA (1989) Competition. Chapman and Hall, London
Koricheva J, Gurevitch J (2013) Place of meta-analysis among other methods of research synthesis. In: Koricheva J, Gurevitch J, Mengersen K (eds) Handbook of meta-analysis in ecology and evolution. Princeton University Press, Princeton, pp 3–13
Koricheva J, Gurevitch J (2014) Uses and misuses of meta-analysis in plant ecology. J Ecol 102:828–844
Koricheva J, Gurevitch J, Mengersen K (2013) Handbook of meta-analysis in ecology and evolution. Princeton University Press, Princeton
Körner C (1998) Alpine plants: stressed or adapted? In: Barker MG (ed) Physiological plant ecology. Blackwell Science, Hoboken, pp 297–311
Lortie CJ (2014) Formalized synthesis opportunities for ecology: systematic reviews and meta-analyses. Oikos 123:897–902
Lortie CJ, Svenning JC (2015) The diversity of diversity studies: retrospectives and future directions. Ecography 38:330–334
Lortie CJ, Turkington R (2002) The effect of initial seed density on the structure of a desert annual plant community. J Ecol 90:435–445
Lortie CJ, Brooker RW, Choler P, Kikvidze Z, Michalet R, Pugnaire FI, Callaway RM (2004a) Rethinking plant community theory. Oikos 107:433–438
Lortie CJ, Brooker RW, Kikvidze Z, Callaway RM (2004b) The value of stress and limitation in an imperfect world: a reply to Körner. J Veg Sci 15:577–580
Lyons KG, Schwartz MW (2001) Rare species loss alters ecosystem function-invasion resistance. Ecol Lett 4:358–365
McIntire EJB (2014) Being a facilitator can be costly: teasing apart reciprocal effects. New Phytol 202:4–6
McIntire EJB, Fajardo A (2014) Facilitation as a ubiquitous driver of biodiversity. New Phytol 201:403–416
McMurray MH, Jenkins SH, Longland WS (1997) Effects of seed density on germination and establishment of a native and an introduced grass species dispersed by granivorous rodents. Am Midl Nat 138:322–330
Michalet R, Brooker RW, Cavieres LA, Kikvidze Z, Lortie CJ, Pugnaire FI, Valiente-Banuet A, Callaway RM (2006) Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol Lett 9:767–773
Michalet R, Maalouf J-P, Choler P, Clément B, Rosebery D, Royer J-M, Schöb C, Lortie CJ (2014) Competition, facilitation and environmental severity shape the relationship between local and regional species richness in plant communities. Ecography 38:335–345
Olsen SL, Töpper JP, Skarpaas O, Vandvik V, Klanderud K (2016) From facilitation to competition: temperature-driven shift in dominant plant interactions affects population dynamics in semi-natural grasslands. Glob Chang Biol: n/a-n/a
Palmblad IG (1968) Competition in experimental populations of weeds with emphasis on the regulation of population size. Ecology 49:26–34
Pescador DS, Chacón-Labella J, de la Cruz M, Escudero A (2014) Maintaining distances with the engineer: patterns of coexistence in plant communities beyond the patch-bare dichotomy. New Phytol 204:140–148
Pugnaire FI, Zhang L, Li R, Luo T (2015) No evidence of facilitation collapse in the Tibetan plateau. J Veg Sci 26:233–242
Schmitt RJ, Holbrook SJ (2003) Mutualism can mediate competition and promote coexistence. Ecol Lett 6:898–902
Schöb C, Michalet R, Cavieres LA, Pugnaire FI, Brooker RW, Butterfield BJ, Cook BJ, Kikvidze Z, Lortie CJ, Xiao S, Al Hayek P, Anthelme F, Cranston BH, García M-C, Le Bagousse-Pinguet Y, Reid AM, le Roux PC, Lingua E, Nyakatya MJ, Touzard B, Zhao L, Callaway RM (2014a) A global analysis of bidirectional interactions in alpine plant communities shows facilitators experiencing strong reciprocal fitness costs. New Phytol 202:95–105
Schöb C, Prieto I, Armas C, Pugnaire FI (2014b) Consequences of facilitation: one plant’s benefit is another plant’s cost. Funct Ecol 28:500–508
Soliveres S, Maestre FT (2014) Plant–plant interactions, environmental gradients and plant diversity: a global synthesis of community-level studies. Perspect Plant Ecol Evol Syst 16:154–163
Sotomayor DA, Lortie CJ (2015) Indirect interactions in terrestrial plant communities: emerging patterns and research gaps. Ecosphere 6:art103-art103
Spiegel O, Nathan R (2012) Empirical evaluation of directed dispersal and density-dependent effects across successive recruitment phases. J Ecol 100:392–404
Stanton-Geddes J, Tiffin P, Shaw RG (2012) Role of climate and competitors in limiting fitness across range edges of an annual plant. Ecology 93:1604–1613
Sthultz CM, Gehring CA, Whitham TG (2007) Shifts from competition to facilitation between a foundation tree and a pioneer shrub across spatial and temporal scales in a semiarid woodland. New Phytol 173:135–145
Tielbörger K, Bilton MC, Metz J, Kigel J, Holzapfel C, Lebrija-Trejos E, Konsens I, Parag HA, Sternberg M (2014) Middle-Eastern plant communities tolerate 9 years of drought in a multi-site climate manipulation experiment. Nat Commun 5:5102
Walker LR, Vitousek PM (1991) An invaded alters germination and growth of a native dominant tree in Hawaii. Ecology 72:1449–1455
Wilson WG, Nisbet RM (1997) Cooperation and competition along smooth environmental gradients. Ecology 78:2994–3017
Wyszomirski T, Weiner J (2009) Variation in local density results in a positive correlation between plant neighbor sizes. Am Nat 173:705–708
Xiao S, Michalet R (2013) Do indirect interactions always contribute to net indirect facilitation? Ecol Model 268:1–8
Acknowledgments
Research was supported by an NSERC postgraduate scholarship and a fellowship from the Blaustein Center for Scientific Cooperation to CJL and an NSERC operating grant to RT. This is a publication of the Mitrani Department of Desert Ecology. We wish to extend special thanks to one referee in particular that provided numerous extremely useful ideas to the implications and interpretation of this model.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Prof. Lauchlan Fraser, Dr. Chris Lortie, Dr. JC Cahill.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
A vignette for the ecological cost–benefit analyses from density series including the calculation of variance of products.
Description
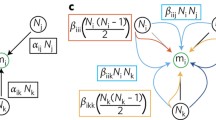
Two functions were developed to conduct ecological cost–benefit analyses on plant data for density series. The equation (Eq. 1) was developed to integrate more than one estimate/measure of plant success, (e.g., fitness, survival, or emergence), represented as expected gain or W as fitness if there are three representative measures within a primary dataset; where W is the product of multiple plant estimates. Variance of W was based on Goodman (1960, 1962) and is calculated with Eq. 2 when there are two products in W and with Eq. 3 when three products are present in W. To calculate W and the Variance of W (Var.W), the mean and variance of the sample populations are required for all density levels. Other factors can also be included in the analysis including species, year, and population. The input data must be a matrix with two columns specified as “mean” and “se” to represent means and variance for plant estimates at the associated density.
Usage
W.2 (x, estimates)
W.3 (x, density, population, year)
Arguments
X—an object of class “data.frame”, with two columns labeled “mean” and “se”.
Estimates—a vector of the plant estimate or measure that is to be multiplied to derive W.
Density—a vector of the plant densities found within rows.
Population—a vector specifying a subset of observations to be aggregated by for W (e.g., species, population, survey method)
Year—an option vector to subset the observations further by year.
Value
W.2 and W.3 each returns an object of class “data.frame” with columns representing the original data except without columns for the estimate, mean, or se. The last two columns of the data frame represent W from Eq. 1, and Var(W) from Eqs. 2 or 3 depending on function used.
Functions
> W.2 <- function(x, estimates){
> est2 <- match(x[,estimates], levels(x[,estimates]))
> x1 <- subset(x, est2==1)
> y1 <- subset(x, est2==2)
> W <- x1$mean*y1$mean
> var.w <- (((x1$mean^2)*y1$se)+((y1$mean^2)*x1$se))-(x1$se*y1$se)
> W.2 <- cbind(x1[,1:(ncol(x1)-3)],W,var.w)
> return(W.2)
> }
>W.3 <- function(x, density, population, year){
>#calculate W
>if(missing(year)){
>W <- setNames(aggregate(x$mean, by=list(x[,density],x[,population]), prod), c(“Density”,”Population”,”W”))
>x[“var.w”] <- x$se/x$mean
>var.w <- setNames(aggregate(x$var.w, by=list(x[,density],data[,population]), sum), c(“Density”,”Population”,”Var.W”))
>var.w[,4] <- var.w[,4]*W[,4]
>metrics <- cbind(W,Var.w=var.w[,4])
>return(metrics)
>}
>else
>{
>W <- setNames(aggregate(x$mean, by=list(x[,density],x[,year],x[,population]), prod), c(“Density”,”Year”,”Population”,”W”))
>}
>#calculate variance
>x[“var.w”] <- x$se/x$mean
>var.w <- setNames(aggregate(x$var.w, by=list(x[,density],data[,year],data[,population]), sum), c(“Density”,”Year”,”Population”,”Var.W”))
>var.w[,4] <- var.w[,4]*W[,4]
>metrics <- cbind(W,Var.w=var.w[,4])
>return(metrics)
>}
Examples
## Lortie & Turkington 2002. J. Ecol.
data(lortie2002)
Cost.benefit <- W.2(lortie2002, estimates= “factor”)
Cost.benefit
data(lortie2002)
Cost.benefit <- W.3(lortie2002, density=“seed.density”, population=“Population”, year=“year”)
Cost.benefit
Rights and permissions
About this article
Cite this article
Lortie, C.J., Filazzola, A., Welham, C. et al. A cost–benefit model for plant–plant interactions: a density-series tool to detect facilitation. Plant Ecol 217, 1315–1329 (2016). https://doi.org/10.1007/s11258-016-0604-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-016-0604-y